Abstract
Spotted knifejaw (Oplegnathus punctatus) is a marine teleost species that is economically important for aquaculture and marine pasture proliferation and shows obvious bisexual growth dimorphism, but molecular sex markers are currently lacking. A 290 bp (base pair) insertion with two fragments (230 bp and 60 bp) was identified in male individuals of O. punctatus based on whole-genome sequencing scanning and structural variation analyses. The gene annotation results showed that the insertion event occurred in the Igfn1 gene of male O. punctatus. The results of amino acid analysis further showed that the insertion event resulted in the functional variation of Igfn1 in male O. punctatus, and recombination caused the inactivation of Igfn1. According to the male-specific insertion information, we designed a PCR-based genetic amplification technique for rapid sex identification in O. punctatus. The results of agarose gel electrophoresis showed that two DNA fragments of 635 bp and 925 bp were amplified in male O. punctatus, while only a single DNA fragment of 635 bp was amplified in female individuals. The sex of individuals identified by this method was consistent with their known phenotypic sex, which will improve sex identification efficiency. This method provides a new DNA marker for rapid sex identification in O. punctatus, which has great significance and application value in monosex breeding and provides new insights for the study of Igfn1 gene recombination and inactivation in male O. punctatus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spotted knifejaw (Oplegnathus punctatus), a member of the Oplegnathidae family of the Perciformes Order, is mainly distributed in 10–100 m deep reefs, gravel, and shell-algae areas in warm tropical waters of the Western Pacific Ocean. O. punctatus is a reef-loving benthic fish whose juveniles show the ecological habit of drifting with seaweed in summer and autumn. O. punctatus is carnivorous and has sharp beak-like teeth that can crush the hard shells of species such as shellfish or sea urchins (Kakizawa et al. 1980). O. punctatus has an aesthetically appealing appearance, with a combination of shiny silver lines and dark brown spots on the fish body. It is known as “dream fish” by some people. O. punctatus is a marine fish with high edible and ornamental value that is favoured by fishing enthusiasts and consumers. In 2014, the Institute of Oceanology, Chinese Academy of Sciences and Shandong Laizhou Mingbo Aquatic Products Co., Ltd. made the first breakthrough in the reproductive regulation and artificial breeding technology of O. punctatus broodstock in China. This made O. punctatus an important new species reared in sea cage and factory culture in this country, and its current market price is relatively high. Furthermore, because of the characteristics of its shellfish-eating behaviour, O. punctatus can clean the net attachments of breeding cages, which could save human resources and cleaning costs. In addition, O. punctatus is a native natural species in China in which resource development and sustainable utilization will have long-term effects, and its breeding prospects are extremely broad.
Because of the growth differences between female and male O. punctatus, which are similar to most other aquacultured fish (Gui and Zhou 2010; Piferrer et al. 2012), sex chromosome-linked markers are of great significance for the genetic improvement of economical traits in O. punctatus aquaculture (Gui and Zhu 2012; Zheng et al. 2013). The chromosomal karyotype of female O. punctatus is 2n = 48, including 46 telocentric chromosomes and 1 pair of subcentral centromeric chromosomes, and the genetic sex type is X1X1X2X2. However, the chromosomal karyotype of male O. punctatus is 2n = 47, including only 44 telocentric chromosomes, 1 pair of subcentral centromeric chromosomes, and 1 giant heterochromatic chromosome, and the genetic sex type corresponds to the X1X2Y pattern (Xue et al. 2016). Based on karyotyping, in situ hybridization, and genome-wide analysis, it was revealed that a chromosomal fusion event occurred in the Neo-Y chromosome of O. punctatus. The Neo-Y chromosome presented significant chromosomal structural changes such as inversion, insertion, and other events (Xiao et al. 2019, 2020; Li et al. 2021). During the breeding of O. punctatus, male fish grow faster than female fish. The preparation of whole male seed of O. punctatus will promote improvements in the quality and efficiency of its aquaculture industry. The development of rapid identification technology for the genetic sex identification of O. punctatus will be helpful for the preservation of its excellent male germplasm (Xiao 2014). In artificial breeding and artificial selection aimed at developing O. punctatus, it is necessary to select high-quality male and female broodstock and achieve accurate sex identification. However, it is difficult to accurately identify the sex of O. punctatus through external morphological characteristics at the juvenile and adult stages. Therefore, the establishment of a rapid sex identification method for O. punctatus will promote the industrialization process by allowing a high percentage of the male fry to be obtained and improve the genetic breeding of O. punctatus.
