Abstract
As an alternative to chemical insecticides, gut bacteria of insects could be used to control insect pests. In this study, bacteria associated with Tuta absoluta, an invasive species that has developed resistance to chemical insecticides, were isolated, and their potential for pest control was investigated. We isolated 13 bacteria from larvae of the pest and identified the isolates on the basis of their morphological, physiological, biochemical, and molecular characteristics as Bacillus thuringiensis (Ta1-8), Staphylococcus petrasii (Ta9), Citrobacter freundii (Ta10), Chishuiella changwenlii (Ta11), Enterococcus casseliflavus (Ta12), and Pseudomonas tremae (Ta13). A laboratory screening test at 109 cfu/ml showed that B. thuringiensis (Bt) isolates caused more than 90% mortality after 3 days. Among the isolates, Bt-Ta1 showed the highest mortality in a short time. The LC50 and LC90 values for Bt-Ta1 were estimated to be 1.2 × 106 and 2 × 109 cfu/ml, respectively. Detailed characterization of Bt-Ta1 revealed that it is one of the serotypes effective on lepidopterans and contains the genes cry1Aa, cry2Aa, and vip3Aa, which encode lepidopteran toxic proteins. Bt-Ta1 isolate has been shown to have the potential to be used in the integrated management of Tuta absoluta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climatic changes increase the chances of occurrence of various invasive insect pests. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), one of the invasive pests, is considered one of the most devastating pests to tomato crops. Tuta absoluta is native to South America and was first reported outside South America in Spain in 2006 (Urbaneja et al. 2008). Since then, it has been reported in almost all tomato-growing countries. The pest attacks the foliage, stems, fruits, and flowers of tomato plants and affects the overall growth and yield of the plant. Damage can reach to 80–100% in the greenhouse and open-field conditions if not controlled (Chidege et al. 2016).
Current control of T. absoluta in tomato-growing areas is mainly based on the use of chemical insecticides. However, in some tomato-growing areas, chemical insecticides are used up to three times a week. Insecticides can generally increase yields by suppressing pest populations. On the other hand, the high number of insecticide applications significantly increases production costs and leads to the emergence of resistant populations over time. Insecticide resistance has been reported worldwide for avermectins, pyrethroids, diamides, benzoylureas, organophosphates, oxadiazines, semicarbazones, and spinosyns (Siqueira et al. 2000; Lietti et al. 2005; Guedes et al. 2019; Langa et al. 2022). In addition, their negative impacts on beneficial insects limit the use of its predators.
Insect pathogenic bacteria are an important alternative for integrated pest management strategies due to their species specificity and environmental safety. Bacteria may be permanently or temporarily associated with their insect host, and such associations may be beneficial or detrimental to the insect. Insect gut bacteria play an important role in nutrition and digestion, detoxification of secondary plant compounds, protection against pathogens, development and reproduction, etc. In addition to these beneficial associations, gut bacteria can become opportunistic pathogens due to physiological or environmental changes that lead to a deterioration of gut microbial diversity (Moran et al. 2005; Engel and Moran 2013). Several studies have investigated the pathogenicity of bacteria associated with the gut microbiota of lepidopterans from different families, such as, Lymantria dispar L. (Lep.: Lymantriidae) (Demir et al. 2012), Spodoptera littoralis (Boisd.) (Lep.: Noctuidae) (Çakici et al. 2014), Antheraea assamensis Helfer (Lep.: Saturniidae) (Haloi et al. 2016), Spodoptera exigua (Hübner) (Lep.: Noctuidae) (Eski et al. 2018), and Spodoptera litura (Fab.) (Lep.: Noctuidae) (Devi et al. 2022).
To date, the effect of entomopathogenic bacteria isolated from different sources on T. absoluta has been studied, but the pathogenicity of the gut bacteria associated with T. absoluta has not been investigated. In this regard, the present study is aimed at determining the pathogenicity of culturable gut bacteria associated with T. absoluta and to present a potential candidate for integrated pest management strategies.
Materials and methods
Insect collection and bacteria isolation
The larvae of the tomato leaf miner were collected from infested greenhouses in Antalya, Turkey. The infested fruits and leaves were carefully dissected, and the larvae were removed. The larvae were sterilized with 70% ethanol for 30 s and then washed three times with sterile distilled water. The larvae were homogenized in 1 ml of PBS (phosphate-buffered saline) using a sterile glass tissue grinder. The homogenized suspension was serially diluted up to 10−7 in PBS solution, and 100 μl of each dilution was spread on TSA (tryptic soy agar) plates. The plates were incubated at 30 ± 2 °C for 4 days. Colonies were differentiated by phenotype and a single representative isolate of each morphotype was sub-cultured on TSA plates (The pure cultures of the bacterial isolates were stored in 20% glycerol at − 80 °C).
