Abstract
The compound known as effective microorganisms (EMs) is widely used in aquaculture to improve water quality, but how they affect the health of Chinese mitten crab (Eriocheir sinensis) is unclear, especially in terms of intestinal microbiota and serum metabolites. In this study, we fed juvenile crabs with an EM-containing diet to explore the effects of EM on the physiological status, intestinal microbiome, and metabolites of E. sinensis. The activities of alanine aminotransferase and alkaline phosphatase were significantly enhanced by EM, indicating that EM supplementation effectively enhanced the antioxidant capacity of E. sinensis. Proteobacteria, Tenericutes, Firmicutes, Bacteroidetes, and Actinobacteria were the main intestinal microbes in both the control and EM groups. Linear discriminant effect size analysis showed that Fusobacteriaceae, Desulfovibrio, and Morganella were biomarkers in the control group, and Exiguobacterium and Rhodobacteraceae were biomarkers in the EM group. Metabolomics analysis revealed that EM supplementation increased cellular energy sources and decreased protein consumption, and oxidative stress. Together, these results indicate that EM can optimize the intestinal microbiome and serum metabolites, thereby benefiting the health of E. sinensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Chinese mitten crab (Eriocheir sinensis) is a commercially important aquaculture species in China. In 2021, the cultural yield of E. sinensis reached 808,274 tons (Fishery Administration Bureau of the Ministry of Agriculture & Villages et al. 2022). Juvenile crabs are susceptible to stress caused by changes in ambient temperature, pH, and ammonia nitrogen concentration (Peng et al. 2019; Qi et al. 2021), and failure to prevent or act quickly to address these stressors may result in huge economic losses. Therefore, it is necessary to take measures to improve the physiological status of juvenile crabs. Currently, common methods to improve the immunity of aquatic organisms include the application of vaccines, prebiotics, and probiotics (Wan et al. 2022). In crustaceans, dietary administration of Fructooligosaccharide, Lactobacillus acidophilu, and Bacillus subtilis have positive effects on growth performance and immunity (Interaminense et al. 2018; Jia et al. 2019; Talpur et al. 2013).
Effective microorganisms (EMs) is a term used to describe a type of compound microbially active agent that is composed of yeast, photosynthetic bacteria, and lactic acid bacteria (Lee et al. 2008). EM is mainly used to regulate the aquatic environment and ecosystem, and numerous studies have confirmed that it is effective in water treatment (Wang et al. 2004; Zarina et al. 2013). To date, EM is mainly used to regulate water quality in aquaculture. The addition of EM to water can improve the ability of crustaceans to resist oxidative stress and enhance crabs’ non-specific immunity (Chu et al. 2021; Zhang et al. 2022). Showed that EM optimized the nutritional composition of the edible tissues of E. sinensis. However, the effect of supplemental EM on intestinal microbiota and the serum metabolism of E. sinensis is unknown.
The intestinal microbiota of aquatic animals is closely related to their health (Semova et al. 2012; Wang et al. 2018). Intestinal microbes are usually affected by the host’s genetics, environment, and diet at different developmental stages (Li et al. 2017; Wang et al. 2019; Wong and Rawls 2012). The main environmental factor is water quality (Wu et al. 2021), and diet can improve the intestinal microbiome by adding different nutrients, further affecting the host’s growth and health (Nikouli et al. 2021; Schmidt et al. 2017). Yoshii et al. (2019) reported that intestinal microbiota play an important role in host metabolism, growth, and immunity, and Sun et al. (2016) found that the correlation between intestinal microbiome communities and metabolic profiles can affect the growth and development of animals.
Reportedly, EM plays an important role in crustacean culture (Chu et al. 2021; Zhang et al. 2022), and we speculate that this effect may be related to intestinal microorganisms and metabolites. The purpose of this study was to test the hypothesis that EM affects the health of E. sinensis by changing the intestinal microbial composition and the serum metabolites.
