Abstract
Sex-specific DNA markers are very helpful for identifying genetic sex and studying sex determination mechanisms in fish. To identify the sex-specific markers of spotted knifejaw (Oplegnathus punctatus), we performed a comparative analysis of the female and male genomes. In this study, an 18 bp insertion was identified in the male genome after verification by sequencing depth and PCR. An effective and rapid method based on PCR was then developed to identify the genetic sex. A male-female-shared primer pair and a male-specific primer were designed for PCR amplification to avoid false-negative phenomena. To examine the primers in practice, we utilized hundreds of spotted knifejaw fish from different groups to identify their genetic sex, and the results were consistent with their phenotypic sex. The male-specific DNA marker would be helpful for artificial breeding, Y chromosome assembly and further study of the sex determination mechanism. This study is the first to identify an effective sex-specific marker in spotted knifejaw.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many marine fishes have a drastic difference in growth rate between sexes, which leads to sexual dimorphism in size (Kobayashi et al. 2013). The growth rate of males is faster than that of females in Nile tilapia (Oreochromis niloticus) (Guerrero III 1975) and yellow catfish (Pelteobagrus fulvidraco), whereas females grow faster than males in flatfish such as Chinese tongue sole (Cynoglossus semilaevis) (Chen et al. 2007) and turbot (Scophthalmus maximus) (Imsland et al. 1997). Therefore, it is very important to conduct sex control breeding and monosex cultivation in aquaculture. Spotted knifejaw (Oplegnathus punctatus) is a newly emerging aquaculture fish species that is mainly distributed in coastal regions of East Asia (Kawato et al. 2017). Although there is no research about sexual dimorphism in spotted knifejaw, barred knifejaw, which belongs to the same genus (Oplegnathus) and shares the same sex chromosome system as spotted knifejaw, is reported to exhibit sexual dimorphism in growth. Furthermore, sexual dimorphism in growth of barred knifejaw is thought to be caused by the sex chromosome system in Oplegnathus (Xiao et al. 2019). Moreover, spotted knifejaw has a long juvenility period about 3–4 years. Thus, it is not easy to identify the sex of fish in appearance and maintain suitable sex proportions of mature spotted knifejaw before and during the breeding season. Developing effective and accurate sex-specific markers is vitally important for studying sexual dimorphism, identifying genetic sex in the breeding process and establishing appropriate breeding strategies for fish yields (Mei and Gui 2015). Furthermore, sex-specific DNA markers can potentially provide important information regarding the sex determination mechanism and evolution of sex chromosomes in fish (Devlin and Nagahama 2002; Berset-Brändli et al. 2006).
Fish have various sex chromosome systems, including XX/XY, ZZ/ZW, XX/XO, ZZ/ZO and X1X1X2X2/X1X2Y (De Almeida-Toledo et al. 2000). Identification of sex-specific markers is an effective approach to determine the sex chromosome systems in fish (Kikuchi and Hamaguchi 2013). Traditional molecular marker techniques, including those involving RAPD, AFLP and SSR, have been used to identify sex-specific markers in fish. Via the RAPD technique, sex-specific DNA markers have been successfully identified and developed for genetic sex identification in African catfish (Clarias gariepinus) (Kovács et al. 2000). Among the traditional molecular marker techniques, the AFLP technique is the most commonly used technique for sex-linked DNA marker identification in fish. Sex-linked AFLP markers have been successfully identified in marine fishes such as rainbow trout (Oncorhynchus mykiss) (Felip et al. 2005), Chinese tongue sole (Cynoglossus semilaevis) (Chen et al. 2007) and barred knifejaw (Oplegnathus fasciatus) (Xu et al. 2013). Moreover, sex-linked (associated) SSR markers have also been identified in marine fish, including yellowtail (Seriola quinqueradiata) (Fuji et al. 2010), Pacific halibut (Hippoglossus stenolepis) (Galindo et al. 2011) and Chinese tongue sole (Cynoglossus semilaevis) (Chen et al. 2012). These approaches for identifying sex-linked DNA markers are effective but time consuming and laborious.