Morphological and biochemical characteristics
Isolates were first screened based on the shape and color of the colony by direct observation using a stereomicroscope. Gram staining was performed according to the procedure described by Claus (1992), and isolates were stained for endospores (Reynolds et al. 2009). Also, using 3-day cultures of endospore-forming isolates, it was observed under the light microscope whether they produce insecticidal crystal proteins. Motility of the isolates was determined by the hanging drop technique (Jain et al. 2020). Biochemical identification of isolates was then performed using the VITEK-2 system (BioMerieux VITEK, St. Louis, MO) according to the manufacturer’s instructions. The findings were evaluated according to Bergey’s Manual of Systematic Bacteriology 1 and 2 (Garrity et al. 2005; de Vos et al. 2009).
16S rDNA gene sequencing
Genomic DNA was extracted from the bacterial isolates using Quick-DNA Fungal/Bacterial MiniPrep Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s protocols. A 1.5-kb region of the bacterial 16S rDNA gene was amplified with primers UNI16S-F (5′-ATTCTAGAGTTTGATCATGGCTCA-3′) and UNI16S-R (5′-ATGGTACCGTGTGACGGGCGGTGTGTA-3′) (Weisburg et al. 1991). Polymerase chain reaction (PCR) was performed with Taq DNA polymerase (New England Biolabs, Hertfordshire, UK) using a temperature profile of initial denaturation at 95 °C for 30 s, followed by 30 cycles of denaturation at 95 °C, annealing at 55 °C for 15 s, initial extension at 68 °C for 60 s, and final extension at 68 °C for 5 min. PCR products were electrophoresed on a 1% agarose gel containing ethidium bromide (0.5 mg/ml), and DNA bands were visualized in a gel documentation system. The amplified PCR products cleaned up from the gel using the NucleoSpin Extract Kit (Macherey-Nagel, Germany) and ligated to the pGEM-T Easy Vector (Promega Co, USA) and then transformed into Escherichia coli JM101 competent cells. After transformation, the plasmid was isolated using the Wizard Plus SV Miniprep DNA Purification System Kit (Promega, France) and sent to MACROGEN (The Netherlands) for sequencing.
Pyhlogeny of bacterial isolates
Sequence assembly was performed using Bioedit software (Hall 2011). Vector contamination was determined and cleaned using VecScreen on NCBI. The edited sequences were compared with the RefSeq database using the nBLAST search tool from NCBI GenBank. The sequences of the isolates were deposited in the GenBank database. The sequences and their closely related species were used for phylogenetic analysis. Multiple sequence alignments were performed using ClustalW, and the phylogenetic tree was constructed using the neighbor-joining algorithm (NJ) with MEGA X (Kumar 2018).
Insect rearing
The larvae of T. absoluta were collected from tomato greenhouses in Ankara, Turkey. Larvae were placed in a cage (50 × 50 × 30 cm) with a tomato plant. Newly emerged adults were transferred to another cage with a tomato plant. The tomato plant used in this study was grown in a greenhouse. The Negris tomato variety was used for the rearing of T. absoluta and the experiments.
Insecticidal potential of the isolates
Bacterial isolates grown overnight at 30 °C in nutrient broth medium were centrifuged at 4500 rpm for 5 min to remove the medium. The pellet was washed twice with sterile distilled water and re-suspended. Then, the bacterial density was determined using a spectrophotometer at 600 nm and adjusted to 1.00 (≈ 1 × 109 cfu/ml) (Ben-Dov et al. 1995). The suspensions were used in the bioassays.
Bioassays were assessed using the leaf disc method recommended by the Insecticide Resistance Action Committee (IRAC 2012). Tomato leaf disks (3 cm) were dipped in the bacterial suspension and allowed to dry on a wire net. When the leaf surface was completely dry, these discs were placed in Petri dishes (3.5 cm) containing moistened cotton. Then, a second instar T. absoluta larva was placed in each Petri dish. Sterile distilled water was used in the control group. At least thirty-two larvae were used for the bioassays. Sterile distilled water was used in the control group. The screening tests were repeated three times. Bioassay was carried out at 25 ± 2 °C, 60–70% relative humidity, and under photoperiod of about 14 h light/10 h dark for 3 days. The mortality rate was recorded daily for three days.