Materials and methods
Experimental design
This experiment was conducted from 7 to 21 August, 2020, at the Freshwater Fisheries Research Center, CAFS (Wuxi, China). Juvenile crabs (average weight, 6.22 ± 0.09 g) were purchased from Suzhou Youhua Ecological Technology Co., Ltd. (Suzhou, China). Six tanks (length × width × height = 44.5 cm × 30 cm × 25 cm) each containing 10 individual juvenile crabs were filled with 10 L of dechlorinated water. After acclimation for 2 weeks, the six tanks were divided into two groups: the control group (CK) and the EM group. Crabs in the CK group were fed 3 g of a commercial diet (JH8653, Jiangsu Hipore Feed Co. LTD, Taizhou, Jiangsu, China), while crabs in the EM group were fed the commercial diet supplemented with 50 g/kg EM (Jiangsu Hengtai Environmental Protection Technology Development Co. Ltd., Wuxi, Jiangsu, China). Table S1 shows the nutrition composition of the commercial diet. Crabs were fed daily at 17:00 during the experiment, and the residual feed and excrement were removed daily before feeding.
Sampling
Samples were collected after EM treatment for 7 days. Crabs were fasted for 24 h before sampling and six crabs were randomly collected from each group. Crabs were anesthetized on ice before hemolymph was collected from the third pereopod. After sitting for 2 h at 4 °C, the hemolymph was centrifuged at 4000 rpm for 10 min, and the supernatant (serum) was collected and stored at − 80 °C. To analyze the intestinal microflora, the surfaces of crabs were sterilized with 70% ethanol. The intestines and hepatopancreas then were aseptically dissected from the musculature, frozen in liquid nitrogen, and stored at − 80 ℃.
Determination of digestive enzyme activities and antioxidant capacity
Intestinal or hepatopancreas samples were weighed and homogenized with saline (100 mg tissue per 900 µl of saline), and the supernatant was used for analysis. Activities of the digestive enzymes lipase (A054-2–1) and amylase (C016-1–1) were measured in intestinal tissues. Hepatopancreas antioxidant parameters, including catalase (CAT; A001-3–2), superoxide dismutase (SOD; A001-3), total antioxidant capacity (T-AOC; A015-2–1), and malondialdehyde (MDA; A003-1–2), were also measured. These indexes were determined using kits purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, Jiangsu, China) (Lei et al. 2021).
Serum biochemical analysis
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactic dehydrogenase (LDH) activities and glucose (GLU) content were measured using a Mindary BS-400 automatic biochemical analyzer (Shenzhen, China) and assay kits purchased from Shenzhen Mindary Bio-medical Electronics Co., Ltd. according to the manufacturer’s instructions (Chu et al. 2021).
Gut microbiota analysis
DNA extraction, PCR amplification, and sequencing
Total microbial DNA was extracted from intestinal contents using the E.Z.N.A.® Stool DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s instructions. The V3–V4 region of the bacterial 16S rDNA was amplified by PCR (95 °C for 5 min followed by 30 cycles at 95 °C for 30 s, 50 °C for 30 s, 72 °C for 60 s, and a final extension at 72 °C for 5 min) using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Sequencing adapters were added to the 5′ ends of primers for PCR amplification. The amplified products were purified, quantified, and homogenized to form a sequencing library. After quality inspection, the qualified library was paired-end (250 bp) sequenced using an Illumina HiSeq 2500 instrument (Biomarker Technologies, Beijing, China) according to standard protocols. We constructed 12 sequencing libraries, including six libraries for the CK group, and six libraries for the EM group.
Bioinformatics analyses
Raw reads were first filtered using Trimmomatic v 0.33 (Bolger et al. 2014) with the cutoff threshold for average base quality score set at 20 over a sliding window of 50 bases. Next, Cutadapt v 1.9.1 (Martin 2011) was used to remove adapter sequences with a 20% maximum allowed error rate and 80% minimum coverage. Pair-end reads were assembled using FLASH v 1.2.11 (Mago and Salzberg 2011) based on sequences that overlapped more than 10 bp and the default maximum allowed error rate. Finally, effective reads were obtained after the identification and removal of chimeric sequences using UCHIME v 8.1 (Knight 2011).