Recently, the development of next-generation sequencing (NGS) technology has allowed researchers to obtain massive genomic information quickly and has provided new opportunities to identify sex-specific markers by comparative analysis of genomic data between males and females. Via NGS-based double digest restriction site-associated DNA sequencing (ddRAD-seq), a total of 17 male-specific loci were identified in Sebastes carnatus and Sebastes chrysomelas rockfishes (Fowler and Buonaccorsi 2016). Moreover, a male-specific marker was successfully identified in the large yellow croaker (Larimichthys crocea) via the whole-genome resequencing technique (Lin et al. 2017).
Spotted knifejaw has the sex determination system of female homogamety (X1X1X2X2) and male heterogamety (X1X2Y) (Li et al. 2016). The Y chromosome is restricted to genetic males; thus, sex-specific DNA fragments should be screened only in the male genome. In this study, we obtained several male-specific DNA fragments by comparative analysis of the female and male genomes of spotted knifejaw. Hereby, a male-specific DNA marker was identified and developed to identify the genetic sex of spotted knifejaw. These results contribute to enhancing fishery management of spotted knifejaw. Furthermore, the ability to screen sex-specific markers provides new insights for sex determination and the evolution of sex chromosomes in fish.
Materials and Methods
Ethical Statement
The collection and handling of the animals in the study were approved by the Chinese Academy of Fishery Science Animal Care and Use Committee, and all animals and experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Chinese Academy of Fishery Sciences.
Fish and Sampling
Approximately 1-year-old spotted knifejaw fish (weighing 300~400 g) from various populations were obtained from Laizhou Mingbo Aquatic Product Co., Ltd., Laizhou, Shandong, China. We collected gonads from 122 individuals and fixed them in 4% PFA (polyformaldehyde) solution for histological sectioning. Moreover, we collected the tail fin clips of the 122 individuals and of another 500 in the ejaculation and ovulation periods of the breeding season and stored them in 95% ethanol at − 20 °C for subsequent DNA extraction.
Genomic DNA Extraction
Genomic DNA was extracted from the clips using a TIANamp Marine Animals DNA Kit (Tiangen, China) according to the manufacturer’s instructions. We used agarose gel electrophoresis to inspect the quality of the DNA and measured their concentrations with a GeneQuant Pro (Pharmacia, USA) RNA/DNA spectrophotometer. All genomic DNA was adjusted to 100 ng/μl and stored at − 20 °C for subsequent experiments.
Histological Sectioning and HE Staining
Histological sectioning and HE staining were performed as described previously (Chen et al. 2007). In brief, ovaries and testes were collected from individual fish and fixed in 4% PFA solution. After fixation, the tissues were dehydrated, embedded in paraffin and sectioned into 6 μm thin slices, after which the sections were stained with haematoxylin and eosin. We then used microscopic inspection of the histological section to identify the phenotypic sex of spotted knifejaw.
Male-Specific Marker Exploitation
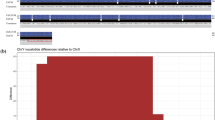
In this work, we used 2 genomic datasets with approximately 20 × coverage from two spotted knifejaw fish (one female and one male) on an Illumina HiSeq platform (Chen SL, unpublished). We used BWA software (Li and Durbin 2010) with the default parameters to align the female and male sequencing reads to the male genome assembly and then used SAMtools software (Li et al. 2009) to convert the aligned files to BAM files and to calculate sequencing depth at each genomic position. We subsequently extracted sequences with more regions covered by reads only of the male genome and not by those of the female genome (Fig. 1a) (Chen et al. 2014). The differences in extracted sequences with male-specific regions between the female and the male were then visualized using the IGV software (Robinson et al. 2011).
PCR Verification of the Male-Specific Marker
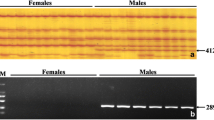
To verify the male-specific marker obtained above, we designed a male-female-shared primer pair (s-F and s-R, Table 1) for the 500 bp flanking sequences of sex-specific fragments using the Geneious R7 software (Kearse et al. 2012). The sex-specific region was amplified with the primer pair using genomic DNA samples from 12 males and 12 females. The cycling conditions were 3 min at 95 °C; 30 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 7 min and, last, incubation at 72 °C for 7 min. All PCR products were analysed on 4% agarose gels by agarose gel electrophoresis. Ten PCR products from 5 male individuals and 5 female individuals were randomly selected. The PCR products were then purified by gel extraction using a gel extraction kit (Vazyme, China), cloned into pEASY-T1 vectors (Transgen, China) and sequenced (Ruibio Biotech, China). The sequences obtained were analysed with the ClustalW method using the Geneious software.