Bacillus thuringiensis strain Ta1 (Bt-Ta1) determined as the most effective isolate in the screening test was used in concentration response experiments. Different concentrations of Bt-Ta1 (from 1010 to 106 cfu/ml) were prepared by serial dilution from stock suspension, and bioassays were conducted as described above.
Statistical analysis
Mortality percentages were calculated and corrected using Abbott’s formula (Abbott 1925). The corrected mortality data were subjected to analysis of variance (one-way ANOVA) followed by comparison of means with the LSD test at α = 0.05 using SPSS 20.0 software. Data normality and homogeneity of variance were checked using the Shapiro-Wilk test and Bartlett’s test, respectively. Concentration-response data were analyzed using probit regression analysis to estimate LC50 and LC90 values. In addition, survival analysis was performed using the Kaplan-Meier method, and survival characteristics were evaluated using log-rank tests with the Holm-Sidak method for multiple comparisons to compare different survival curves.
Detailed characteristics of Bacillus thuringiensis Ta1
aroE and gyrB gene sequencing
The gyrB gene (a housekeeping gene encoding the DNA gyrase subunit B protein, topoisomerase type I) and the aroE gene (a housekeeping gene encoding shikimate dehydrogenase) were partially amplified using the primer pairs gyrB-F1/gyrB-R1 and aroE-F1/aroE-R1 (Soufiane and Côté 2009). PCR was performed with an initial denaturation at 95 °C for 30 s, followed by 30 cycles consisting of 95 °C for 30 s, 47 °C for 30 s, and 68 °C for 1 min, and a final extension for 5 min at 68 °C. After confirmation of the amplified products by 1% agarose gel electrophoresis, cloning of the amplified products, selection of transformants, and determination of nucleotide sequences were performed as previously described.
Insecticidal gene profile
PCR was performed to screen cry and vip type genes using oligonucleotide primer pairs described by Jain et al. (2012) and Hernandez-Rodrigues et al. (2009) (Table 1). The amplified products were electrophorezed through a 1% agarose gel and visualized by ethidium bromide staining. The PCR products of each gene were purified using a PCR clean-up kit (Macherey-Nagel, Düren, Germany) and sequenced at Macrogen Inc. (The Netherlands).
Microscopy of spore-crystal mixture
A loopful of Bt-Ta1 was inoculated into 100 ml T3 medium (Martin and Travers 1989) to promote sporulation and incubated at 30 °C until complete lysis of the cells (approximately 72 h). The spore-crystal mixture was collected by centrifugation at 4 °C. The pellet was washed at least three times with ice-cold 0.5 M NaCl to remove extracellular components. Finally, the spore crystal pellet was suspended in sterile distilled water and examined using a phase contrast microscope (Nikon Eclipse Ni, Japan) according to the spore crystal staining method described by Smirnoff (1962). In addition, the spore-crystal mixture was examined with a scanning electron microscope (Zeiss Evo LS10, Germany) for the morphology of the insecticidal crystal proteins.
Results
Thirteen different bacteria (Ta1 to Ta13) associated with T. absoluta larvae were obtained. They were first distinguished by macroscopic and microscopic observation. Eight spore-forming Gram-positive bacilli, three non-spore-forming bacilli, and two Gram-positive cocci were determined. The biochemical characteristics determined with the VITEK-2 compact system are listed in Table 2 for the spore-forming bacilli and in Table 3 for Gram-negative and Gram-positive non-spore-forming bacilli. In addition, the predicted species for the isolates based on the VITEK-2 identification system and their probability and confidence are presented in Table 4.
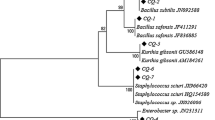
Comparison of the partial sequence of the 16S rDNA genes with the RefSeq database using nBLAST showed that the first eight isolates (Ta1 to Ta8) had 99.87% sequence similarity with Bacillus cereus group. The observation of insecticidal crystal proteins in light microscopy in these isolates indicated that they were B. thuringiensis. On the other hand, the partial 16S rDNA sequence of Ta9 isolate showed 99.72% identity to the strain of Staphylococcus petrasii NCTC13830. The partial 16S rDNA sequence of Ta10 isolate showed 99.53% identity to the strain of Citrobacter freundii FDAARGOS. The partial 16S rDNA sequence of Ta11 isolate showed 99.84% identity to the strain of Chishuiella changwenlii BY4. The partial 16S rDNA sequence of Ta12 isolate showed 99.86% identity to the strain of Enterococcus cassaliflavus EC20. The partial 16S rDNA sequence of Ta13 isolate showed 98.93% identity to the strain of Pseudomonas tremae PA-1-12B. Phylogenetic analysis based on the 16S rDNA sequence also confirmed the molecular characterization (Fig. 1). The sequence data were deposited in the GenBank database under accession numbers MK517625 to MK517637.