Quality of each library was evaluated by calculating the average length, GC content, Q20, Q30, and effective ratio. Effective reads at 97.0% similarity level were clustered to obtain operational taxonomic units (OTUs) using USEARCH v10.0 (Edgar 2013). The subsequent analyses were based on OTUs. Taxonomy classification was performed using Naive Bayesian classifier at 70% confidence with Silva as the reference database (Callahan et al. 2016). Unweighted UniFrac distance metrics were used to estimate alpha-diversity (Bolyen et al. 2019). To investigate differences in bacterial community abundance between two groups, t-tests were performed on species abundance values between groups using Metastats (White et al. 2009).
Nontargeted metabolomic analysis
The serum samples were added to three times their volume of methanol, fully shaken and homogenized, then centrifuged at 13,000 rpm for 20 min at 4 °C to obtain the supernatant. The supernatants were transferred to new polypropylene tubes and processed through a nitrogen-purging instrument. The dried samples were redissolved in 50 μL of methanol. After centrifugation, the supernatant was used for analysis following an untargeted metabolomics approach using a liquid chromatography-mass spectrometry system (Thermo Fisher Scientific, Waltham, MA, USA). The system was equipped with an Accucore C18 column (2.6 μm, 50 mm × 2.1 mm, Thermo Fisher Scientific). Separation was achieved using the procedure, i.e., 0 min, 60% B; 2 min, 60% B; 9 min, 100% B; 17 min, 100% B; and 18 min, 60% B at a flow rate of 50 μL/min, where B is methanol and A is aqueous formic acid (0.025% (v/v) formic acid). The mobile phase was washed and rebalanced after the process was completed, and the total running time was 25 min. The injection volume in mass spectrometry mode was 2 μL, and the samples were scanned in positive and negative ion modes. The ionization parameters were analyzed following the method described by Jia et al. (2021). Data were analyzed in Compound Discoverer 3.1 software (Thermo Fisher Scientific, Waltham, MA, USA). Specific methods of data processing and multivariate statistical analysis followed those of Song et al. (2020), including Pareto scale principal component analysis and orthogonal partial least squares discriminant analysis.
Statistical analysis
The data are presented as the mean ± standard deviation of measurements of six replicates for each group. Normal distribution and homogeneity of variance of data were tested with Shapiro–Wilk and Levene tests (α = 0.05), respectively. Two-tailed t-test was used to compare the results between different groups and P < 0.05 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0. (IBM Corporation, Armonk, NY, USA).
Results
Digestive enzyme activities
Intestinal lipase and amylase activities did not differ significantly between the CK and EM groups (Fig. 1). These results showed that dietary EM supplementation had little effect on intestinal digestion.
Serum biochemical parameters
Serum ALT and ALP activities in the EM group were significantly higher than those in the CK group (Table 1). In contrast, AST and LDH activities and GLU content in the serum of E. sinensis did not differ significantly between the two groups (Table 1).
Antioxidant capacity
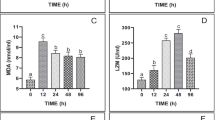
The SOD and CAT activities and T-AOC in the hepatopancreas samples from the EM group were significantly lower than those of the CK group (Fig. 2a–c). However, the MDA content did not differ significantly between the two groups (Fig. 2d).
Gut microbiome composition
16S rDNA sequencing
In total, 849,040 effective reads were obtained from the 12 libraries. On average, 70,753 effective reads were obtained from each library, and the number of effective sequences accounted for 89% of the raw reads. The Shannon index tended to be flat, indicating that the amount of sequencing data was reasonable and the sequencing depth was sufficient. The rank abundance curve showed that the samples contained relatively high levels of biodiversity (Fig. S1).
