Abstract
One paradox of the trophic biochemistry of the deep sea giant tubeworm Riftia pachyptila, endemic to hydrothermal vent sites and nourished by polyunsaturated fatty acid (PUFA) deficiency chemolitoautotrophic sulfide-oxidizing bacteria, is the source of their PUFAs. Biosynthesis of PUFA starts with two precursors C18:2n-6 and C18:3n-3, which cannot be biosynthesized by most animals due to lack of ω6- and ω3-desaturase; thus, C18:2n-6 and C18:3n-3 are generally essential fatty acids for animals. Here, we characterized a gene derived from the R. pachyptila located by hydrothermal vent, which encoded a novel ω3-desaturase (Rp3Fad). The gene was identified by searching the R. pachyptila transcriptome database using known ω3-desaturases, and its predicted protein showed 37–45% identical to ω3-desaturases of fungus and microalgae, and only 31% identitical to nematode Caenorhabditis elegans ω3-desaturase. Expression in yeast Saccharomyces cerevisiae showed that the Rp3Fad could desaturate C18:2n-6 and C18:3n-6 into C18:3n-3 and C18:4n-3, respectively, displaying a Δ15 activity similar to plant ω3-desaturase, but it showed no activity towards C20 n-6 PUFA substrates, differing from the well-characterized C. elegans ω3-desaturases. Δ5, Δ6, Δ8, and Δ12 activity were also tested, resulting in no corresponding production. The function of ω3-desaturase identified in R. pachyptila could produce C18:3n − 3 used in synthesis of n − 3 series PUFAs, suggesting an adaption to PUFA deficiency environment in deep sea hydrothermal vent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-chain polyunsaturated fatty acids (PUFAs) and their derivatives (such as prostaglandins, leukotrienes, and thromboxanes) are essential components of membrane phospholipids or involved in signaling and the regulation of lipid metabolism, inflammatory response, and cell division (Simopoulos 1999; Zeyda et al. 2002; Kawamoto et al. 2009; Calder 2010; Vrablik and Watts 2013). Synthesis of long-chain PUFAs is involved in a group of membrane-bound desaturases and elongating enzymes, which started with linoleic acid (LA, 18:2n-6) for the n-6 series, and α-linolenic acid (ALA, 18:3n-3) for the n-3 series. ω6 (or Δ12)-desaturase catalyzes the conversion of C18:1n-9 into LA, which can then be further desaturated by ω3 (or Δ15)-desaturase to produce ALA. However, with certain exceptions, animals in general lack both ω3-desaturase and ω6-desaturase; thus, these two fatty acids (FAs) are considered as essential dietary components (Pereira et al. 2003).
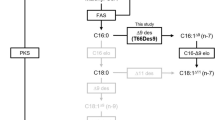
As one of the most studied species living at deep sea hydrothermal vents, the giant tubeworm Riftia pachyptila mainly depends on chemolitoautotrophic sulfide-oxidizing bacteria located intracellularly in the trophosome to satisfy its nutritional needs (Cavanaugh et al. 1981; Zal et al. 1996a, 1996b, 1998). Sulfide-oxidizing bacteria derived from the hydrothermal vent sediment and water of the habitats or isolated from the host usually contained mainly saturated and monounsaturated fatty acids (MUFAs) and possibly provided limited PUFAs to its primary consumers although trace C18 PUFAs (generally LA) were detected in several species (Conway and Capuzzo 1991; Fullarton et al. 1995a; Fullarton et al. 1995b; Zhang et al. 2005; Li et al. 2011). However, in addition to bacterial source FAs, R. pachyptila had been reported to contain a high percent of PUFAs such as Arachidonic acid (AA, C20:4n-6) and Eicosapentaenoic acid (EPA, C20:5n-3) (Phleger et al. 2005). These findings raised the hypothesis that the giant tubeworm has the capability to desaturate and elongate bacterial FAs to obtain long-chain PUFAs AA and EPA although some marine polar bacteria and deep sea bacteria could produce either EPA or DHA (Phleger et al. 2005). Synthesis of AA and EPA from the PUFA precursors LA and ALA through either conventional “Δ6 pathway” or the alternative “Δ8-pathway” had been exclusively reported in marine animals (Pereira et al. 2003; Monroig et al. 2013; Liu et al. 2014). Since intermediate FAs C20:2n-6 and C20:3n-6 were detected in body tissues of the giant tubeworm (Phleger et al. 2005), we speculated that R. pachyptila possibly possess a “Δ8 pathway” to synthesize long-chain PUFAs. However, unlike plant and some fungus, most animals are unable to introduce a double bond at the Δ12 and Δ15 position on the C18 FAs due to lack of ω6-desaturase and ω3-desaturase and cannot de novo synthesize LA and ALA served as precursors for long-chain PUFA synthesis (Pereira et al. 2003, 2004). It was unclear how R. pachyptila obtained these two essential FAs used for further desaturation and elongation.