The results of the bioassays with a concentration of 1 × 109 cfu/ml are shown in Fig. 2. Mortality was significantly higher when B. thuringiensis isolates were used (p < 0.05). All Bt isolates caused more than 90% mortality in larvae of T. absoluta after 72 h of application. Isolates Ta1, Ta2, Ta4, Ta6, Ta7, and Ta8 also caused complete mortality. However, isolates other than B. thuringiensis showed low virulence resulting in less than 15% mortality (F = 1420, df = 12, p < 0.05) (Fig. 2). In the concentration-response experiment with isolate Ta1, complete mortality was observed at a concentration of 1010 cfu/ml, while mortality was 53% at a concentration of 106 cfu/ml. The survival analysis also showed that there was a significant difference between concentrations and control group (Fig. 3). The LC50 and LC90 values for Bt-Ta1 were estimated to be 1.2 × 106 and 2 × 109 cfu/ml, respectively.
Screening test of bacteria associated with Tuta absoluta under laboratory conditions at a concentration of 1 × 109 cfu/ml. Corrected mortality indicates Abbott-corrected mortality of pooled data from three experiments. Data were analyzed by analysis of variance followed by LSD test (least significant difference) at the p < 0.05 level as a post hoc comparison of mortality means. Mean values marked with the same letter are not significantly different from each other. Error bars indicate the standard deviation
For detailed characterization of Bt-Ta1 isolate, which showed the high virulence, the gyrB and aroE genes were amplified. It yielded a single amplicon of 2 kbp (later determined at 1923 bp) and 0.7 kbp (755 bp) in length for gyrB and aroE, respectively. The sequences generated for the gyrB and aroE genes are available in the GenBank under accession numbers MN010643 and MN010642, respectively. The concatenated partial sequences of 16S rDNA, gyrB, and aroE had a length of 4108 nucleotides. A bootstrapped maximum likelihood phylogenetic tree was inferred from the alignment of the concatenated nucleotide sequences. Bt-Ta1 was clustered with Bt kurstaki, Bt higo, Bt thompsoni, Bt kenyae, and Bt indiana (Fig. 4). Furthermore, Bt-Ta1 isolate was found to contain cry1, cry2, and vip3 genes, which are toxic to lepidopterans. The partial sequence of the cry1 and cry2 genes showed 99% sequence similarity with the cry1Aa and cry2Aa genes, respectively, deposited in the NCBI/GenBank database. The phylogenetic tree generated by MEGA X showed that cry1 and cry2 (our isolate) belong to the same branch as cry1Aa and cry2Aa (Fig. 5). Similarly, the partial sequence of the vip3 gene showed 99% similarity with the vip3Aa gene, and this was also confirmed by phylogenetic analysis (Fig. 6). The sequence data were deposited in the GenBank database under accession numbers OM022279, OL956537, and OL956538, respectively. Microscopic examination also revealed that the isolate produces bipyramidal and cubic insecticidal crystal proteins (Fig. 7).
Maximum- likelihood phylogenetic tree based on the concatenated partial sequences of genes included in the MLST scheme of Soufiane and Côté (2009). The phylogenetic position of Bt-Ta1 (indicated by a black diamond) is shown among type strains of B. cereus group. Bootstrap values above 50% are indicated at each branch point. Bars, 0.02 substitutions per site
Discussion
Microorganisms that colonize the insect gut include protists, fungi, archaea, and bacteria, which play an important role in insect growth, development, and reproduction (Engel and Moran 2013). In addition to their functional role, indigenous gut microbes have also been described as pathogenic in insects (Chandel et al. 2013; Devi et al. 2022; Eski et al. 2018; Secil et al. 2012). In the present study, based on morphological, biochemical, and molecular characteristics, thirteen culturable bacteria associated with the gut microbiota of T. absoluta were identified, and their efficacy on T. absoluta was evaluated. Our results showed that a variety of bacterial phyla belonging to Firmicutes, Bacteroidetes, and Proteobacteria were present in the gut of T. absoluta. However, the dominant phylum was Firmicutes, which is consistent with the results of a previous study (Wang et al. 2022). On the other hand, studies of the gut bacteria of 30 species of Lepidoptera revealed that most gut bacterial families belong to Proteobacteria (Voirol et al. 2018). Meanwhile, the gut microbiota of Lepidoptera varies considerably depending on developmental stage, diet, and environment.