At present, the female or male genetic sex of O. punctatus is assessed by adopting the “trisomy method” based on combined amplification with three primers (Li et al. 2020). The annealing temperatures of the three primers used in this method are different, which will result in an inconsistent PCR amplification efficiency. The size of the genetic male-specific target band obtained by this method is 222 bases (bp), and this band can be easily confused with primer-dimers (10–250 bp) generated during the PCR amplification process. Therefore, this is a technical problem that urgently needs to be solved in the artificial breeding and aquaculture industry to excavate new genetic sex-specific molecular markers of O. punctatus and establish molecular technology for the efficient and rapid identification of male and female genetic sex in O. punctatus.
Immunoglobulin-like and fibronectin type III domain-containing 1 (Igfn1) is a novel eukaryotic translation elongation factor 1A (eEF1A)-binding protein that is specifically expressed in skeletal muscle (Mansilla et al. 2008). Studies have shown that the Igfn1 gene is mainly involved in protein translation, skeletal muscle contraction, muscle development, the skeletal muscle cytoskeleton, and intracellular calcium balance (Kilpinen et al. 2010). Compared with healthy children, the expression of the Igfn1 gene is significantly downregulated in the skeletal muscle of children with congenital muscular dystrophy with selective deletion of the collagen sarcolemma, and the downregulation of this gene could affect the synthesis of skeletal muscle protein (Paco et al. 2013). Another study showed that in cultured myotubes from hypercatabolic trauma patients, Igfn1 gene expression inhibits apoptosis and promotes cell proliferation (Bosutti et al. 2007). It has been reported that the Igfn1 gene is mainly involved in the stress-induced activation of muscle MAPK signalling (Hao et al. 2019; Panasyuk et al. 2008; Sanges et al. 2012). Furthermore, the transcription of the Igfn1 gene is strongly positively correlated with MSTN signalling. It was shown that the inhibition of MSTN signalling leads to muscle hypertrophy and downregulation of the Igfn1 gene. Conversely, enhancement of the MSTN signalling pathway can lead to muscle atrophy and marked upregulation of Igfn1 expression (Rahimov et al. 2011; Chen et al. 2014).
Materials and Methods
Ethics Statement
All experiments strictly abided by the experimental animal guidance policy of the experimental animal ethics committee of the Institute of Oceanology, Chinese Academy of Sciences (Permit Number: IOCAS202206).
Experimental Materials
O. punctatus individuals were obtained from the Fish Breeding Base of the Institute of Oceanology, Chinese Academy of Sciences (Haihe Aquatic Seedling Co., Ltd., Weihai Wendeng). To verify the feasibility of male and female sex marking in O. punctatus, we anaesthetized 12 males and 12 females of adult O. punctatus with MS-222, dissected and collected their muscle tissues, and preserved the tissue samples in liquid nitrogen for further analysis.
DNA Extraction
A Tiangen Marine Animal DNA Extraction Kit was used to extract DNA from the muscle tissue of O. punctatus with known biological sex based on histology, followed by 0.8% agarose gel electrophoresis to verify the integrity of genomic DNA. The DNA supernatant was subjected to the measurement of OD value with a UV spectrophotometer and finally placed in a − 20 °C freezer for future use.
Comparative Genomic Analysis
Third-generation PacBio whole-genome sequencing technology was used to complete the sequencing and assembly of the whole genomes of the female and male O. punctatus and to obtain high-quality chromosome-level genomes of female and male O. punctatus. The reference genome CNGB accession code used for analysis in this study is CNP0001488 (Li et al. 2021).