In this study, we used the extensive information now available from the R. pachyptila to identify genes encoding desaturases with Δ12 and Δ15 activity. This deep sea organism was predicted to desaturate and elongate C18: 1n-7 based on its FA profiles and living habits (Phleger et al. 2005). Here, we described the isolation and characterization of a transcript from R. pachyptila, which encoded a ω3-desaturase with activity on C18 PUFA substrates. Heterologous expression of this gene in yeast demonstrated its ability to desaturate LA and GLA (γ-linolenic acid, C18:3n-6) into ALA and C18:4n-3, respectively, with no activity on C18:1n-9 or C20 PUFA substrates. To our knowledge, this is the first report of an animal ω3-desaturase derived from deep sea hydrothermal vent.
Materials and Methods
Identification of the R. pachyptila ω3-Desaturase Coding Sequence
The R. pachyptila 454 sequencing sequence read archive (SRA) database was downloaded from NCBI website (http://www.ncbi.nlm.nih.gov) under GenBank accession number. SRX098351 assembled with iAssembler (v1.3.2) after sequence trimmed, then the assembled R. pachyptila EST database was BlastX searched against with nematode Caenorhabditis elegans ω3-desaturase (GenBank accession number. NP_001023560) and several other organism ω3-desaturases as the query sequences. A 1033 bp EST sequence was obtained to show only a 31% similarity with C. elegans ω3-desaturase. The DNA sequence corresponding to the putative coding DNA sequence (CDS) of the R. pachyptila ω3-desaturase (Rp3Fad) was used to search against the assembled R. pachyptila EST database resulting in no homologous sequence with high identity. The putative CDS of Rp3Fad was synthesized (Takara, Dalian, China) and sequenced to confirm the right sequence.
Heterologous Expression of Rp3Fad in Yeast and Fatty Acid Analysis by GC
The open reading frame (ORF) of the Rp3Fad was amplified from the synthesized sequence using the high-fidelity DNA polymerase kit (PrimeStar® HS DNA polymerase, Takara, Dalian, China). Forward primer GGGAA GCTTAACACAATGCTGAGCGACGTGTGCCA (Hind III) and reverse primer GCTCTAGATCTAGTAATACGCATGTAGCATCGT (XbaI) containing restriction sites were used to perform the PCR, and the PCR amplifications involved 30 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 5 s, and extension at 68 °C for 90 s. After PCR, the DNA fragments were purified, digested with the corresponding restrictive endonucleases, and ligated into similarly digested pYES2 vector (Invitrogen, CA, USA). The recombinant plasmids were transformed into yeast S. cerevisiae strain INVSc1 accordingly to the method of Gietz et al. (1992). Recombinant yeast was cultivated in S. cerevisiae minimal medium minus uracil (SCMM−uracil) broth using galactose for induction of gene expression as described previously (Hastings et al. 2001). Cultures were supplemented with PUFA substrates at final concentrations of 0.5 μM for C18 and 0.75 μM for C20 according to Li et al. (2010). After cell disruption, total lipids were extracted with chloroform/methanol (2:1, v/v), containing 0.01% butylated hydroxytoluene as antioxidant. Fatty acid methyl esters (FAMEs) were prepared, extracted, and purified by thin-layer chromatography on silica gel 60 plate (Merck, Germany). FAMEs were analyzed using gas chromatography (Agilent 6890N, Agilent, US) equipped with a hydrogen flame ionization detector and a capillary column (30 m × 0.25 mm × 0.25 μm, DB-WAX, Agilent, US). The proportion of substrate FA converted to desaturation product was calculated from the gas chromatograms as 100·[product area/(product area + substrate area)].