Based on the morphological, biochemical, and molecular characteristics of the spore-forming Bacillus isolates, it was determined that they are bacteria from the Bacillus cereus group. The microorganisms of the Bacillus cereus group consist of at least eight closely related species: B. anthracis, B. cereus, B. thuringiensis, B. mycoides, B. pseudomycoides, B. weihenstephanensis, B. cytotoxicus, and B. toyonensis. The bacterium Bacillus thuringiensis can be easily distinguished from other members of the group by the insecticidal crystal proteins it produces during sporulation. The detection of insecticidal crystal proteins in our isolates under the light microscope showed that the isolates are B. thuringiensis. It is an important entomopathogenic bacterium that is toxic to many insects and has been used for decades to control agricultural pests. It is an important alternative for the control of pests that have developed resistance to conventional insecticides such as Tuta absoluta. Aynalem et al. (2021) tested 34 B. thuringiensis strains isolated from different habitats and reported that although most isolates were pathogenic against second larval stage, B. thuringiensis strain AAUF6 had the highest virulence with a mortality of 75% after 7 days of inoculation with 108 cfu/ml bacterial suspension. Hafsi et al. (2012) reported that the commercially formulated B. thuringiensis strain achieved a mortality rate of 72.5% against the second larval stage of T. absoluta in Tunisia. Similarly, Hasan and Yusuf showed that among the 12 Bt isolates obtained from dead larvae of T. absoluta, the B1 isolate had the highest insecticidal activity with a mortality rate of 60% against second larval stage of the pest at a concentration of 8.9 × 109 spores/ml. In our study, all Bt isolates showed a mortality rate of more than 90% against second instar larvae after 72 h of treatment at a concentration of 109 cfu/ml. As can be seen from the studies, the differences in mortality rates are largely related to the virulence of the bacterial isolates. Insecticidal crystal proteins expressed during sporulation are important virulence factors for B. thuringiensis, and virulence depends on the type of crystal toxins and their expression level. In their study in Ethiopia, Aynalem et al. (2021) showed that strain AAUF6, which produces Cry2 and Cry9, and strain AAUMF9, which produces Cry1 toxin, were more effective against T. absoluta than Bt isolates expressing other toxins. When several Bt collections were screened for a range of cry genes, 40–50% of the strains showed activity against one or more Lepidoptera species, and the Lepidoptera-specific active proteins belonged to the Cry1, Cry2, and Cry9 groups (Schnepf et al. 2005). It was obvious that our results were in agreement with those of previous studies, and the important role of cry1 and cry2 as determinants of pathogenicity against Lepidoptera pests was confirmed by the current results. Another virulence factor of Bt is the Vip proteins produced during the vegetative stage. The Vip proteins, which do not show sequence homology with the Cry proteins, are classified into four groups (Vip1, Vip2, Vip3, and Vip4) according to their sequence homology. The Vip3 proteins have already been described as toxic to lepidopterans such as T. absoluta (Sellami et al. 2015), S. frugiperda (Caccia et al. 2014), H. armigera (Seifinejad et al. 2007), and Lobesia botrana (Palma et al. 2013). In our study, the isolate Bt-Ta1, which contains the vip3 gene, was found to have a toxic effect on T. absoluta.
Isolate Ta9, Gram-positive, non-spore-forming and motile, was defined as Staphylococcus haemolyticus according to the VITEK-2 identification system. However, after comparing the 16s partial gene sequence with the NCBI RefSeq database, the isolate showed 99.72% sequence similarity with S. petrasii strain NCTC13830. This was also confirmed by phylogenetic analysis. In addition, biochemical properties of Ta9 were found to be compatible with the type strain Staphylococcus petrasii SEQ110. Since S. petrasii is not found in the VITEK-2 database, it is assumed that it can be identified as one of the closest species, S. haemolyticus. Staphylococcus petrasii has been isolated from various human clinical specimens. It is an opportunistic pathogen that causes mild to fatal infections, especially in immunocompromised patients. In this study, S. petrasii was isolated from an insect for the first time and its virulence on an insect against Tuta asoluta was assessed for the first time. It turned out that its virulence was quite low (18.75%) and it was not promising as a biocontrol agent.