Development and Confirmation of the Genetic Sex Identification Technique
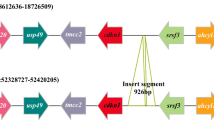
In practical aquaculture, we need to identify the genetic sex of a spotted knifejaw en masse with a simple and rapid method in accordance with the allele-specific polymerase chain reaction (ASPCR) principle (Wallace et al. 1997) to avoid false-negative results. We designed one male-specific primer (male-s-F) for the sequence spanning the male-specific marker and another two shared primers (mf-s-F and mf-s-R) for the flanking sequence using the Geneious software (Table 1). PCR was performed with male-specific primers using genomic DNA samples from spotted knifejaw (51 males and 47 females). The cycling conditions were 3 min at 95 °C; 30 cycles of 95 °C for 30 s, 57 °C for 30 s and 72 °C for 7 min and, last, incubation at 72 °C for 7 min. The PCR products were analysed on 1% agarose gels by agarose gel electrophoresis.
Results
Screening of Male-Specific Markers by Comparative Analysis of Whole-Genome Sequencing
In this work, we previously sequenced and assembled the whole genomes of female and male spotted knifejaw fish. By comparing the sequencing reads of the female and male genomes, we obtained a large amount of male-specific sequences ranging from 1 bp to 50 bp, which were covered by reads of only the male genome and not by those of the female genome. As a result, a total of 1967 male-specific regions (insertion type structural variation) in 5 contigs were identified and considered sex-specific DNA marker candidates (Fig. 1b). Moreover, we filtered the candidates according to sequencing depth and duplication. We subsequently confirmed that, in males, the sequencing depth of the candidates (~ 10 ×) was half of the sequencing depth of autosomal sequences (~ 20 ×). Since the male individuals were heterozygous for male-specific sequences, the sequences that occurred only in the male individuals should be the male-specific markers.
Verification of the Male-Specific Markers
To avoid the individual-specific or population-specific phenomenon that occurs in the detection of male-specific markers, 98 individuals from various populations of spotted knifejaw were used for verification of the male-specific markers, and their phenotypic sex was identified by histological observations of the testes and ovaries (Fig. 2). We obtained flanking 500 bp fragments of male-specific markers according to the sequence locations and designed the respective primers using the Geneious software. Last, we isolated an 18 bp insertion specific to the male (probably located on the Y chromosome) by PCR and agarose electrophoresis (Fig. 3a). Two bands were observed in the 12 male samples on 4% agarose gels, whereas only one band was observed in the 12 female samples (Fig. 3b). After sequencing, two sequences of 152 bp and 134 bp were obtained from males, and one sequence of 150 bp was obtained from females. The longer sequence contained the 18 bp fragment, while the shorter sequence was identical in both the males and females (Fig. 3c). These results suggested that the 18 bp insertion was male specific and could be developed to identify the genetic sex in spotted knifejaw aquaculture.
Isolation of a male-specific marker. a Visualization of the male-specific marker by the IGV software. The predicted male-specific insertion is shown in the red frame. The male-specific sequence used for specific primer design is shown in the blue dotted frame. b Identification of the male-specific marker via gel electrophoresis. The DNA marker is indicated by the letter M. c Sequence alignment of bands from female and male spotted knifejaw
Development of an Identification Technique for Genetic Sex
In this study, three primers were used for the PCR experiment (Fig. 4a). By using agarose gel electrophoresis, we observed two bands in the male samples and one band in the female samples on a 1% agarose gel. The size of the long band was identical and was approximately 550 bp in both the males and females, while the size of the short band was approximately 250 bp in the males (Fig. 4b). The genetic sex of 98 individuals that were approximately 1 year old was consistent with their phenotypic sex, which further verified the male-specific marker. The genetic sex of 500 spotted knifejaw fish in the breeding season was identical to the phenotypic sex observed in the ejaculation and ovulation periods. In this way, the genetic sex could be identified quickly and stably with no false-negative phenomenon.