Sequence and Phylogenetic Analyses
The multiple sequence alignment was performed using ClustalX 2.1 and phylogenetic trees were made in MEGA 6 package on the basis of putative protein sequence of ω3-desaturase from the R. pachyptila and other organisms using the neighbor-joining method (Tamura et al. 2013). Confidence in the resulting phylogenetic tree branch topology was measured by bootstrapping through 1000 iterations.
Results
Identification and Characterization of R. pachyptila ω3-Desaturase
BLASTX analysis of R. pachyptila EST transcriptome using C. elegans ω3-desaturase (fat-1, GenBank accession number: NP_001023560) as query revealed a transcript with 1587 bp in length. The putative R. pachyptila ω3-desaturase (GenBank accession number: KY399781), designated as Rp3Fad, encoding a putative peptide of 403 amino acids, was 52% identitical to Lottia gigantea hypothetical protein LOTGIDRAFT_74451 (XP_009063368) which was not functionally characterized. It shared 37–45% identity to ω3-desaturase of fungus and microalgae, and only 31% identity to well functionally characterized C. elegans ω3-desaturase (Fig. 1). Sequence analysis also showed that this peptide possessed three diagnostic histidine-rich boxes motif found to be present in all membrane-bound desaturases (Fig. 1). Phylogenetic analysis showed that Rp3Fad and some uncharacterized putative ω3-desaturase proteins of marine invertebrate grouped together, then clustered with some fungus ω3-desaturases, and the node support is very high (100) (Fig. 2).
Amino acid sequence comparison of R. pachyptila ω-desaturase (Rpω3) with other ω3-desaturases. The alignment includes amino acid sequences from C. elegans (Ceω3; NP_001023560), Saprolegnia diclina (Sdω3; AAR20444), and Triticum aestivum (Taω3; BAA28358). Identical residues are shaded black. The underlines indicate the three histidine-rich boxes
Phylogenetic tree comparing the deduced aa sequences of the giant tubeworm R. pachyptila ω3-desaturase with other organisms ω3 and ω6-desaturases. The alignment was generated by the Clustal W program, and the tree was constructed using the neighbor-joining method with MEGA6.0 software. The horizontal branch length is proportional to aa substitution rate per site. The numbers represent the frequencies with which the tree topology presented was replicated after 1000 iterations
Functional Characterization of Rp3Fad in Yeast
To determine the functionality of the putative Rp3Fad, yeast transformed with pYES2 vector alone or containing Rp3Fad cDNA ORF were grown in the presence of various PUFA substrates in SCMM−uraci media, and the FA profile of yeast was extracted and analyzed by gas chromatography (Fig. 3). The FA profile of yeast cell with empty plasmid was characterized of only C16:0, C16:1n-7, C18:0, and C18:1n-9 (Hastings et al. 2001). To check for ω3-desaturase activity on C18 PUFAs, the substrates LA or GLA were exogenously added to the cultures individually, which were then converted into ALA and C18:4n-3, respectively, indicating that Rp3Fad possessed a ω3-desaturase activity (Fig. 3d, f), and 3.4% of LA and 4.2% of GLA were desaturased into ALA and C18:4n-3, respectively (Table 1). When C20 PUFA substrates C20:2n-6, C20:3n-6, or C20:4n-6 (AA) were added to the cultures, no ω3 FA was produced (Table 1). None Δ6-, Δ8, or Δ5-production was detected when yeast transformed with the pYES2-Rp3Fad recombinant plasmid was grown in the presence of Δ6- (C18:2n-6, C18:3n-3), Δ8- (C20:2n-6, C20:3n-3), or Δ5- (C20:3n-6) substrates Additionally, Rp3Fad did not convert endogenous C18:1n-9 into C18:2n-6 in the recombinant yeast culture, indicating that Rp3Fad did not possess the Δ12 activity.
