Abstract
Optimal CT pulmonary angiography (CTPA) is a prerequisite for accurate diagnosis and management of suspected venous thromboembolic disease (VTE) in the emergency department (ED). However, a certain proportion of CTPA studies are diagnostically limited or non-diagnostic due to various technical causes. In this study, we analyze the incidence and cause of suboptimal CTPA studies in the ED and assess the need for additional imaging. Reports of 1444 consecutive CTPAs performed in an ED on adult patients over a 25-month period beginning November 30, 2011, were reviewed. The observed suboptimal CTPA rate was 4.2 % (60/1444). The most common causes of limited or non-diagnostic CTPA in the ED were related to timing of contrast bolus or IV infiltration (26/60, 43.4 %), respiratory motion (16/60, 26.7 %), multifactorial causes (10/60, 16.7 %), and patient motion (8/60, 13.3 %). Of the 60 studies included, only 7 patients (11.7 %) underwent additional diagnostic imaging during the same hospital visit for VTE, while 3 patients (5.0 %) underwent additional imaging for suspected VTE over the next 2 months. A total of 2/60 (3.4 %) patients had documented acute PE on additional imaging performed either on the same hospital visit or within 2 months. Regardless of the factors contributing to suboptimal CTPA, only a very small proportion of patients receive additional imaging to evaluate for VTE, either on the same visit or during the next 2 months (16.7 %, 10/60 patients). A small number (3.4 %) of these patients have documented acute PE within 2 months when additional imaging tests were performed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolic disease (VTE) is a significant cause of morbidity and mortality in the USA, with an incidence of approximately 300,000 people annually. It is responsible for 50,000 deaths each year [1]. Because of the significant associated mortality [2–5], prompt diagnosis is a requisite for appropriate management of patients with suspected VTE. CT pulmonary angiography (CTPA) is considered the most sensitive and specific modality in the evaluation of acute VTE in the emergency setting [6]. The high accuracy of CTPA has made it the imaging study of choice in the evaluation of patients with suspected VTE [7] (Figs. 1, 2, and 3).
Axial CT images at the level of the main pulmonary artery (a, c) and at the level of lung bases (b, d) in four different patients demonstrate the most common causes of suboptimal CTPA, including a poor contrast bolus, b respiratory motion, c multifactorial causes, and d patient motion. Factors contributing to suboptimal CTPA studies classified as multifactorial include any combination of suboptimal contrast bolus, respiratory motion or patient motion, patient habitus, streak artifact, and atelectasis
A 39-year-old male with pleuritic chest pain and hypoxia underwent CTPA in the ED, which was non-diagnostic due to suboptimal timing of contrast bolus (a, axial; b, coronal). The patient was admitted to the hospital, and a V/Q scan performed on the following day (c, Xe-133 ventilation scan) was positive for acute PE (d, arrow, Tc-99 MAA perfusion scan)
A 74-year-old female with stage IV lung adenocarcinoma and prior left pneumonectomy presenting with shortness of breath. CTPA in the ED was limited by respiratory motion at the right lung base (a). The patient returned 62 days later, at which time a second CTPA (b, axial, c, coronal) was positive for acute PE (arrows). The acute PE diagnosed on the second scan was thought to represent a new acute PE, and not a missed PE from the prior limited study
Due to the high mortality associated with untreated acute VTE, clinicians in the emergency department (ED) maintain high clinical suspicion and operate with a low threshold to order a CTPA, at times resulting in overuse of CTPA [8, 9]. Furthermore, the symptoms of acute VTE overlap with many unrelated medical conditions, further contributing to the widespread use of CTPA in the ED. Although the vast majority of CTPA are of diagnostic quality, a certain proportion will be non-diagnostic for a variety of reasons. Previously, with older model scanners that used 4-slice and 16-slice multidetector computed tomography (MDCT), the main factors that resulted in an indeterminate CTPA study were motion artifact (74 %) and poor contrast enhancement of the pulmonary arteries (40 %) [10]. Scanner technology has evolved over the past 10–15 years, and with widespread use of 64-slice and 128-slice scanners, the factors that limit diagnostic quality CTPA may have evolved.