Isolate Ta10, a motile Gram-negative bacterium, was defined as Citrobacter freundii by the VITEK-2 identification system. The partial sequence of 16S rDNA gene and its phylogenetic analysis also confirmed that the isolate was C. freundii. This bacterium, which can cause respiratory and urinary tract infections in humans, has already been isolated from various insects, and its pathogenicity has been reported. Eski et al. (2018) found that C. freundii strain Sn4 associated with the gut microbiota of Sesamia nonagrioides L. (Noctuidae: Lepidoptera) was pathogenic to the host and had a virulence of 60% in 3rd instar larvae. However, in this study, C. freundi strain Ta10 had the lowest virulence (9.37%) among the isolated bacteria. Polenogova et al. (2022) also reported that C. freundii did not cause significant mortality in L. decemlineata, which was only 4% on the sixth day after exposure. However, feeding L. decemlineata larvae with C. freundii may lead to histological changes in gut tissues and modulation of immune responses, increasing the pathogenicity of B. thuringiensis.
Isolate Ta11, a non-motile Gram-negative, rod-shaped bacterium, was defined as Chryseobacterium indologenes by the VITEK-2 identification system. However, the partial sequence of the 16S rDNA gene showed high sequence similarity with Chishuiella changwenlii strain BY4. The phylogenetic tree confirmed that Ta11 isolate was a strongly supported clade with a bootstrap value of 100%. Furthermore, the biochemical properties of the Ta11 isolate were compatible with the reference strain Chishuiella changwenlii BY4. Since C. changwenlii is not found in the VITEK-2 database, it is possible that the VITEK-2 identification system made a wrong identification. Although it is not well documented, C. changwenlii has previously been isolated from the foregut of Bactrocera dorsalis (Diptera: Tephritidae) (Yao et al. 2022). The pathogenicity of the bacterium in insects was demonstrated for the first time in this study, and its virulence was found to be quite low (15.62%).
Ta12 isolate, Gram-positive, non-spore-forming cocci, was identified as Enterococcus casseliflavus by both the VITEK-2 identification system and 16S rDNA gene sequencing. The bacterium is rarely isolated in clinical specimens but can cause a variety of invasive infections in immunocompromised or chronically ill patients. It has previously been isolated from stored product pests (Channaiah et al. 2010), lepidopterans, Hyles euphorbiae and Brithys crini (Vilanova et al. 2016), and Manduca sexta (Lepidoptera, Sphingidae). Thakur et al. (2015) also reported its pathogenicity. They showed that E. casseliflavus strain SL10 isolated from the gut of Spodoptera litura (Lepidoptera: Noctuidae) caused 13% larval mortality in S. litura. Similarly, E. casseliflavus strain Ta12 caused 12.5% mortality against the larvae of T. absoluta in our study.
Gram-negative, non-spore-forming, motile bacterium Ta13 was not identified using the VITEK-2 identification system. However, the isolate was identified as Pseudomonas tremae by 16S rDNA gene sequencing and its phylogenetic analysis. Although other pseudomonads have already been isolated from various insects, P. tremae, which is known as a plant pathogen, was detected in insects for the first time, and its pathogenicity was determined in this study. It is obvious that it is not promising as a biological control agent, as it caused only 15.6% larval mortality.
Conclusion
Thirteen bacteria associated with the gut microbiota of T. absoluta larvae were identified, and their pathogenicity in the pest was studied under laboratory conditions. Among them, Bt-Ta1 may prove to be a suitable microbial control agent that could be used as part of integrated pest management strategy of T. absoluta. In further studies, the Bt-Ta1 isolate should be formulated to overcome the adverse effects of environment such as UV radiation and temperature, and its virulence should be tested under greenhouse or field conditions to validate the results.
