Abstract
Purpose
Magnetic resonance imaging (MRI) is a sensitive imaging tool which lacks the burden of ionizing radiation. It is not established as primary diagnostic tool in traumatic brain injury (TBI). The purpose of this study was to evaluate the usefulness of MRI as initial imaging modality in the emergency management of mild pediatric TBI.
Methods
Children (0–18 years, sub-divided in four age-groups) with mild TBI who received MRI in the emergency department were identified. Clinical characteristics and trauma mechanisms were evaluated retrospectively. Univariate and multivariate logistic regression analyses were used to identify clinical factors that might be indicative for trauma sequelae on MRI scans.
Results
An institutional case series of 569 patients (322 male/247 female; age < 18years; (GCS ≥ 13), who received MRI for mild TBI, was analyzed. Multi-sequence imaging (including T2, T2*, FLAIR, and diffusion-weighted sequences) was feasible without sedation in 96.8% of cases (sedation, 1.8%; general anesthesia, 1.4%). MRI revealed trauma-associated findings in 13% of all cases; incidental findings were detected in 4.7%. In our cohort, GCS deterioration, scalp hematoma, clinical signs of skull base fractures, and horseback riding accidents were related to structural trauma sequelae on MRI.
Conclusions
MRI is a practical primary imaging tool for evaluating children with mild TBI in the emergency department. The presented analyses demonstrated that in our institution, MRI imaging is performed frequently in the emergency department. It resulted mostly in normal findings. This may reflect uneasiness of when to perform imaging in mild TBI and appears retrospectively as an “overdo.” There are clinical factors that are more likely associated with MRI-positive findings. Their reliability has to be evaluated in prospective studies in order to formulate further decision rules of when to perform MRI imaging or not.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) occurs frequently in children and young adults and is a major cause of mortality and morbidity [1, 2]. Due to the availability and practicability, computed tomography (CT) remains the first-line imaging modality for the evaluation of TBI sequelae. However, large cohort studies have proven that CT-related ionizing radiation bears a serious risk to induce malignancies in children in the long-time course after imaging [3,4,5,6]. To avoid these potentially negative consequences, magnetic resonance imaging (MRI) is increasingly recognized as an alternative modality in pediatric TBI [7].
By investigating children who primarily received CT scans followed by rapid MRI within 48 h, Mehta et al. concluded that rapid MRI represents a promising imaging technique for detecting TBI sequelae [8]. This was confirmed by Sheridan et al. who demonstrated reasonable sensitivity and specificity of fast MRI sequences to detect brain lesions after trauma [9]. Young et al. demonstrated similar detection rates of intracranial injury by using MRI in non-sedated toddlers compared to acquisition of cranial CT scans [10]. Buttram et al. reported a three times higher detection rate of MRI for intraparenchymal lesions compared to CT scans, including diffuse axonal injury (DAI) [11]. In all these studies, MRI was used as supplementary investigation in patients who initially received a CT scan for TBI evaluation.
Despite the advantages of MRI, this imaging modality is not used routinely as initial diagnostic tool in the management of acute TBI in children. One reason for this is that MRI is still considered too cumbersome and impracticable in young children (German guidelines AWMF “Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V.“; http://www.awmf.org/uploads/tx_szleitlinien/024-018l_S2k_Schaedel-Hirn Trauma_im_Kindesalter, web link as of January 10, 2018). Furthermore, there is still no gold standard of this imaging modality for detecting TBI-related pathologies. However, it bears the potential to become the imaging tool of choice in mild TBI in which there is often uncertainty about the need of imaging to not miss potential cranial trauma sequelae. The latter is considered in the current national guidelines, which recommend cranial imaging and hospital admission besides others in case of “uncertainty in regard to behavioral changes of the child by the parents’ impression” (web link as of January 10, 2018).
To address this dilemma, the Pediatric Emergency Care Applied Research Network (PECARN) provides clinical decision rules in order to identify children who are at low risk for relevant TBI and in whom imaging, i.e., cranial CT, can be omitted [12]. Despite these efforts, the use of cranial CT scans in the setting of mild TBI did actually not decline: Due to the uncertainty of potentially overlooked TBI sequelae, clinicians often decide to perform imaging taking the risks associated with ionizing radiation [5, 6, 13].