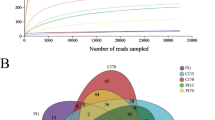
Of the 1546 OTUs identified, 1394 were detected in the CK group, 1217 were found in the EM group, and 1065 were shared between the two groups (Fig. 3). The OTUs belonged to 2 kingdoms, 33 phyla, 79 classes, 176 orders, 296 families, 553 genera, and 452 species.
Microbial community composition
The microflora of the samples was analyzed by taking the average of the community composition ratios. At the phylum level, eight and six main phyla were detected in the CK and EM groups, respectively (> 1% mean relative abundance). Proteobacteria, Tenericutes, Firmicutes, Bacteroidetes, and Actinobacteria were the main gut flora in both groups. Proteobacteria was the most abundant phylum in the EM group, with a relative abundance of 31.09%, followed by Tenericutes (24.97%), Firmicutes (14.01%), Bacteroidetes (15.07%), and Actinobacteria (9.16%). In the CK group, the most abundant phylum was Proteobacteria (30.58%), followed by Tenericutes (22.06%), Firmicutes (16.31%), Bacteroidetes (13.49%), and Actinobacteria (10.01%) (Fig. 4a). At the genus level, the dominant flora in the EM group were Candidatus Bacilloplasma (18.81%), Acinetobacter (6.59%), and uncultured bacterium_f_Mycoplasmataceae (5.48%). In the CK group, Candidatus Bacilloplasma (21.18%), Cupriavidus (5.61%), and Ralstonia (4.81%) were the most abundant flora (Fig. 4b).
Microbial diversity
Table 2 shows the estimates of community richness (ACE and Chao1) and diversity (Simpson and Shannon). No significant differences were found between the two groups.
Linear discriminant (LDA) effect size (LEfSe) analysis of the crabs’ gut bacteria at a default logarithmic LDA score of 3 was used to identify biomarkers with statistical differences between the two groups. In the CK group, Fusobacteriaceae, Desulfovibrio, and Morganella were biomarkers. In the EM group, Family_XII, Exiguobacterium Rhodobacteraceae, and Rhodobacterales were biomarkers (Fig. 5).
Serum metabolome profile
The principal component analysis plot showed that the first two principal components accounted for 95.47% of the total variance and the sample points were separated (Fig. 6a). In the current study, the values of the parameters of the model R2X, R2Y, and Q2Y were 0.688, 0.998, and 0.662, respectively, indicating a good model fit and acceptable predictability (Fig. 6b).
In total, 273 metabolites were identified in serum samples, and the abundance of 40 metabolites differed significantly between the CK and EM groups. Among them, 11 metabolites were downregulated (log2FC < 0) and five metabolites were upregulated (log2FC > 0) (Table 3). Upon the pathway enrichment analysis of differential metabolites, a total of 19 significantly enriched metabolic pathways were identified, including arachidonic acid metabolism, vascular smooth muscle contraction, and platelet activation (Figure S2).
Correlation between gut microbiota and serum metabolites
To better understand the correlation between the different metabolites and gut microbiota of E. sinensis, we performed Spearman’s correlation analysis (Fig. 7). Spearman’s correlation analysis results revealed a significant association between some of the microbes and metabolites. For example, 2-hydroxybutanoic acid was positively associated with uncultured_bacterium_f_Mycoplasmataceae, Moraxellaceae, and Roseimarinus but negatively correlated with Dysgonomonas, Candidatus Bacilloplasma, Lactobacillaceae, and Acinetobacter (P < 0.05). Pyruvic acid was positively associated with uncultured_bacterium_f_Mycoplasmataceae and Moraxellaceae, and arachidonic acid was negatively correlated with Roseimarinus.