Data Availability
Datasets are available from the corresponding author on reasonable request.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2):265–267
Aynalem B, Muleta D, Venegas J, Assefa F (2021) Isolation, molecular characterization and pathogenicity of native Bacillus thuringiensis, from Ethiopia, against the Tomato leafminer, Tuta absoluta: detection of a new high lethal phylogenetic group. Microbiol Res 250:126802
Ben-Dov E, Boussiba S, Zaritsky A (1995) Mosquito larvicidal activity of Escherichia coli with combinations of genes from Bacillus thuringiensis subsp. israelensis. J Bacteriol 177(10):2851–2857
Caccia S, Chakroun M, Vinokurov K, Ferré J (2014) Proteolytic processing of Bacillus thuringiensis Vip3A proteins by two Spodoptera species. J Insect Physiol 67:76–84
Çakici FÖ, Sevim A, Demirbağ Z, Demir İ, Özkan Çakici F, Sevim A, Demirbaǧ Z, Demir İ (2014) Investigating internal bacteria of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) larvae and some Bacillus strains as biocontrol agents. Turkish J Agric For 38(1):99–110
Chandel K, Mendki MJ, Parikh RY, Kulkarni G, Tikar SN, Sukumaran D, Prakash S, Parashar BD, Shouche YS, Veer V (2013) Midgut microbial community of Culex quinquefasciatus mosquito populations from India. PLoS ONE 8(11):e80453
Channaiah LH, Subramanyam B, McKinney LJ, Zurek L (2010) Stored-product insects carry antibiotic-resistant and potentially virulent enterococci. FEMS Microbiol Ecol 74:464–471
Chidege M, Al-zaidi S, Hassan N, Julie A, Kaaya E, Mrogoro S (2016) First record of tomato leaf miner Tuta absoluta (meyrick) (lepidoptera: Gelechiidae) in Tanzania. Agric Food Secur 5(1):1–7
Claus D (1992) A standardized Gram staining procedure. World J Microbiol Biotechnol 8(4):451–452
de Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer K-H, Whitman WB (2009) The Firmicutes. In: Whitman WB, Parte AC (eds) Bergey's manual of systematic bacteriology. Springer, New York
Demir I, Eryüzlü E, Demirbaǧ Z (2012) A study on the characterization and pathogenicity of bacteria from Lymantria dispar L. (Lepidoptera: Lymantriidae). Turkish J Biol 36(4):459–468
Devi S, Saini HS, Kaur S (2022) Assessing the pathogenicity of gut bacteria associated with Tobacco caterpillar Spodoptera litura (Fab.). Sci Rep 12:8257
Engel P, Moran NA (2013) The gut microbiota of insects – diversity in structure and function. FEMS Microbiol Rev 37(5):699–735
Eski A, Demir İ, Güllü M, Demirbağ Z (2018) Biodiversity and pathogenicity of bacteria associated with the gut microbiota of Beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Microb Pathog 121:350–358
Garrity G, Bell J, Lilburn T (2005) Pseudomonales. In: Garrity G, Brenner D, Krieg N, Staley J (eds) Bergey’s manual of systematic bacteriology, vol 2, 2nd edn. Part B. Springer, New York
Guedes RNC, Roditakis E, Campos MR, Haddi K, Bielza P, Siqueira HAA, Tsagkarakou A, Vontas J, Nauen R (2019) Insecticide resistance in the tomato pinworm Tuta absoluta: patterns, spread, mechanisms, management and outlook. J Pestic Sci 92(4):1329–1342
Hafsi A, Abbes K, Chermiti B, Nasraoui B (2012) Response of the tomato miner Tuta absoluta (Lepidoptera: Gelechiidae) to thirteen insecticides in semi-natural conditions in Tunisia. EPPO Bull 42(2):312–316
Hall T, Biosciences I, Carlsbad C (2011) BioEdit: an important software for molecular biology. GERF Bull Biosci 2(1):60–61
Haloi K, Kalita MK, Nath R, Devi D (2016) Characterization and pathogenicity assessment of gut-associated microbes of Muga silkworm Antheraea assamensis Helfer (Lepidoptera: Saturniidae). J Invertebr Pathol 138:73–85
Hernandez-Rodriges CS, Boets A, Van Rie J, Ferre J (2009) Screening and identification of vip genes in Bacillus thuringiensis strains. J Appl Microbiol 107(1):219–225
IRAC (2012) Tuta absoluta susceptibility test method 022. https://irac-online.org/methods/tuta-absoluta-larvae/Accessed [02 June 2023].