Functional characterization of the R. pachyptila putative ω3-desaturase (Rp3Fad) in yeast S. cerevisiae. FAME were extracted from yeast transformed with pYES2 vector alone (a, c, e) or the constructs pYES2-Rp3Fad (b, d, f), and grown in the presence of FA substrate (peak number) C18:2n-6 (5) or C18:3n-6 (7). Peaks 1–4 represent the main endogenous FAs of yeast, namely C16:0, C16:1n, C18:0, and C18:1n-9, respectively. Based on retention times, PUFA productions were identified as C18:3n-3 (d, peak 6) and C18:4n-3 (f, peak 8). Vertical axis: FID response. Horizontal axis: retention time
Discussion
As a primary consumer feeding on sulfide-oxidizing bacteria which is usually deficiency of PUFAs, the mouthless vent tubeworm R. pachyptila was detected to contain a high amount of PUFAs (Phleger et al. 2005). The vent tubeworms lacked a mouth and a gut (Cavanaugh et al. 1981; Zal et al. 1996A), an input of PUFAs from photic zone was excluded. This observation led to the prediction that R. pachyptila produces biologically active PUFAs themselves, or obtain them from endosymbiotic bacteria. Since LA and ALA are particularly interesting FAs which cannot be formed de novo by most animals (Pond et al. 2002), some FA desaturase enzymes that functioned as ω3- and/or ω6-desaturase might exist in R. pachyptila that convert monounsaturated FAs into LA and ALA providing PUFA precursors. By BlastX searching, a transcript encoding the putative ω3-desaturase was identified and functionally characterized in yeast, which revealed that the encoded protein could convert LA and GLA into ALA and C18:4n-3, respectively, displaying a Δ15 activity. None Δ5, Δ6, or Δ8 production was detected in the presence of corresponding FA substrates. In addition, no desaturased production was detected when C20:3n-6 and C20:4n-6 were exogenously added. These results indicated that this protein was an ω3-desaturase with activity towards C18 PUFA substrates.
Like other ω3-desaturases, sequence analysis showed that the Rp3Fad contained three histidine-rich motifs and did not possess a fused cytochrome b5 domain at its N-terminus (Shanklin et al. 1994; Pereira et al. 2004). The Rp3Fad and several other predicted ω3-desaturase from invertebrate grouped together, and then clustered with fungus ω3-desaturase in phylogenetic tree; however, this peptide did not cluster closely with the functionally characterized animal ω3-desaturase from the nematode C. elegans (Spychalla et al. 1997; Meesapyodsuk et al. 2000). It seemed that the Rp3Fad and some fungus ω3-desaturases evolve from the same origin. Unlike the nematode (Meesapyodsuk et al. 2000) and fungus (Pereira et al. 2004; Sakuradani et al. 2005) ω3-desaturase that could catalyze both C18 and C20 PUFA substrates, Rp3Fad only displayed activity towards C18 PUFA substrates, exhibiting almost the same substrate specificity to high plant ω3-desaturases (Sakuradani et al. 2005).
Since no Δ12 production was determined when Rp3Fad was functionally characterized in the yeast, we had also searched for a possible ω6-desaturase in the R. pachyptila transcriptome dataset using plant and C. elegans ω6-desaturases as the queries. Two another predicted fatty acid desaturases with low similarity to the queries were identified, which instead showed a high similarity to invertebrate Δ5 desaturase. ω3-desaturase in some species (such as C. elegans) displayed a high similarity to the same source ω6-desaturase. The Rp3FAD was used as the query to search against the giant tubeworm transcriptome dataset, but no more homolog was obtained, except the Rp3FAD transcript. The Rp3FAD from this species might be the only enzyme necessary for the synthesis of PUFA precursors; thus, the source of LA for the giant vent tubeworm was undefined. A minor percent of LA were detected in both symbiotic (Fullarton et al. 1995b) and free-living (Jacq et al. 1989; Temara et al. 1991) forms sulfur-oxidizing bacteria, and some amount of C18:2n PUFAs were also detected at exterior of the chimney wall where tubeworms resided (Li et al. 2011). Even though nothing was known about FA synthesis of endosymbiotic bacteria resided in R. pachyptila, these microorganisms might possibly contribute LA to its host.