Based on the current national guidelines of the AWMF, every patient who was admitted to the emergency department of our hospital with TBI-related symptoms like severe or prolonged headaches, vomiting, behavioral changes due to parents’ or caregivers’ impression (especially in children less than 24 months of age), or after falls from significant height (> 1.5 m) or accidents involving high velocity, received cranial imaging (AWMF guidelines; link provided above, and European guidelines, [14]). To avoid ionizing radiation in patients younger than 19 years of age, it is the policy of our hospital to perform MRI as first imaging modality instead of CT imaging in mild TBI (24 h/day). Because MRI is not an established tool in this setting, we now sought to investigate the usefulness of this approach in respect to practicability like need of sedation and duration of the examination. In this context, we also aimed to identify MRI sequences, which might be suitable candidates in future standardized protocols. In this context, indicators that may predict structural trauma sequelae in MRI imaging and direct clinical decision-making on when to use cranial imaging in children after mild TBI were statistically analyzed.
Material and methods
Ethical approval was obtained from the Clinical Research Ethics Board of the Christian-Albrechts-University, Kiel, Germany (AZ 431/15).
Identification of study groups and clinical data acquisition
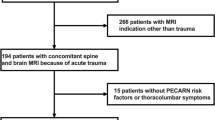
The prospectively maintained electronic databases of our hospital were queried for all patients younger than or at age 18 years who were admitted for mild TBI (GCS 13-15) (January 2009 to July 2016). Patients who received cranial MRI as initial imaging modality as part of their emergency assessment were included in the study. Their charts were reviewed retrospectively for age, sex, type of injury, clinical features, further clinical course, and duration of hospital stay. Headache and amnesia were evaluated in children older than 4 years of age. Children with suspected non-accidental injury were excluded.
Imaging protocols
MRI of children who were included in the study was re-evaluated by a neuroradiologist. Examinations were performed on either 1.5 Tesla or 3 Tesla scanners (Achieva; Philips Healthcare, Best, The Netherlands). Data acquisition included single-shot sequences, fast sequences, or higher resolution sequences as performed in routine protocols.
Protocols included diffusion-weighted images (DWI), T2-weighted images, FLAIR-images (Fluid attenuation inversion recovery), and T2*-weighted images or BOLD-images (Blood-oxygen-level dependent).
In incompliant children, T2-weighted imaging was acquired with single-shot technique. In cases of assumed mid face injury, additional STIR (Short-tau inversion recovery)-sequences were performed. In cases with incidental findings, respective additional sequences were performed.
Statistical analysis
Statistical evaluation was performed by SPSS (version 22; IBM Inc., Somers, NY, USA) and the python packages Scikit-learn (Pedregosa et al, Scikit-learn: Machine Learning in Python, JMLR 12, pp. 2825–2830, 2011) and Statsmodels (http://statsmodels.sourceforge.net/). For the logistic regression model, we defined the appearance of any trauma finding on the MRI scan as the primary outcome measure. We set up a univariate and multivariate binary logistic regression model to test the given variables from the acquired clinical data.
Results
Clinical characteristics
Five hundred sixty-nine patients met the inclusion criteria, i.e., who received a cranial MRI during primary care in the emergency department of our hospital for mild TBI. Patients received imaging in accordance to the current national guidelines of TBI management in our country (details are provided in the introduction). Patient demographics are shown in Table 1. Clinical characteristics of patients included in this study are summarized in Table 2. The mean age was 9.45 ± 4.43 years with 322 (56.6%) males and 247 (43.4%) females. Patients were further subdivided into the following age groups: 0–12 months (13 patients; 2.3%), 1–5 years (122 patients, 21.4%), 6–11 years (216 patients; 38.0%), and 12–18 years (218 patients; 38.3%). The GCS was 15 in 91.0% of the patients, 14 in 4.7%, and 13 in 4.2%. GCS deterioration occurred in five cases (0.9%). Seizures occurred in nine cases. The mean time from presentation in the emergency department to imaging took 60 min (median, 48 min; minimum of 10 min and maximum of 194 min).