Male-Specific Marker Exploitation
The genomes of O. punctatus females and males were aligned by genome-wide scanning with TBtools (Chen et al. 2020), and the sequencing depth of each genomic location was calculated. Subsequently, we extracted sequences showing male-specific sex differences. The ChX1F/M and ChYM chromosomes were compared by Mauve software to identify sites of chromosomal structural variation, such as sex-related insertions and deletions (Darling et al. 2004). Nucleotide differences in ChYM Igfn1 relative to ChX1F/M Igfn1 were visualized with RStudio. The applied R language command was as follows: if(!require namespace("devtools")), install.packages ("devtools"), devtools::install_github ("git@github.com:YuLab-SMU/ggmsa.git"), library (ggmsa), fas = list.files (system.file("112.fas","GVariation", package = "ggmsa"), pattern = "fas", full.names = TRUE), x = seqdiff (fasta = "D:/ggmsa/112.fas"), plot (x). GSV hotspots, GC contents, gene densities, and chromosome position information were obtained from the O. punctatus genome database; Igfn1 gene positions were marked on the chromosomes, and the collinear relationships between the genes on sex chromosomes were obtained from the genome database; and the results were visualized using TBtools software. The nucleotide sequences of Igfn1 genes on ChX1F/M and ChYM of O. punctatus were aligned using Jalview software (Waterhouse et al. 2009). The Igfn1 gene nucleotide sequence patterns on ChX1F/M and ChYM were visualized with IBS software (Liu et al. 2015).
Genetic Sex Identification in O. punctatus
Compared with the female O. punctatus ChX1F/M, a 290 bp insertion occurred in the DNA sequence of male O. punctatus ChYM. This fragment was a DNA marker unique to the Y chromosome of male O. punctatus, and its presence or absence could be used to identify the genetic sex of O. punctatus. Therefore, we designed a pair of primers (ChXY_F:5′-AAACAGAGGACATTCAAGCCG-3′; ChXY_R: 5′-GTTGCTGCCCTTCTTGCGT-3′) targeting this fragment with Primer5 software. Sex-specific regions were amplified with the two primer pairs described above using genomic DNA samples from 12 male and 12 female O. punctatus. The 25 µL PCR system had the following components: 5.0 µL 10 × buffer, 4.0 µL dNTPs, 0.5 µL rTaq enzyme (5 U/µL), 2 µL primers (ChXY_F:1 and ChXY_R:2 1 µL each), 2.0 µL DNA template, and 11.5 µL ddH2O. The touch-down PCR amplification program was as follows: 95 °C 3 min, 61–59 °C (− 1 °C) 30 s, 72 °C 1 min 30 s, 3 cycles; 95 °C 30 s, 59.5 °C 30 s, 72 °C 1 min 30 s, 28 cycles; 72 °C 10 min; hold at 15 °C. Thereafter, 3.0 µL of 10 × loading buffer was added to each PCR sample; 1.5% agarose gel electrophoresis was performed at a 110 V constant voltage for 30 min, and the PCR products were analysed according to the gel imaging bands. In addition, according to the expected band positions of male and female individuals, simulated images of the gel electrophoresis results were drawn with TBtools to compare their consistency with the actual gel electrophoresis bands.
Results
Screening of Male-Specific Markers of O. punctatus
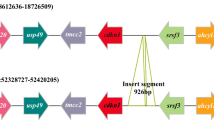
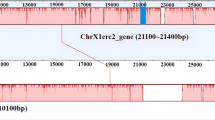
The Neo-Y chromosome of O. punctatus males was homologous to the female X1 chromosome, and a large insertion region was observable through comparative genomic bioinformatics analysis (Fig. 1). The male and female marker fragments were annotated as the Igfn1 gene through genome annotation (Fig. 2). As shown in Fig. 3, the target length of the Igfn1 gene on chromosome X1 was 635 bp, whereas the target length of the Igfn1 gene containing the insertion fragment on chromosome Neo-Y was 925 bp. The sequences of Igfn1 genes on ChX1F/M and ChYM are shown in Supplementary file 1. The amino acid sequence encoded by the Igfn1 gene was also identified in males and females. In addition, amino acid sequence variants and recombination inactivation were found in the Igfn1 genes of males in which insertions occurred (Fig. 4). The CDS and amino acid sequence of the Igfn1 gene are shown in Supplementary file 2. Two large fragment insertions were found between loci 164 and 221 of ChYM, with sizes of 230 bp and 60 bp, respectively (Fig. 5a). Compared with the female X1 chromosome (ChX1F/M), the DNA sequence of the male Neo-Y chromosome (ChYM) carried an insert with a DNA sequence of 290 bp, which was a unique DNA marker of the Y chromosome of O. punctatus. Moreover, ChX1F/M could serve a genetic marker of both sexes of O. punctatus.