FA profile of vent and seep animals harboring chemoautotrophic bacteria was exclusively analyzed, and the origin of PUFAs was also discussed in several invertebrates, indicating that vent and seep animals had the capability to de novo synthesize PUFAs (Pond et al. 2002; Phleger et al. 2005). The ω3-desaturase identified in this study gave the first molecular proof of the existence of enzyme that involved in ALA biosynthesis in hydrothermal vent animals. The hydrothermal vent ecosystem is relatively isolated from photic zone (Wakeham et al. 1997), and PUFAs are relatively insufficient in the primary producer chemoautotrophic bacteria (Pond et al. 2002; Li et al. 2011). The products of a ω3-desaturases can provide the precursor (and/or intermediate) for synthesis of n-3 series PUFAs, which could compensate shortage of n-3 PUFAs in natural diet. Identification of the ω3-desaturases in R. pachyptila indicated that this organism is highly adapted to the ω3-PUFA deficiency environment in hydrothermal vent.
References
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2:355–374
Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB (1981) Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: possible chemoautotrophic symbionts. Science 213:340–342
Conway N, Capuzzo JM (1991) Incorporation and utilization of bacterial lipids in the Solemya-Velum Symbiosis. Mar Biol 108:277–291
Fullarton JG, Dando PR, Sargent JR, Southward AJ, Southward EC (1995a) Fatty-acids of hydrothermal vent Ridgeia-Piscesae and inshore bivalves containing symbiotic bacteria. J Mar Biol Assoc UK 75:455–468
Fullarton JG, Wood P, Sargent JR (1995b) Fatty acid composition of lipids from sulphuroxidizing and methylotrophic bacteria from thyasirid and lucinid bivalves. J Mar Biol Assoc UK 75:445–454
Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20:1425
Hastings N, Agaba M, Tocher DR, Leaver MJ, Dick JR, Sargent JR, Teale AJ (2001) A vertebrate fatty acid desaturase with Δ5 and Δ6 activities. PNAS 98:14304–14309
Jacq E, Prieur D, Nichols P, White DC, Porter T, Geesey GG (1989) Microscopic examination and fatty-acid characterization of filamentous bacteria colonizing substrata around subtidal hydrothermal vents. Arch Microbiol 152:64–71
Kawamoto J, Kurihara T, Yamamoto K, Nagayasu M, Tani Y, Mihara H, Hosokawa M, Baba T, Sato SB, Esaki N (2009) Eicosapentaenoic acid plays a beneficial role in membrane organization and cell division of a cold-adapted bacterium, Shewanella livingstonensis Ac10. J Bacteriol 191:632–640
Li Y, Monroig O, Zhang L, Wang S, Zheng X, Dick JR, You C, Tocher DR (2010) Vertebrate fatty acyl desaturase with Delta4 activity. PNAS 107:16840–16845
Li JW, Zhou HY, Peng XT, Fu MY, Chen ZQ, Yao HQ (2011) Abundance and distribution of fatty acids within the walls of an active deep-sea sulfide chimney. J Sea Res 65:333–339
Liu H, Zhang H, Zheng H, Wang S, Guo Z, Zhang G (2014) PUFA biosynthesis pathway in marine scallop Chlamys Nobilis reeve. J Agric Food Chem 62:12384–12391
Meesapyodsuk D, Reed DW, Savile CK, Buist PH, Ambrose SJ, Covello PS (2000) Characterization of the regiochemistry and cryptoregiochemistry of a Caenorhabditis elegans fatty acid desaturase (FAT-1) expressed in Saccharomyces cerevisiae. Biochemistry 39:11948–11954
Monroig O, Tocher DR, Navarro JC (2013) Biosynthesis of polyunsaturated fatty acids in marine invertebrates: recent advances in molecular mechanisms. Mar Drugs 11:3998–4018
Pereira SL, Leonard AE, Mukerji P (2003) Recent advances in the study of fatty acid desaturases from animals and lower eukaryotes. Prostaglandins Leukot Essent Fatty Acids 68:97–106
Pereira SL, Huang YS, Bobik EG, Kinney AJ, Stecca KL, Packer JC, Mukerji P (2004) A novel omega3-fatty acid desaturase involved in the biosynthesis of eicosapentaenoic acid. Biochem J 378:665–671