Trauma mechanisms
The most common trauma mechanisms included falls, especially in the younger age groups (0–12 months and 1–5 years), followed by sports-related accidents mostly in the older age groups (6–12 and 12–18 years). Accidents in which children were run over or struck by vehicles were also more common in the older age groups as well as motor vehicle accidents, bicycle-related accidents, assaults, and horseback riding accidents.
MRI protocols and sequences
Depending on the MRI findings and the patients’ compliance, scanning times lasted from 30 s to 35 min with an average of 7:54 min. The standard protocol (T2, FLAIR, T2*, and DWI) in single-shot technique took 2 min, 4 h and 30 min in fast acquisition technique, and 11 h and 45 min in the routine protocol. The most frequent MRI sequences performed during initial imaging included T2-weighted images, performed in 565 patients (99%), diffusion-weighted imaging (DWI) in 547 patients (96%), FLAIR in 546 patients (96%), and T2*-weighted/BOLD-Imaging in 515/59 patients (91/10%). Standard protocols are summarized in Table 3.
Fast acquisition technique was used in more than 60% of the cases. Single-shot technique was only needed in 3% of the patients, who became uncooperative during the exam. STIR sequences for detecting midface injuries were performed in 46 patients (8%). Additional sequences that were performed to further specify MRI findings included T1-weighted images (43 patients), Time of flight (TOF)-angiography (6 patients), phase contrast angiography (PCA) (4 patients), MR-spectroscopy (1 patient), and high-resolution T2-weighted images (3 patients).
Sedation and general anesthesia
Details on required sedation and general anesthesia are summarized in Table 4. In the youngest age group (0–12 months), one child (7.7%) required sedation and one child (7.7%) required general anesthesia. In the age group 1–5 years, five children (4.1%) required sedation and four children required (3.3%) general anesthesia. Four children aged 6–12 years (1.9%) required sedation and three (1.4%) required general anesthesia.
Trauma sequelae and incidental findings
Thirteen percent of all cases exhibited cranial and intra-cranial trauma-related structural lesions in the initial MRI. Two patients underwent surgery for epidural hematoma and cranial vault fracture; two patients underwent surgery for depressed cranial vault fracture. Further trauma findings are detailed in Table 5. Combined trauma sequelae were frequently detected.
Incidental findings were detected in 4.7%. Two children had to undergo further neurosurgical intervention due to the incidental finding of a pilocytic astrocytoma. One patient underwent surgery for cavernoma. Incidental findings are summarized in Table 6.
Patients’ further clinical course
69.7% of the patients were admitted in the hospital for 24-h TBI observation, 7.5% stayed for 48 h, and 12.3% stayed longer than 48 h. Patients were admitted in accordance to the current guidelines (AWMF; details provided in the introduction). In 7.2%, parents refused admission and children were discharged directly from the emergency department.
Patients with post-traumatic lesion-positive MRI had a longer hospital stay (average 86.49 ± 54.38 h) than patients without MRI findings (28.56 ± 23.41 h; independent t test: t = 15.864, p < 0.001). Patients with trauma sequelae were followed up in the outpatient clinic (16% of the presented TBI cohort).
Univariate and multivariate binary logistic regression
In the univariate binary logistic regression analysis, the following variables were significantly related to the presence of trauma-related lesions on MRI: GCS deterioration (odds ratio 10.415, confidence interval between 1.711 and 63.415; p = 0.011), scalp hematoma (OR 1.845, CI 1.126–3.022, p = 0.015), clinical signs of skull base fracture (OR 7.477, CI 2.864–19.518, p < 0.001), clinical signs of cranial vault fracture (OR 3.972, CI 1.883–8.379, p < 0.001), horseback riding accidents (OR 3.427, CI 1.602–7.331, p = 0.002). Sports-related accidents (OR 0.233, CI 0.072–0.759, p = 0.016) and headache (OR 0.435 CI 0.243–0.779, p = 0.005) showed odds ratios below 1 and a negative regression coefficient.