Alignment of ChX1F/M and ChYM nucleotide sequences. a Alignment of ChX1F/M and ChYM nucleotide sequences. “ + ” indicates that the sequences of Igfn1 on ChX1F/M are inconsistent with those of Igfn1 on ChYM; the male-specific 290 bp insert is shown in dark grey. The primer ChXY_F and the primer ChXY_R are indicated by in the red frame. b ChYM Igfn1 nucleotide differences relative to ChX1F/M Igfn1. The x-axis corresponds to the nucleotide position along the genome, and the y-axis is the numbers of nucleotide differences between the genomes; bars indicate the number of base differences according to individual nucleotides (A, T, C, or G) and all four nucleotides (dark red bar)
Gene collinearity circle diagram of sex chromosome associations in male and female individuals. a represents GSV (genetic structural variation) hotspots (500–5000); b represents GC content; c represents gene density; d represents chromosomes. The blue lines represent the collinear relationships between ChX1 and ChY, the green lines represent the collinear relationships between ChX2 and ChY, the yellow box indicates where Igfn1 is located on ChY, and the red box indicates where Igfn1 is located on ChX1
Genomic collinearity analysis of the Igfn1 gene on ChX1F/M and the Igfn1 gene on ChYM. The blue and green areas in the figure represent the positions of Igfn1 on ChX1F/M and ChYM, respectively; ChX1F/M Igfn1-635 bp and ChYM Igfn1-925 bp represent enlarged images of ChX1F/M Igfn1 and ChYM Igfn1, respectively; PCR-ChX1F/M-Igfn1-635 bp and PCR-ChYM-Igfn1-925 bp represent PCR amplified fragments; the light green area in the figure represents the 290 bp insert in Igfn1 on ChYM
Igfn1 gene insertion recombination mode and amplification mode. a Pattern diagram of Igfn1 nucleotide sequence insertion events on ChX1F/M and ChYM. Primer positions are indicated by triangular arrows; regions with the same colour represent homologous regions, and different colours represent insertion site information. Marker: DL 2000 DNA Maker; ♂: physiological male; ♀: physiological female; b image of the PCR product patterns of male and female O. punctatus following 1.5% agarose gel electrophoresis. c Image of the PCR products of male and female O. punctatus following agarose gel electrophoresis. Individuals showing two bands (635 bp and 925 bp) in the figure are genetic males that were also histologically identified as males, and individuals showing a single band (635 bp) are genetic females that were also histologically identified as females
Verification of the Male-Specific Marker O. punctatus
As shown in Fig. 5b, c, two DNA fragments of 635 bp and 925 bp were amplified in the 12 genetic male individuals of O. punctatus (X1X2Y), and the 925 bp fragment was a genetic male-specific marker fragment, while in the genetic 12 female (X1X1X2X2) individuals, only a single DNA fragment of 635 bp was amplified. Male individuals harboured a 290 bp Igfn1 gene insertion, while female individuals showed no DNA fragment insertion. The original agarose gel electrophoresis image is shown in Supplementary file 3. The genetic sex of the PCR-validated individuals showed consistency with their phenotypic sex, further validating the applicability of male-specific markers.
Development of an Identification Technique for Genetic Sex
Based on the principles of this study, the sex of O. punctatus was identified in aquaculture by agarose gel electrophoresis. Male individuals had two bands while female individuals had only one band. The short band, approximately 635 bp, was present in both males and females. However, that long band that was approximately 925 bp existed only in male individuals. The genetic sex of O. punctatus detected by this method during the breeding period was consistent with the observed phenotypic sex. Therefore, the accuracy of the marker was further verified.
Discussion
In mammals (Ruckstuhl and Neuhaus 2002) and birds (Shaffer et al. 2001), it has been found that most males grow faster than females following birth. In reptiles (Cox et al. 2009) and fish, patterns of both females growing faster than males and males growing faster than females are observe. Tongue sole (Cynoglossus semilaevis Günther), Bastard halibut (Paralichthys olivaceus), and Argus fish (Scatophagus argus) exhibit greater growth in females than in males. In contrast, Nile tilapia (Oreochromis niloticus) males grow approximately 40% faster than females (Zhu 2012), and Banded catfish (Pelteobagrus fulvidraco) also exhibit the phenomenon of males showing greater growth than females (Liu et al. 2007; Liu 1997). The growth of O. punctatus shows significant sex differences under both natural and aquaculture conditions, with male body weight exceeding female body weight. Many studies have shown that in populations with larger males, intraspecies competition, foraging, and protection of breeding sites for males to survive and obtain mating rights are more intense than in other populations (Ros et al. 2004). Therefore, the larger size of male O. punctatus than female O. punctatus may be directly related to reproduction and foraging.