In theory, once sufficient clinical suspicion exists to request a CTPA for a patient in the ED, the diagnosis must be either confirmed or excluded. In reality, however, a certain percentage of CTPA will be either suboptimal or non-diagnostic and the ED clinician treating the patient may or may not opt to pursue further diagnostic workup for acute VTE. The purpose of this study is to examine the incidence of factors that contribute to suboptimal or non-diagnostic CTPA studies in the ED at an academic urban level 1 trauma center. A second question addressed in this study, which may have larger implications, is what happens to the patients after a suboptimal or indeterminate CTPA in the ED. Since the decision to treat patients with subsegmental pulmonary emboli is controversial [11], patients should at least be adequately evaluated through the level of the segmental pulmonary arteries in order to confidently confirm or exclude acute VTE that warrants treatment.
Methods
This retrospective study was approved by the Institutional Review Board and was conducted in accordance with the Health Insurance Portability and Accountability Act of 1996; informed consent was waived. Diagnostic radiology reports from 1444 consecutive CTPA studies performed in an ED on adult patients over a 25-month period beginning on November 30, 2011, were reviewed. Subjects were included if the impression of the final radiology report indicated that the examination was described as suboptimal or indeterminate through the level of the main, lobar, or segmental pulmonary arteries or if the examination was declared non-diagnostic. Of the 1444 examinations performed during this period, 60 met the criteria for inclusion (males, n = 31; females, n = 29; mean age = 53.7 years, range 22.1–86.7 years). Medical records for this group were reviewed, and descriptive statistics were calculated on the factors limiting scan quality.
All CTPA studies were performed on a 64-slice scanner (LightSpeed VCT; GE Medical Systems, Milwaukee, WI) using standard CTPA protocol at our institution which includes at first a localizer sequence acquired through the level of the carina followed by a timing bolus of 20 cm3 of intravenous contrast injected at 4–5 cm3/s using a power injector (Medrad Stellant, Bayer HealthCare, Whippany NJ). For the timing bolus, sequential axial scans at the level of the main pulmonary artery are acquired at a 2-s interval until the peak Hounsfield unit measurement is obtained. Subsequently, 60 cm3 of IV contrast is injected at a rate of 4–5 cm3/s using a power injector for the CTPA scan with a saline chaser; 5 s are added to the measured peak enhancement time after contrast injection. Contiguous axial slices from the lowest margin of the diaphragm to the lung apices during breath hold at end expiration are acquired. Our protocol requires a working peripheral IV cannula or line of at least 20 gauge, preferably at antecubital fossa, and use of Isovue-370 (Iopamidol Injection 76 %; Bracco Diagnostics Inc., Monroe Township, NJ) intravenous administration. Additional parameters for the CTPA scan are as follows: 0.625 mm slice thickness with 1.25- and 3.75-mm reconstructions using 40 % adaptive statistical iterative reconstruction, or ASIR, 120 kV, automated mA (min 200–max 650), noise index 23, 0.4 s rotation, and a pitch of 1.375/55 (40 mm detector coverage). Soft tissue reformats in coronal and sagittal planes and maximum intensity projection (MIP) reformats in axial and coronal planes were created.
Medical records were reviewed to determine whether or not patients underwent additional testing for acute VTE, either with repeat CTPA or a ventilation-perfusion (V/Q) scan, during the same hospital visit and over the subsequent 2 months. The observed rate of suboptimal CTPA was calculated. In addition, the frequency of the most common causes of suboptimal CTPA was determined. Follow-up data from the medical record was reviewed to determine what percentage of patients with suboptimal CTPA in the ED underwent subsequent diagnostic imaging to evaluate for acute VTE, either during the same hospital admission or during the following 2 months. The rates of subsequently diagnosed CTPA were determined.
Results
The observed rate of suboptimal CTPA from the ED was 4.2 % (60/1444). The most common causes of suboptimal or non-diagnostic CTPA in the ED were related to the suboptimal timing of IV contrast bolus or IV infiltration (26/60, 43.4 %), respiratory motion (16/60, 26.7 %), multifactorial causes (10/60, 16.7 %), and patient motion (8/60, 13.3 %).