Age significantly correlated with the appearance of trauma-related MRI findings (OR 0.915, CI 0.867–0.967, p = 0.002, pseudo-R2 = 0.023). In the age group of younger children, there was a higher incidence of MRI lesions as indicated by a negative regression coefficient (β = − 0.088). The results of the univariate binary logistic regression are shown in Table 7.
The results of the multivariate binary logistic regression are summarized in Table 8. To summarize, the variables GCS deterioration (OR = 10.499, CI = 1.325–83.190; p = 0.026), scalp hematomas (OR = 1.889, CI = 1.118–3.188, p = 0.017), clinical signs of skull base fracture (OR = 7.863, CI = 2.924–21.144, p = 0.001), horseback riding accidents (OR = 3.244, CI = 1.434–7.337, p = 0.005), and with a negative regression coefficient headache (OR = 0.494 CI 0.269–0.910, p = 0.024) had an impact in the outcome variable.
Discussion
In our study, we evaluated MRI as an initial imaging tool in pediatric patients who were admitted with mild TBI in the emergency department. A single institute cohort of 569 pediatric and juvenile patients up to the age of 18 years was analyzed.
Though MRI is considered more challenging in children in view of time and monitoring aspects, only 1.8% of patients needed sedation and 1.4% general anesthesia for imaging. This was mostly related to the fact that sufficient imaging data could be obtained in less than 10 min of scanning time. Furthermore, medical doctors or parents accompanied younger children in the MRI to soothe and support or held those patients in position. Despite the limiting factor that most of the patients were 6 years and older (6–18 years = 76.3%)—with probably less need of sedation and general anesthesia in these age groups compared to younger children—RI can be considered as alternative to CT imaging in the acute trauma setting.
In the local radiology department T2, DWI, FLAIR, and T2*-weighted sequences were used frequently in the acute setting of imaging TBI in children. In trauma-positive MRI, these sequences were sufficient to detect trauma sequelae. Average imaging time was 8 min. So far, no standardized trauma MRI protocol has been established and acquisition techniques were dependent on children’s injury patterns and compliance. Based on our institutional analyses, one could consider evaluating a standardized protocol including T2 or FLAIR, T2*, and DWI in a future prospective study. Due to the rigorous effort of our hospital to avoid ionizing radiation in patients less than 19 years of age, there was the limitation that no CT images were available on patients who initially received MRI imaging. Thus, no statement regarding compatibility of these different imaging techniques can be offered. However, the use of similar MRI protocols (including triplane T2-weighted and susceptibility weighted sequences [10], single-shot T2-, single-shot diffusion- and single-shot FLAIR-weighted sequences [8], and rapid acquisition of axial, sagittal, coronal T2-weighted fast spin echo images [9]) have been shown to sufficiently detect relevant intracranial trauma sequelae compared to CT scans (also refer to the introduction). Total scanning times for MRI in these studies were 1–3 min. Along with our analyses, MRI protocols including T2, DWI, FLAIR, and T2* sequences appear suitable to become standard protocols for the initial evaluation of pediatric TBI.
While previous studies demonstrated MRI superiority in diagnosing intraparenchymal lesions compared to CT [11] and a higher detection rate for EDH, SDH, and SAH [10], there is still controversy going on regarding bone-associated pathologies. In this respect, MRI is often considered an unreliable tool to detect skull fractures [10, 15]. However, this view is increasingly challenged by accumulating evidence suggesting similar detection rates of skull fractures in CT and MRI [8]. In our case series, MRI picked up common TBI sequelae including cranial vault skull fractures in 16 and skull base fractures in four cases. For there were no CT scans available due to aforementioned reasons, we cannot exclude that skull base fractures or other bone-related trauma sequelae were missed on MRI, especially in children who did not present with associated clinical signs. Still patients with negative MRI did not develop problems in their further clinical course and there was no necessity for surgical treatment. This might be a hint that there were at least no significant or clinically relevant bone-associated TBI sequelae missed with MRI in our cohort.