Discussion
Effects of EM on serum biochemical parameters
Serum biochemical parameters are regarded as indicators of physiological disorders resulting from stress. ALT is an important amino transferase that plays an important role in protein metabolism, and it also is an indicator of liver function (Peng et al. 2018; Xie et al. 2021). ALP is a constituent of lysosomes and is directly involved in the transfer of phosphate groups and the metabolism of phosphate esters. It also promotes the removal of foreign bodies and is an important detoxification enzyme in animals (Chu et al. 2021). In this study, ALT and ALP activities were significantly higher in the EM group than in the CK group. After adding EM, the activity of ALP in the cells was stimulated to increase. It was released into the serum so that the activity of ALP in the serum was also enhanced, promoting the metabolism of substances in the cells and thereby maintaining the healthy physiological state of E. sinensis.
Effects of EM on antioxidant capacity
In animal cells, the production and removal of reactive oxygen species (ROS) are maintained in a balanced state under stable living conditions or without severe stress (Wan et al. 2022). When crabs suffer from oxidative stress, harmful products such as superoxide, hydroxyl radical, peroxy radical, and hydrogen peroxide appear (Cheng et al. 2021; Schock et al. 2010). The antioxidant system includes SOD and CAT, which clear ROS to protect the host from oxidative damage (Fu et al. 2017).
In this study, the SOD and CAT activities and T-AOC of the CK group were significantly higher than those of the EM group, possibly because the EM group had a benign environment for crab growth (Dobrzynski et al. 2022; Li et al. 2020; Pujiastuti and Suwartha 2017). EM may diffuse into the water either through direct dissolution or indirect excretion by crabs. Wang et al. (2021) reported that EM reduced the total nitrogen and total phosphorus content of aquaculture ponds, whereas E. sinensis in the CK group were vulnerable to oxidative stress and fluctuating antioxidant indexes. Thus, feeding E. sinensis with EM-supplemented diets may effectively enhance their resistance to oxidative stress.
Effects of EM on the intestinal microbiota
Intestinal microbes play an important role in the growth and development of their host animal (Wang et al. 2011). Wang et al. (2019) previously reported that Proteobacteria, Bacteroidetes, Firmicutes, and Tenericutes were the dominant phyla in intestinal samples from E. sinensis. Similarly, Proteobacteria and Tenericutes were the most abundant taxa in both the EM and CK groups in this study. Interestingly, although there was no difference in alpha diversity between the two groups, the species differences analyzed by LEfSe were statistically significant and several groups were distinctive (i.e., biomarkers) in the CK and EM groups.
Fusobacteriaceae, Morganella, and Desulfovibrio were the taxa that may serve as biomarkers of the CK group. Fusobacteriaceae are known to be positively correlated with inflammation. For example, fecal microbiota from people with cirrhosis and from inflammatory colorectal polyps from miniature dachshunds contained higher levels of Fusobacteriaceae compared with controls (Bajaj et al. 2012; Igarashi et al. 2016). Morganella is a human pathogen that is also lethal to the Mexican fruit fly and to some fish (Cifuentes et al. 2022; Reshma et al. 2018; Sontowski and van Dam 2020), and Desulfovibrio has been associated with mucosal inflammation (Earley et al. 2015). Although these microbes have been studied mainly in humans and less in crustaceans, we propose that they may also have negative effects on crustacean hosts. In the present study, the CK group contained potential pathogenic bacteria, indicating disruption of the microbiota balance in E. sinensis.
Potential biomarkers in the EM group included Exiguobacterium and Rhodobacteraceae. Manan et al. (2022) reported that Exiguobacterium could be used as a potential probiotic due to its function in the uptake and metabolism of nutrients, and Sombatjinda et al. (2014) found that it improved the growth and survival of the shrimp Penaeus vannamei. Rhodobacteraceae, the dominant group of gut microbiota in Litopenaeus vannamei, has been identified as an indicator of shrimp health (Gao et al. 2022a, b). In the current study, the levels of beneficial bacteria in the EM group were significantly higher than those in the CK group. EM is rich in beneficial bacteria, and it can control harmful bacteria and improve the immunity of animals (Laskowska et al. 2017; Li et al. 2022; Xing et al. 2007). Therefore, EM is beneficial to the health of E. sinensis because it improves the intestinal microbiome.