Discussion
In teleost fishes, the sex determination mechanism is diverse and complicated; as such, investigations of this mechanism are important to both basic theory and artificial breeding programmes. Sex markers can lay a foundation for the identification of sex chromosomes and the study of sex determination mechanisms. The X1X1X2X2/X1X2Y multiple sex chromosome system has been reported in fish species and has attracted the attention of many researchers (Sun et al. 2013; Pennell et al. 2015; Xu et al. 2019; Cai et al. 2019). More than 20 species, including barred knifejaw (Oplegnathus fasciatus), present multiple sex chromosome systems (Cioffi and Bertollo 2010). A male-specific marker of a deletion of 8 bp and several single-nucleotide polymorphism (SNP) locations have been identified by the AFLP approach in barred knifejaw, and specific primers applicable in genetic sex identification have been developed (Xu et al. 2013). Closely related to barred knifejaw, spotted knifejaw also has the X1X1X2X2/X1X2Y type of multiple sex chromosome system. Moreover, spotted knifejaw is a commercially important marine aquaculture fish of the family Oplegnathidae and is promoted on a large scale in China. Sex-specific markers could help in the breeding of a large amount of spotted knifejaw for aquaculture farming. Since the sex ratio of adult fish influences pairing behaviour and male-female interactions, sex-specific markers could be used to guide artificial breeding programmes (Székely et al. 2014).
Comparative genome analysis represents an ideal approach to rapidly screen sex-specific markers with subtle differences, even single-nucleotide differences, and provides a valuable resource for molecular marker exploitation (Woram et al. 2003). In previous work, we sequenced the genomes of female and male spotted knifejaw fish (Chen SL, unpublished). To identify sex-specific markers, we performed a comparative analysis of the whole genomes of the female and male to find the genetic differences in the heteromorphic sex chromosome (Y chromosome) of the males. The male-specific markers are considered to be located on the Y chromosome because the Y chromosome exists exclusively in genetic males. Therefore, male-specific markers will help to assemble and identify sex chromosomes in future studies.
Sex-linked DNA variations, including indel events, lead to the formation and evolution of sex chromosomes (Charlesworth and Charlesworth 2000). In large yellow croaker, a 15 bp deletion was screened in the third intron of the dmrt1 gene based on the comparative analysis of the resequenced genomes between the males and females (Lin et al. 2017). In other research on spotted knifejaw, 895 unigenes expressed specifically in males and 469 unigenes expressed specifically in females were screened by comparative transcriptome analysis (Du et al. 2017). However, no sex-specific markers were identified successfully. In this study, we identified a male-specific marker of an 18 bp insertion in a sex-linked region without successful annotation in spotted knifejaw. These results reveal that not all sex-specific markers are located in the gene region and that sex-specific markers always reflect the evolution of sex chromosomes.
In practical aquaculture, development of a rapid and effective genetic sex identification method is urgently needed. Consequently, the ASPCR principle has been used to develop sex-specific markers to identify genetic sex. In the present study, we designed three primers based on the sex-specific marker and its flanking sequence. By the use of the mixed primers, PCR amplification and agarose gel electrophoresis were conducted to observe the number of bands. There are many advantages of this PCR-based method, such as the avoidance of false-negative phenomena, easy implementation and low requirements for reaction conditions. More importantly, the results of the identification are accurate and can be obtained rapidly (Brugmans et al. 2003; Ennis and Gallagher 1994).
The sex of teleosts is mostly determined by genetic factors; however, environmental factors and hormones also influence the sex of some fish species (Baroiller and D'cotta 2001). The sex reversal phenomenon is ubiquitous in marine fish such as Chinese tongue sole (Cynoglossus semilaevis), leading to inconsistencies between genetic sex and phenotypic sex (Ji et al. 2010). As reported, no sex reversal phenomenon occurred in barred knifejaw, which also belongs to the family Oplegnathidae and has the same sex chromosome system as spotted knifejaw (Xu et al. 2013). For practical use, we further examined the genetic sex of approximately 500 spotted knifejaw fish, which were identical to the phenotypic sex observed in the ejaculation and ovulation periods. Although sex reversal was not detected in spotted knifejaw in our research, the influence of environmental factors on sex determination should be further studied and confirmed.
Conclusion
We exploited a male-specific marker of an 18 bp insertion using comparative analysis of the genomes and successfully identified the marker for the genetic sex identification of spotted knifejaw. We further developed an effective and rapid method based on PCR for the practical detection of genetic sex. A male-specific DNA marker would be helpful for artificial breeding, Y chromosome assembly and further study of the sex determination mechanism. To the best of our knowledge, this study is the first to report the exploitation of sex-specific markers and the detailed method of genetic sex identification in spotted knifejaw.