Jain A, Jain R, Jain S (eds) (2020) Basic techniques in biochemistry, microbiology and molecular biology. Humana, New York NY
Jain D, Kachhwaha S, Jain R, Kothari SL (2012) PCR based detection of cry genes in indigenous strains of Bacillus thuringiensis isolated from the soils of Rajasthan. Indian J Biotechnol 11(4):491–494
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Langa TP, Dantas KCT, Pereira DL, de Oliveira M, Ribeiro LMS, Siqueira HAA (2022) Basis and monitoring of methoxyfenozide resistance in the South American tomato pinworm Tuta absoluta. J Pestic Sci 95(1):351–364
Lietti MMM, Botto E, Alzogaray RA (2005) Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop Entomol 34(1):113–119
Martin PAW, Travers RS (1989) Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol 55(10):2437–2442
Moran NA, Russell JA, Koga R, Fukatsu T (2005) Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl Environ Microbiol 71(6):3302
Palma L, De Escudero IR, Maeztu M, Caballero P, Muñoz D (2013) Screening of vip genes from a Spanish Bacillus thuringiensis collection and characterization of two Vip3 proteins highly toxic to five lepidopteran crop pests. Biol Control 66(3):141–149
Polenogova OV, Noskov YA, Artemchenko AS, Zhangissina S, Klementeva TN, Yaroslavtseva ON, Khodyrev VP, Kruykova NA, Glupov VV (2022) Citrobacter freundii, a natural associate of the Colorado potato beetle, increases larval susceptibility to Bacillus thuringiensis. Pest Manag Sci 78:3823–3835
Reynolds J, Moyes R, Breakwell DP (2009) Differential staining of bacteria: Endospore stain. Curr Protoc Microbiol 15(1)
Schnepf HE, Lee S, Dojillo J, Burmeister P, Fencil K, Morera L, Nygaard L, Narva KE, Wolt JD (2005) Characterization of Cry34/Cry35 binary insecticidal proteins from diverse Bacillus thuringiensis strain collections. Appl Environ Microbiol 71(4):1765–1774
Secil ES, Sevim A, Demirbag Z, Demir I (2012) Isolation, characterization and virulence of bacteria from Ostrinia nubilalis (Lepidoptera: Pyralidae). Biologia 67:767–776
Seifinejad A, Salehi Jouzani GR, Hosseinzadeh A, Abdmishani C (2007) Characterization of Lepidoptera-active cry and vip genes in Iranian Bacillus thuringiensis strain collection. Biol Control 44(2):216–226
Sellami S, Cherif M, Abdelkefi-Mesrati L, Tounsi S, Jamoussi K (2015) Toxicity, activation process, and histopathological effect of Bacillus thuringiensis vegetative insecticidal protein Vip3Aa16 on Tuta absoluta. Appl Biochem Biotechnol 175(4):1992–1999
Siqueira HAA, Guedes RNC, Picanco MC (2000) Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric For Entomol 2(2):147–153
Smirnoff WA (1962) A staining method for differentiating spores, crystals and cells of Bacillus thuringiensis (Berliner). J Insect Pathol 4:384–386
Soufiane B, Côté JC (2009) Discrimination among Bacillus thuringiensis H serotypes, serovars and strains based on 16S rRNA, gyrB and aroE gene sequence analyses. Anton Leeuw Int J G 95(1):33–45
Thakur A, Dhammi P, Saini HS, Kaur S (2015) Pathogenicity of bacteria isolated from gut of Spodoptera litura (Lepidoptera: Noctuidae) and fitness costs of insect associated with consumption of bacteria. J Invertebr Pathol 127:38–46
Urbaneja A, Vercher R, Navarro V, Garcia Mari F, Porcuna J (2008) La polilla del tomate, Tuta absoluta. Phytoma Espana 194:16–23
Vilanova C, Baixeras J, Latorre A, Porcar M (2016) The generalist inside the specialist: gut bacterial communities of two insect species feeding on toxic plants are dominated by Enterococcus sp. Front Microbiol 7:1005
Voirol PLR, Frago E, Kaltenpoth M, Hilker M, Fatouros NE (2018) Bacterial symbionts in Lepidoptera: their diversity, transmission, and impact on the host. Front Microbiol 9:556
Wang H, Xian X, Gu Y, Castañé C, Arnó J, Wu S, Wan F, Liu W, Zhang G, Zhang Y (2022) Similar bacterial communities among different populations of a newly emerging invasive species, Tuta absoluta (Meyrick). Insects 13(3):252
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703
Yao Z, Cai Z, Ma Q, Bai S, Wang Y, Zhang P, Guo Q, Lemaitre B, Zhang H (2022) Compartmentalized PGRP expression along the dipteran Bactrocera dorsalis gut forms a zone of protection for symbiotic bacteria. Cell Rep 41(3):111523
Author information
Authors and Affiliations
Contributions
Ardahan Eski: performing experiments, data analysis, phylogenetic analysis, and writing draft manuscript. Pervin Erdoğan: performing bioassays and data analysis. Zihni Demirbağ: supervising reviewing and editing of the manuscript. İsmail Demir: molecular experiments, phylogenetic analysis, and writing draft manuscript. All authors have approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eski, A., Erdoğan, P., Demirbağ, Z. et al. Isolation and identification of bacteria from the invasive pest Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and evaluation of their biocontrol potential. Int Microbiol 27, 631–643 (2024). https://doi.org/10.1007/s10123-023-00418-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00418-1