Despite the introduction of clinical decision rules of when to skip imaging for TBI (PECARN) [12] applying cranial CT for mild TBI did not decline: Due to the fear of potentially missing TBI sequelae, clinicians often decide to perform imaging (i.e., CT) thereby accepting the risks of associated ionizing radiation [5, 6, 13]. Also in our institutional series, it became obvious that most MRI performed for TBI turned out to be negative (82%). Imaging was performed in line with the German guidelines (AWMF; details outlined in the introduction). Thereby all children who received imaging also fulfilled at least one criterion of potentially being PECARN positive (i.e., altered behavior, headaches, significant trauma mechanisms).
To also address the emerging criticism that MRI is a diagnostic “overdo” in the trauma setting, we analyzed our series with regard to clinical clues and trauma mechanisms that might be indicative for structural TBI sequelae in MRI imaging. Thereby, GCS deterioration, scalp hematomas, and clinical signs of skull base and cranial vault fractures, injury mechanisms involving horseback riding correlated with the appearance of evident trauma sequelae on MRI headache, a PECARN criterion, and other sports-related accidents correlated negatively to positive MRI findings. This inconsistent finding was most probably due to a bias introduced by age as only children older than 4 years of age were reliably accessible to an evaluation for headache. Also, sports-related accidents appeared exclusively in the two older age groups. This was related to a selection bias, which also became evident by the negative correlation coefficient for age and GCS at admission: Younger children who were investigated via MRI exhibited severer clinical symptoms than older children. Due to this study weakness, it is not possible to reliably define age-dependent clinical predictors (like headaches and sports activities) for MRI positive findings at this time point. For now, the potentially identified clinical predictors have to be evaluated further prospectively for different age groups in order to formulate a decision tree which might reduce unnecessary imaging in mild TBI.
Another critical point of MRI in children is the need for sedation with associated technical monitoring efforts. Narcotics were considered risk factors for cognitive deficits in children [16, 17], and there has been a recent warning of the FDA concerning general anesthetic and sedation drugs “for lengthy periods of time or over multiple surgeries or procedures” that “may negatively affect brain development in children younger than 3 years” (https://www.fda.gov/Drugs/DrugSafety/ucm554634.htm; as of April 27, 2017. By using rapid MRI sequences, this problem was overcome in most of the patients in our case series (3.2% of patients needed sedation or general anesthesia). Thereby, one crucial factor was the handling of young children during imaging acquisition: By getting involved, parents, caregivers, accompanying doctors or technical assistants, and most children tolerated MRI without sedation. Although there are no data on cranial ultrasound in TBI right now, this modality might be an alternative in very young children (less than 1 year) with no need for sedation and easy accessibility. In regard to the latter point, the authors are aware of the limited availability of MRI in other institutions. However, despite the limitations of our study, at places were MRI is available, it should be considered a serious alternative to CT scans in children when imaging becomes necessary in mild TBI management.
Conclusions
MRI is a practicable imaging tool for evaluating children with mild TBI in the emergency department. Along with previous studies, our analyses established the basis to prospectively evaluate standardized MRI protocols as initial imaging tool. Pediatric TBI factors that predict structural findings on imaging have to be specified and evaluated further for the daily routine in the emergency department to avoid unnecessary imaging.