References
Baroiller J-F, D'cotta H (2001) Environment and sex determination in farmed fish. Comp Biochem Physiol Part C Toxicol Pharmacol 130:399–409
Berset-Brändli L, Jaquiéry J, Dubey S, Perrin N (2006) A sex-specific marker reveals male heterogamety in European tree frogs. Mol Biol Evol 23:1104–1106
Brugmans B, Van Der Hulst RG, Visser RG, Lindhout P, Van Eck HJ (2003) A new and versatile method for the successful conversion of AFLP™ markers into simple single locus markers. Nucleic Acids Res 31:e55–e55
Cai M, Zou Y, Xiao S, Li W, Han Z, Han F, Xiao J, Liu F, Wang Z (2019) Chromosome assembly of Collichthys lucidus, a fish of Sciaenidae with a multiple sex chromosome system. Sci Data 6:132
Charlesworth B, Charlesworth D (2000) The degeneration of Y chromosomes. Philos Trans R Soc Lond B Biol Sci 355:1563–1572
Chen S-L, Li J, Deng S-P, Tian Y-S, Wang Q-Y, Zhuang Z-M, Sha Z-X, Xu J-Y (2007) Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis). Mar Biotechnol 9:273–280
Chen S-L, Ji X-S, Shao C-W, Li W-L, Yang J-F, Liang Z, Liao X-L, Xu G-B, Xu Y, Song W-T (2012) Induction of mitogynogenetic diploids and identification of WW super-female using sex-specific SSR markers in half-smooth tongue sole (Cynoglossus semilaevis). Mar Biotechnol 14:120–128
Chen S, Zhang G, Shao C, Huang Q, Liu G, Zhang P, Song W, An N, Chalopin D, Volff JN, Hong Y, Li Q, Sha Z, Zhou H, Xie M, Yu Q, Liu Y, Xiang H, Wang N, Wu K, Yang C, Zhou Q, Liao X, Yang L, Hu Q, Zhang J, Meng L, Jin L, Tian Y, Lian J, Yang J, Miao G, Liu S, Liang Z, Yan F, Li Y, Sun B, Zhang H, Zhu Y, Du M, Zhao Y, Schartl M, Tang Q, Wang J (2014) Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet 46:253–260
Cioffi M, Bertollo L (2010) Initial steps in XY chromosome differentiation in Hoplias malabaricus and the origin of an X 1 X 2 Y sex chromosome system in this fish group. Heredity 105:554
De Almeida-Toledo LF, Daniel-Silva MDFZ, Lopes C, Toledo-Filho SDA (2000) Sex chromosome evolution in fish. II. Second occurrence of an X1X2Y sex chromosome system in Gymnotiformes. Chromosom Res 8:335–340
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Du X, Wang B, Liu X, Liu X, He Y, Zhang Q, Wang X (2017) Comparative transcriptome analysis of ovary and testis reveals potential sex-related genes and pathways in spotted knifejaw Oplegnathus punctatus. Gene 637:203–210
Ennis S, Gallagher T (1994) A PCR-based sex-determination assay in cattle based on the bovine amelogenin locus. Anim Genet 25:425–427
Felip A, Young WP, Wheeler PA, Thorgaard GH (2005) An AFLP-based approach for the identification of sex-linked markers in rainbow trout (Oncorhynchus mykiss). Aquaculture 247:35–43
Fowler BL, Buonaccorsi VP (2016) Genomic characterization of sex-identification markers in Sebastes carnatus and Sebastes chrysomelas rockfishes. Mol Ecol 25:2165–2175
Fuji K, Yoshida K, Hattori K, Ozaki A, Araki K, Okauchi M, Kubota S, Okamoto N, Sakamoto T (2010) Identification of the sex-linked locus in yellowtail, Seriola quinqueradiata. Aquaculture 308:S51–S55
Galindo HM, Loher T, Hauser L (2011) Genetic sex identification and the potential evolution of sex determination in Pacific halibut (Hippoglossus stenolepis). Mar Biotechnol 13:1027–1037
Guerrero Iii RD (1975) Use of androgens for the production of all-male Tilapia aurea (Steindachner). Trans Am Fish Soc 104:342–348
Imsland A, Folkvord A, Grung G, Stefansson S, Taranger G (1997) Sexual dimorphism in growth and maturation of turbot, Scophthalmus maximus (Rafinesque, 1810). Aquac Res 28:101–114
Ji X, Chen S, Ma H, Jiang Y, Yang J, Dong X (2010) Natural sex reversal of female Cynoglossus semilaevis in rearing populations. J Fish China 34:322–327