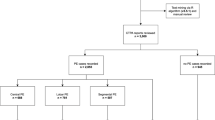
Of the 60 subjects whose CTPA examinations were declared suboptimal or non-diagnostic, only 7 patients (11.7 %) underwent additional diagnostic imaging to evaluate for acute VTE during the same hospital visit, with 5 patients (8.3 %) undergoing repeat CTPA and 2 patients (3.3 %) evaluated with V/Q scans. Only one patient was diagnosed with acute PE on the same hospital visit after positive findings 1 day later on a V/Q scan. Over the following 2 months, 3 patients out of the initial 60 (5.0 %) underwent additional imaging for suspected VTE, all with CTPA. From this group, a single examination performed exactly 2 months later (62 days) was positive for acute PE. Altogether, a total of 2/60 (3.4 %) patients had documented acute PE on additional imaging performed either on the same hospital visit or within 2 months, as outlined in Fig 4.
Discussion
CT pulmonary angiography is widely accepted as the examination of choice in the evaluation of suspected VTE in the ED, with sensitivity and specificity of 90 and 95 %, respectively [12–14]. The suboptimal CTPA rate in ED patients in our study was 4.2 %, slightly lower than but comparable to the 2005 study by Jones et al. [10] who reported a rate of 6.6 %. A suboptimal CTPA may fail to diagnose VTE with resultant fatality from untreated acute pulmonary embolism. Although the exact mortality of untreated pulmonary embolism is a matter of debate, the previously reported rate ranges between 5 and 30 % [2, 4, 15, 16].
In our study, the primary cause of suboptimal CTPA was attributed to poor opacification of the pulmonary arteries with intravenous contrast. This finding differs from Jones’ finding that motion artifact is the main culprit in suboptimal CTPA [10], a discrepancy that is likely attributable to the use of 64-slice multidetector CT at our institution and the use of 4- to 16-slice scanners in their study. Other authors using a single detector scanner have also found suboptimal contrast opacification of the pulmonary arteries to be the main cause [17], though it should be noted that their study had a substantially higher rate of indeterminate or suboptimal CTPA rate of 35.7 % (46/129 patients).
Regardless of the factors contributing to a suboptimal CTPA, only a very small proportion of patients received additional imaging to evaluate for VTE, either on the same visit or during the next 2 months (16.7 %, 10/60 patients). Aside from one study examining outcomes in cancer patients with suboptimal CTPA [18], a review of the literature reveals one study which specifically examines the outcomes of patients from the general population who undergo a suboptimal CTPA [10]. A small proportion (3.4 %) of these patients were diagnosed with acute PE within 2 months when additional imaging tests were performed. In our study, high clinical suspicion led clinicians to pursue a V/Q scan immediately that led to a diagnosis of PE for one patient, while the other patient was diagnosed 2 months later after re-presenting with persistent symptoms. In the patient who underwent CTPA 2 months later, the clot appeared to represent an acute PE rather than a chronic embolus that had been missed on the prior scan, suggesting that a repeat scan may not have changed the course of this patient’s care.
Limitations
Our study has several limitations. First, the retrospective nature of our study presents several possible biases. We were unable to prospectively collect risk stratification data, such as modified Well’s criteria scores, which could contribute to our understanding of how this subset of patients is managed. Second, even though a standard CTPA protocol is utilized, we are unable to ensure that the exact same technical parameters were used in each case including the type of IV catheter. Lastly, the actual CTPA reports were reviewed from the medical records rather then re-evaluating the images with preset criteria for quality of each scan. These studies were reviewed by multiple radiologists with 2 to 15 years of experience as a part of routine clinical care. This may have resulted in interobserver variability with potential inconsistency of categorizing a scan into a suboptimal study.
Conclusions
The number of patients diagnosed with acute VTE has risen dramatically since the year 2000, likely in large part due to advances in MDCT imaging with CTPA [19]. Appropriateness Criteria from the American College of Radiology for evaluation of patients with acute chest pain and suspected pulmonary embolism are readily available [20]. Because of the complex nature of VTE and the way emergency medicine is practiced, there is a solid base of literature that strives to optimize the appropriate use of CTPA in the ED [8, 21]. However, at this time, no consensus exists to guide clinicians in the evaluation of patients after a suboptimal CTPA is performed in the ED. In order to move towards evidence-based approach in this clinical scenario, we must first understand how often repeat imaging is performed presently. The decision to repeat CTPA or pursue alternative testing with V/Q scan may depend on local availability of services, consideration of radiation dose [22], and the clinical acuity of a given patient. In order to minimize suboptimal CTPA, a properly timed contrast bolus and peripheral IV line are of paramount importance.