Phleger CF, Nelson MM, Groce AK, Cary SC, Coyne KJ, Nichols PD (2005) Lipid composition of deep-sea hydrothermal vent tubeworm Riftia pachyptila, crabs Munidopsis subsquamosa and Bythograea thermydron, mussels Bathymodiolus sp. and limpets Lepetodrilus spp. Comp Biochem Physiol B Biochem Mol Biol 141:196–210
Pond DW, Allen CE, Bell MV, Van Dover CL, Fallick AE, Dixon DR, Sargent JR (2002) Origins of long-chain polyunsaturated fatty acids in the hydrothermal vent worms Ridgea piscesae and Protis hydrothermica. Mar Ecol Prog Ser 225:219–226
Sakuradani E, Abe T, Iguchi K, Shimizu S (2005) A novel fungal omega3-desaturase with wide substrate specificity from arachidonic acid-producing Mortierella Alpina 1S-4. Appl Microbiol Biotechnol 66:648–654
Shanklin J, Whittle E, Fox BG (1994) Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787–12794
Simopoulos AP (1999) Essential fatty acids in health and chronic disease. Am J Clin Nutr 70:560S–569S
Spychalla JP, Kinney AJ, Browse J (1997) Identification of an animal omega-3 fatty acid desaturase by heterologous expression in Arabidopsis. PNAS 94:1142–1147
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Temara A, Deridder C, Kaisin M (1991) Presence of an essential polyunsaturated fatty-acid in intradigestive bacterial symbionts of a deposit-feeder echinoid (Echinodermata). Comp Biochem Physiol B Biochem Mol Biol 100:503–505
Vrablik TL, Watts JL (2013) Polyunsaturated fatty acid derived signaling in reproduction and development: insights from Caenorhabditis elegans and Drosophila melanogaster. Mol Reprod Dev 80:244–259
Wakeham SG, Hedges JI, Lee C, Peterson ML, Hernes PJ (1997) Compositions and transport of lipid biomarkers through the water column and surficial sediments of the equatorial Pacific Ocean. Deep-Sea Res II Top Stud Oceanogr 44:2131–2162
Zal F, Lallier FH, Wall JS, Vinogradov SN, Toulmond A (1996a) The multi-hemoglobin system of the hydrothermal vent tube worm Riftia pachyptila. I reexamination of the number and masses of its constituents. J Biol Chem 271:8869–8874
Zal F, Lallier FH, Green BN, Vinogradov SN, Toulmond A (1996b) The multi-hemoglobin system of the hydrothermal vent tube worm Riftia pachyptila. II complete polypeptide chain composition investigated by maximum entropy analysis of mass spectra. J Biol Chem 271:8875–8881
Zal F, Leize E, Lallier FH, Toulmond A, Van Dorsselaer A, Childress JJ (1998) S-Sulfohemoglobin and disulfide exchange: the mechanisms of sulfide binding by Riftia pachyptila hemoglobins. PNAS 95:8997–9002
Zeyda M, Staffler G, Hořejší V, Waldhäusl W, Stulnig TM (2002) LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J Biol Chem 277:28418–28423
Zhang CL, Huang ZY, Cantu J, Pancost RD, Brigmon RL, Lyons TW, Sassen R (2005) Lipid biomarkers and carbon isotope signatures of a microbial (Beggiatoa) mat associated with gas hydrates in the Gulf of Mexico. Appl Environ Microbiol 71:2106–2112
Acknowledgements
This work was supported by National Natural Science Foundation of China (41606152, 41576127), Strategic Priority Research Program of CAS (XDB06010104), Knowledge Innovation Program of the Chinese Academy of Sciences (CAS) (SIDSSE–201401, SIDSSE–QN–201407), “Hundred Talents Program” of CAS (SIDSSE–BR–201401, Y510021), and Major scientific and technological projects of Hainan Province (ZDKJ2016009 and ZDKJ2016012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, H., Wang, H., Cai, S. et al. A Novel ω3-Desaturase in the Deep Sea Giant Tubeworm Riftia pachyptila . Mar Biotechnol 19, 345–350 (2017). https://doi.org/10.1007/s10126-017-9753-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-017-9753-9