Genetically and morphologically distinct X and Y chromosomes generally evolve independently in animals (Charlesworth 1996). Chromosomal structural variation is critical for maintaining the formation and evolution of sex chromosomes (Charlesworth and Charlesworth 2000). In this study, we designed male-specific DNA marker primers based on a Y chromosome insertion event identified in O. punctatus and observed the number of bands of products amplified by polymerase chain reaction and agarose gel electrophoresis to effectively identify males and females. Therefore, a PCR method for the rapid identification of the genetic sex of O. punctatus was established, and the method can be used to distinguish the genetic sex of O. punctatus quickly, accurately, and efficiently. There are obvious sex differences in the growth rate of O. punctatus during the breeding process, and the growth rate of males is faster than that of females. Males generally take 2 to 2.5 years to reach sexual maturity, while females generally take more than 3 years. Therefore, according to the polymerase chain reaction method presented in this paper, the genetic sex of an individual can be quickly and accurately identified by simply obtaining fin rays from juveniles during the early stages of O. punctatus growth. This finding guides the artificial genetic breeding of O. punctatus (Szekely et al. 2014; Gong et al. 2022).
At present, some progress has been made in research on the effect of Igfn1 on skeletal muscle, and these research results have enabled a deeper understanding of the role of Igfn1 in muscle cell proliferation, differentiation, and muscle protein synthesis and decomposition. The Igfn1 gene produces various proteins through alternative splicing, which are mainly found in skeletal muscle and heart (Baker et al. 2010). To explore the potential role of Igfn1, Xiang Li et al. applied nonselective knockdown of shRNA and specific targeting of Igfn1 exon 13 by CRISPR/Cas9 mutagenesis in C2C12 cells. Reduced expression of the common 3’-UTR in Igfn1 variants resulted in complete blunting of myoblast fusion but did not prevent the expression of differentiation markers. These findings suggest that Igfn1_v1 plays a role in the fusion and differentiation of myoblasts in vitro (Li et al. 2017). Therefore, the inactivation of the expression of Igfn1 after insertion in O. punctatus males may affect the function of male skeletal muscles and myoblasts.
Conclusion
In this paper, we performed screening and obtained the Igfn1 gene as a sex marker from the whole-genome sequence of O. punctatus. The Igfn1 gene of male individuals has a 290 bp DNA insertion fragment compared with that of female individuals, and the Igfn1 marker gene was further used for genetic sex identification in O. punctatus. According to this method, a pair of primers were designed, and their products from males and females were separated by agarose gel electrophoresis. Two DNA fragments of 635 bp and 925 bp were amplified in physiologically male individuals, but only a single DNA fragment of 635 bp was amplified in physiologically female individuals. This method of male and female identification reduces the time required to accurately identify the genetic sex of O. punctatus and improves the efficiency of sex detection.