References
Giuntini C, Di Ricco G, Marini C, Melillo E, Palla A (1995) Pulmonary embolism: epidemiology. Chest 107(1 Suppl):3S–9S
Calder KK, Herbert M, Henderson SO (2005) The mortality of untreated pulmonary embolism in emergency department patients. Ann Emerg Med 45(3):302–310
Egermayer P (1996) The mortality of untreated pulmonary embolism. Chest 110(1):303
Engelke C, Rummeny EJ, Marten K (2006) Pulmonary embolism at multi-detector row CT of chest: one-year survival of treated and untreated patients. Radiology 239(2):563–575
Polo Friz H, Molteni M, Del Sorbo D, et al. (2015) Mortality at 30 and 90 days in elderly patients with pulmonary embolism: a retrospective cohort study. Intern Emerg Med 10(4):431–436
Parikh N, Morris E, Babb J, et al. (2015) MDCT diagnosis of acute pulmonary embolism in the emergent setting. Emerg Radiol 22(4):379–384
Mamlouk MD, van Sonnenberg E, Gosalia R, et al. (2010) Pulmonary embolism at CT angiography: implications for appropriateness, cost, and radiation exposure in 2003 patients. Radiology 256(2):625–632
Crichlow A, Cuker A, Mills AM (2012) Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad Emerg Med 19(11):1219–1226
Molaee S, Ghanaati H, Safavi E, Foroumandi M, Peiman S (2015) Computed tomography pulmonary angiography for evaluation of patients with suspected pulmonary embolism: use or overuse. Iran J Radiol 12(3):e22383
Jones SE, Wittram C (2005) The indeterminate CT pulmonary angiogram: imaging characteristics and patient clinical outcome. Radiology 237(1):329–337
Mehta D, Barnett M, Zhou L, et al. (2014) Management and outcomes of single subsegmental pulmonary embolus: a retrospective audit at North Shore Hospital, New Zealand. Intern Med J 44(9):872–876
Perrier A, Roy PM, Sanchez O, et al. (2005) Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 352(17):1760–1768
Stein PD, Fowler SE, Goodman LR, et al. (2006) Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 354(22):2317–2327
van Belle A, Buller HR, Huisman MV, et al. (2006) Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 295(2):172–179
Carson JL, Kelley MA, Duff A, et al. (1992) The clinical course of pulmonary embolism. N Engl J Med 326(19):1240–1245
Stein PD, Henry JW, Relyea B (1995) Untreated patients with pulmonary embolism. Outcome, clinical, and laboratory assessment. Chest 107(4):931–935
Gosselin MV, Rassner UA, Thieszen SL, Phillips J, Oki A (2004) Contrast dynamics during CT pulmonary angiogram: analysis of an inspiration associated artifact. J Thorac Imaging 19(1):1–7
Hayes SA, Soff GA, Zabor EC, Moskowitz CS, Liu CC, Ginsberg MS (2014) Clinical consequences of an indeterminate CT pulmonary angiogram in cancer patients. Clin Imaging 38(5):637–640
Schissler AJ, Rozenshtein A, Schluger NW, Einstein AJ (2015) National trends in emergency room diagnosis of pulmonary embolism, 2001-2010: a cross-sectional study. Respir Res 16:44
Bettmann MA, Baginski SG, White RD, et al. (2012) ACR Appropriateness Criteria(R) acute chest pain—suspected pulmonary embolism. J Thorac Imaging 27(2):W28–W31
Cooper J (2015) Improving the diagnosis of pulmonary embolism in the emergency department. BMJ Qual Improv Rep 4(1)
Sodickson A, Baeyens PF, Andriole KP, et al. (2009) Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 251(1):175–184
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ᅟ
This retrospective study was approved by the Institutional Review Board and was conducted in accordance with the Health Insurance Portability and Accountability Act of 1996; informed consent was waived.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bates, D.D.B., Tkacz, J.N., LeBedis, C.A. et al. Suboptimal CT pulmonary angiography in the emergency department: a retrospective analysis of outcomes in a large academic medical center. Emerg Radiol 23, 603–607 (2016). https://doi.org/10.1007/s10140-016-1425-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-016-1425-y