Kawato Y, Yamashita H, Yuasa K, Miwa S, Nakajima K (2017) Development of a highly permissive cell line from spotted knifejaw (Oplegnathus punctatus) for red sea bream iridovirus. Aquaculture 473:291–298
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Kikuchi K, Hamaguchi S (2013) Novel sex-determining genes in fish and sex chromosome evolution. Dev Dyn 242:339–353
Kobayashi Y, Nagahama Y, Nakamura M (2013) Diversity and plasticity of sex determination and differentiation in fishes. Sex Dev 7:115–125
Kovács B, Egedi S, Bártfai R, Orbán L (2000) Male-specific DNA markers from African catfish (Clarias gariepinus). Genetica 110:267–276
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26:589–595
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079
Li P, Cao D, Liu X, Wang Y, Yu H, Li X, Zhang Q, Wang X (2016) Karyotype analysis and ribosomal gene localization of spotted knifejaw Oplegnathus punctatus. Genet Mol Res 15:gmr15049159
Lin A, Xiao S, Xu S, Ye K, Lin X, Sun S, Wang Z (2017) Identification of a male-specific DNA marker in the large yellow croaker (Larimichthys crocea). Aquaculture 480:116–122
Mei J, Gui J-F (2015) Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci China Life Sci 58:124–136
Pennell MW, Kirkpatrick M, Otto SP, Vamosi JC, Peichel CL, Valenzuela N, Kitano J (2015) Y fuse? Sex chromosome fusions in fishes and reptiles. PLoS Genet 11:e1005237
Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29:24
Sun XX, Xu DD, Lou B, Li SL, Zhan W, Zhang YR, Xin J (2013) Development and characterization of novel microsatellite markers in the rock bream fish Oplegnathus fasciatus. Genet Mol Res 12:6462–6465
Székely T, Weissing FJ, Komdeur J (2014) Adult sex ratio variation: implications for breeding system evolution. J Evol Biol 27:1500–1512
Wallace RB, Pal BK, Ugozzoli LA, Wu DY (1997) Allele specific polymerase chain reaction. Google Patents
Woram RA, Gharbi K, Sakamoto T, Hoyheim B, Holm L-E, Naish K, Mcgowan C, Ferguson MM, Phillips RB, Stein J (2003) Comparative genome analysis of the primary sex-determining locus in salmonid fishes. Genome Res 13:272–280
Xiao Y, Xiao Z, Ma D, Liu J, Li J (2019) Genome sequence of the barred knifejaw Oplegnathus fasciatus (Temminck & Schlegel, 1844): the first chromosome-level draft genome in the family Oplegnathidae. Gigascience 8. https://doi.org/10.1093/gigascience/giz013
Xu D, Lou B, Xu H, Li S, Geng Z (2013) Isolation and characterization of male-specific DNA markers in the rock bream Oplegnathus fasciatus. Mar Biotechnol 15:221–229
Xu D, Sember A, Zhu Q, Oliveira EA, Liehr T, Al-Rikabi ABH, Xiao Z, Song H, Cioffi MB (2019) Deciphering the origin and evolution of the X1X2Y system in two closely-related Oplegnathus species (Oplegnathidae and Centrarchiformes). Int J Mol Sci 20. https://doi.org/10.3390/ijms20143571
Funding
This work was supported by grants from the Special Scientific Research Funds for Central Non-profit Institutes, Yellow Sea Fisheries Research Institute (20603022019018), AoShan Talents Cultivation Program Supported by Qingdao National Laboratory for Marine Science and Technology (No.2017ASTCP-OS15), the Taishan Scholar Climbing Project Fund of Shandong, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Xu, H., Xu, W. et al. Isolation of a Male-Specific Molecular Marker and Development of a Genetic Sex Identification Technique in Spotted Knifejaw (Oplegnathus punctatus). Mar Biotechnol 22, 467–474 (2020). https://doi.org/10.1007/s10126-020-09966-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-020-09966-3