References
Baker J, Riley G, Romero MR, Haynes AR, Hilton H, Simon M, Hancock J, Tateossian H, Ripoll VM, Blanco G (2010) Identification of a Z-band associated protein complex involving KY, FLNC and IGFN1. Exp Cell Res 316:1856–1870
Bosutti A, Scaggiante B, Grassi G, Guarnieri G, Biolo G (2007) Overexpression of the elongation factor 1A1 relates to muscle proteolysis and proapoptotic p66((ShcA)) gene transcription in hypercatabolic trauma patients. Metab-Clin Exp 56:1629–1634
Charlesworth B (1996) The evolution of chromosomal sex determination and dosage compensation. Curr Biol 6:149–162
Charlesworth B, Charlesworth D (2000) The degeneration of Y chromosomes. Philos Trans R Soc Lond B Biol Sci 355:1563–1572
Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202
Chen JL, Walton KL, Winbanks CE, Murphy KT, Thomson RE, Makanji Y, Qian HW, Lynch GS, Harrison CA, Gregorevic P (2014) Elevated expression of activins promotes muscle wasting and cachexia. Faseb J 28:1711–1723
Cox RM, Stenquist DS, Calsbeek R (2009) Testosterone, growth and the evolution of sexual size dimorphism. J Evol Biol 22:1586–1598
Darling AC, Mau B, Blattner FR, Perna NT (2004) Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403
Gong J, Li BJ, Zhao J, Zhou ZX, Ke QZ, Zhu QH, Xu DD, Zhou T, Xu P (2022) Sex-specific genomic region identification and molecular sex marker development of rock bream (Oplegnathus fasciatus). Mar Biotechnol 24:163–173
Gui J, Zhu Z (2012) Molecular basis and genetic improvement of economically important traits in aquaculture animals. Chin Sci Bull 57:1751–1760
Gui JF, Zhou L (2010) Genetic basis and breeding application of clonal diversity and dual reproduction modes in polyploid Carassius auratus gibelio. Science China-Life Sciences 53:409–415
Hao Y, Xin HR, Zhang C, Gu XH (2019) Immunoglobulin-like and fibronectin type III domain containing 1 involved in the regulation of skeletal muscle stress damage. Acta Agriculturae Boreali-Sinica 34:380-385
Kakizawa Y, Kamishikiryo K, Shirato M, Maehara S, Fujii H, Iesato S (1980) The tooth development of the parrot perch, Oplegnathus fasciatus, (family Oplegnathidae, Teleostei). J Nihon Univ Sch Dent 22:211–226
Kilpinen S, Ojala K, Kallioniemi O (2010) Analysis of kinase gene expression patterns across 5681 human tissue samples reveals functional genomic taxonomy of the kinome. PLoS ONE 5:14
Li M, Xu H, Xu WT, Zhou Q, Xu XW, Zhu Y, Zheng WW, Li WS, Pang ZF, Chen SL (2020) Isolation of a male-specific molecular marker and development of a genetic sex identification technique in spotted knifejaw (Oplegnathus punctatus). Mar Biotechnol 22:467–474
Li M, Zhang R, Fan GY, Xu WT, Zhou Q, Wang L, Li WS, Pang ZF, Yu MJ, Liu Q, Liu X, Schartl M, Chen SL (2021) Reconstruction of the origin of a Neo-Y sex chromosome and its evolution in the spotted knifejaw, Oplegnathus punctatus. Mol Biol Evol 38:2615–2626
Li X, Baker J, Cracknell T, Haynes AR, Blanco G (2017) IGFN1_v1 is required for myoblast fusion and differentiation. PLoS ONE 12:24
Liu HQ, Cui SQ, Hou CC, Xu J, Chen HX (2007) YY Supermale generated gynogenetically from XY female in Pelteobagrus fulvidraco (Richardson). Acta Hydrobiologica Sinica 718-725. https://doi.org/10.3321/j.issn:1000-3207.2007.05.018
Liu SP (1997) A study on the biology of pseudobagrus fulvidraco in Poyang Lake. Chin J Zool 11-17. https://doi.org/10.13859/j.cjz.1997.04.005
Liu W, Xie Y, Ma J, Luo X, Nie P, Zuo Z, Lahrmann U, Zhao Q, Zheng Y, Zhao Y, Xue Y, Ren J (2015) IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31:3359–3361
Mansilla F, Dominguez CAG, Yeadon JE, Corydon TJ, Burden SJ, Knudsen CR (2008) Translation elongation factor eEF1A binds to a novel myosin binding protein-C-like protein. J Cell Biochem 105:847–858
Paco S, Kalko SG, Jou C, Rodriguez MA, Corbera J, Muntoni F, Feng L, Rivas E, Torner F, Gualandi F, Gomez-Foix AM, Ferrer A, Ortez C, Nascimento A, Colomer J, Jimenez-Mallebrera C (2013) Gene expression profiling identifies molecular pathways associated with collagen VI deficiency and provides novel therapeutic targets. PLoS ONE 8:15
Panasyuk G, Nemazanyy I, Filonenko V, Negrutskii B, El’skaya, A.V. (2008) A2 isoform of mammalian translation factor eEF1A displays increased tyrosine phosphorylation and ability to interact with different signalling molecules. Int J Biochem Cell Biol 40:63–71