Abbreviations
- BOLD:
-
Blood-oxygen-level dependent
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DAI:
-
Diffuse axonal injury
- DWI:
-
Diffusion-weighted imaging
- EDH:
-
Epidural hematoma
- FLAIR:
-
Fluid attenuation inversion recovery
- GCS:
-
Glasgow Coma Scale
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratio
- SAH:
-
Subarachnoid hemorrhage
- SDH:
-
Subdural hematoma
- STIR:
-
Short-tau inversion recovery
- TBI:
-
Traumatic brain injury
References
Keenan HT, Bratton SL (2006) Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci 28:256–263. https://doi.org/10.1159/000094152
Pal’a A, Kapapa M, Posovszky C, Röderer G, König R, Woischneck D, Wirtz CR, Kapapa T (2015) Head injury in children: has a change in circumstances caused an increase in treatment numbers? J Child Neurol 30:1153–1158. https://doi.org/10.1177/0883073814554655
Brenner DJ (2002) Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol 32:224–228. https://doi.org/10.1007/s00247-002-0671-1
Brenner DJ, Hall EJ (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284. https://doi.org/10.1056/NEJMra072149
Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Craft AW, Parker L, Berrington de González A (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505. https://doi.org/10.1016/S0140-6736(12)60815-0
Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, Feigelson HS, Roblin D, Flynn MJ, Vanneman N, Smith-Bindman R (2013) The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 167:700–707. https://doi.org/10.1001/jamapediatrics.2013.311
Cohen AR, Caruso P, Duhaime A-C, Klig JE (2015) Feasibility of “rapid” magnetic resonance imaging in pediatric acute head injury. Am J Emerg Med 33:2–5. https://doi.org/10.1016/j.ajem.2015.03.052
Mehta H, Acharya J, Mohan AL, Tobias ME, LeCompte L, Jeevan D (2016) Minimizing radiation exposure in evaluation of pediatric head trauma: use of rapid MR imaging. AJNR Am J Neuroradiol 37:11–18. https://doi.org/10.3174/ajnr.A4464
Sheridan DC, Newgard CD, Selden NR, Jafri MA, Hansen ML (2017) QuickBrain MRI for the detection of acute pediatric traumatic brain injury. J Neurosurg Pediatr 19:259–264. https://doi.org/10.3171/2016.7.PEDS16204
Young JY, Duhaime AC, Caruso PA, Rincon SP (2016) Comparison of non-sedated brain MRI and CT for the detection of acute traumatic injury in children 6 years of age or less. Emerg Radiol 23:325–331. https://doi.org/10.1007/s10140-016-1392-3
Buttram SD, Garcia-Filion P, Miller J et al (2015) Computed tomography vs magnetic resonance imaging for identifying acute lesions in pediatric traumatic brain injury. Hosp Pediatr 5:79–84. https://doi.org/10.1542/hpeds.2014-0094
Kuppermann N, Holmes JF, Dayan PS, Hoyle JD, Atabaki SM, Holubkov R, Nadel FM, Monroe D, Stanley RM, Borgialli DA, Badawy MK, Schunk JE, Quayle KS, Mahajan P, Lichenstein R, Lillis KA, Tunik MG, Jacobs ES, Callahan JM, Gorelick MH, Glass TF, Lee LK, Bachman MC, Cooper A, Powell EC, Gerardi MJ, Melville KA, Muizelaar JP, Wisner DH, Zuspan SJ, Dean JM, Wootton-Gorges SL (2009) Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 374:1160–1170. https://doi.org/10.1016/S0140-6736(09)61558-0
Berdahl CT, Vermeulen MJ, Larson DB, Schull MJ (2013) Emergency department computed tomography utilization in the United States and Canada. Ann Emerg Med 62:486–494.e3. https://doi.org/10.1016/j.annemergmed.2013.02.018
Vos PE, Alekseenko Y, Battistin L, Ehler E, Gerstenbrand F, Muresanu DF, Potapov A, Stepan CA, Traubner P, Vecsei L, von Wild K, European Federation of Neurological Societies (2012) Mild traumatic brain injury. Eur J Neurol 19:191–198. https://doi.org/10.1111/j.1468-1331.2011.03581.x
Roguski M, Morel B, Sweeney M, Talan J, Rideout L, Riesenburger RI, Madan N, Hwang S (2015) Magnetic resonance imaging as an alternative to computed tomography in selected patients with traumatic brain injury: a retrospective comparison. J Neurosurg Pediatr 15:529–534. https://doi.org/10.3171/2014.PEDS14128
Malviya S, Voepel-Lewis T, Eldevik OP et al (2000) Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 84:743–748. https://doi.org/10.1093/bja/aer407
Wang X, Xu Z, Miao CH (2014) Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: an updated systematic review with meta-regression. PLoS One 9:e85760. https://doi.org/10.1371/journal.pone.0085760
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Rights and permissions
About this article
Cite this article
Cohrs, G., Huhndorf, M., Niemczyk, N. et al. MRI in mild pediatric traumatic brain injury: diagnostic overkill or useful tool?. Childs Nerv Syst 34, 1345–1352 (2018). https://doi.org/10.1007/s00381-018-3771-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-3771-4