Piferrer F, Ribas L, Diaz N (2012) Genomic approaches to study genetic and environmental influences on fish sex determination and differentiation. Mar Biotechnol 14:591–604
Rahimov F, King OD, Warsing LC, Powell RE, Emerson CP, Kunkel LM, Wagner KR (2011) Gene expression profiling of skeletal muscles treated with a soluble activin type IIB receptor. Physiol Genomics 43:398–407
Ros AFH, Becker K, Canario AVM, Oliveira RF (2004) Androgen levels and energy metabolism in Oreochromis mossambicus. J Fish Biol 65:895–905
Ruckstuhl KE, Neuhaus P (2002) Sexual segregation in ungulates: a comparative test of three hypotheses. Biol Rev 77:77–96
Sanges C, Scheuermann C, Zahedi RP, Sickmann A, Lamberti A, Migliaccio N, Baljuls A, Marra M, Zappavigna S, Reinders J, Rapp U, Abbruzzese A, Caraglia M, Arcari P (2012) Raf kinases mediate the phosphorylation of eukaryotic translation elongation factor 1A and regulate its stability in eukaryotic cells (vol 3, e276, 2012). Cell Death Dis 3:1
Shaffer SA, Weimerskirch H, Costa DP (2001) Functional significance of sexual dimorphism in Wandering Albatrosses, Diomedea exulans. Funct Ecol 15:203–210
Szekely T, Weissing FJ, Komdeur J (2014) Adult sex ratio variation: implications for breeding system evolution. J Evol Biol 27:1500–1512
Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191
Xiao Y, Xiao Z, Ma D, Zhao C, Liu L, Wu H, Nie W, Xiao S, Liu J, Li J, Herrera-Ulloa A (2020) Chromosome-level genome reveals the origin of Neo-Y chromosome in the male barred knifejaw Oplegnathus fasciatus. iScience 23:101039
Xiao YS, Xiao ZZ, Ma DY, Liu J, Li J (2019) Genome sequence of the barred knifejaw Oplegnathus fasciatus (Temminck & Schlegel, 1844): the first chromosome-level draft genome in the family Oplegnathidae. GigaScience 8:8
Xiao ZZ (2014) Study on population genetics and culture biology of Oplegndthus fasciatus. Ocean University of China.
Xue R, An H, Liu QH, Xiao ZZ, Wang YF, Li J (2016) Karyotype and Ag-NORs in male and female of Oplegnathus Punctatus. Oceanologia et Limnologia Sinica. Oceanologia Et Limnologia Sinica 47:626–632
Zheng X, Kuang Y, Lv W, Cao D, Zhang X, Li C, Lu C, Sun X (2013) A consensus linkage map of common carp (Cyprinus carpio L.) to compare the distribution and variation of QTLs associated with growth traits. Science China-Life Sciences 56:351–359
Zhu YY (2012) Screening of the AFLP marker linked to the sex locus of Oreochromis niloticus and association between polymorphsims of two genes and growth traits. Huazhong Agricultural University.
Acknowledgements
The authors thank the Institute of Oceanology, the Chinese Academy of Sciences for the infrastructural support, Prof. Zhizhong Xiao for the experimental materials, and Prof. Jun Li for his guidance.
Funding
This work was supported by grants from the National Key Research and Development Program (2018YFD0901204), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (GML2019ZD0402), Key Deployment Projects of Center for Ocean Mega-Science, Chinese Academy of Sciences (Frontier Cross-category, COMS2020Q05), China Agriculture Research System (CARS-47), Major Agricultural Application Technology Innovation Project of Shandong Province (SD2019YY01), STS project (KFZD-SW-106, ZSSD-019, 2017T3017, and KFJ-STS QYZX-020), Qingdao National Laboratory for Marine Science and Technology (2018SDKJ0502-2 and 2015ASKJ02), and National Natural Science Foundation of China (No. 31672672).
Author information
Authors and Affiliations
Contributions
YSX and JL conceived and designed the project and revised the manuscript. YTM, JL and YSX performed the genomic investigations and wrote the manuscript. ZZX, YDW, and HXZ participated in data analysis, discussion, and figure preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Xiao, Y., Xiao, Z. et al. Identification of Male-Specific Molecular Marker and Development of PCR-Based Genetic Sex Identification Technique in Spotted Knifejaw (Oplegnathus punctatus). Mar Biotechnol 24, 969–978 (2022). https://doi.org/10.1007/s10126-022-10160-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-022-10160-w