Effects of EM on the metabolites of E. sinensis
Compared to the CK group, the EM group had lower serum concentrations of valine and arachidonic acid. Valine is an essential amino acid required to assemble body protein. A higher release of valine in the serum may suggest degradation of structural proteins (Gillis and Ballantyne 1996). Yu et al. (2021) reported that reducing valine promotes metabolic health in mice. This indicated that the EM was beneficial to metabolic health of E. sinensis by decreasing protein consumption.
Recent studies showed that mannose can be used as a source of cellular energy and that has immune regulatory functions (Davis and Freeze 2001; Zhang et al. 2021). The mannose level was significantly higher in the EM group than in the CK group, indicating that the physiological function of E. sinensis could be kept stable by increasing cellular energy sources. Mannose also increased the Bacteroidetes to Firmicutes ratio in the gut microbiota in our study, which is a signature associated with the lean phenotype (Sharma et al. 2018). However, the relationship between the ratio and the health of crabs needs to be studied further. 2-Hydroxybutanoic acid is correlated with the synthesis of glutathione, which is a well-known antioxidative factor (Ma et al. 2017). The 2-hydroxybutanoic acid level in the EM group was significantly higher than that in the CK group, indicating that crabs with dietary EM supplementation had higher antioxidant ability than control crabs.
Because the CK group had lower levels of metabolites due to oxidative stress, it had a higher antioxidant index than the EM group. This is consistent with the SOD, CAT, and T-AOC data. Similar results were found in Macrobrachium nipponense (Sun et al. 2018) and L. vannamei (Parrilla-Taylor and Zenteno-Savín, 2011). Overall, the metabolomics analysis showed that feeding E. sinensis with an EM-supplemented diet was beneficial to crab health, mainly by increasing cellular energy sources and decreasing protein consumption, and oxidative stress.
Gut microbes may be associated with metabolites
With the emergence of the microbiota-gut-brain axis concept (i.e., the communication pathways that enable the interaction of intestinal microbiota with the central nervous system of the host), it has become accepted that the gut microbiota may play an important role in the development of disease. Many studies have found that changes in gut-brain axis interactions are related to neurodegenerative diseases, eating disorders, stress response, and behavioral changes (Quigley 2017; Ratsika et al. 2021; Sun et al. 2016; Wen et al. 2021). Intestinal microbiota interact with the host to produce metabolites, which act as intermediates or end-products of microbial metabolism (Dong et al. 2022). In the current study, certain microorganisms (e.g., Moraxellaceae, Lactobacillaceae, Acinetobacter) were correlated with serum metabolites (2-hydroxybutanoic acid, pyruvic acid, arachidonic acid). Therefore, we speculate that EM may affect the microbial composition of E. sinensis through the microbiota-gut-brain axis and then regulate the metabolites and ultimately affect the physiological state and function of the crabs.
Conclusion
Overall, the addition of EM to the diet of juvenile E. sinensis helped enhance the crabs’ resistance to oxidative stress and optimize their intestinal microbiome. Furthermore, metabolomic analysis revealed that EM contributes to E. sinensis health by increasing cellular energy sources and reducing protein consumption, and oxidative stress. However, further studies are needed to determine the optimal concentration of EM in the diet. The results of this study provide a scientific basis for understanding the effects of EM in E. sinensis aquaculture.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. The raw data are available at https://www.ncbi.nlm.nih.gov/sra/PRJNA875292.
References
Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM (2012) Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 302:168–175. https://doi.org/10.1152/ajpgi.00190.2011
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Caporaso JG (2019) Reproducible interactive scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Cheng CH, Ma HL, Liu GX, Deng YQ, Feng J, Jie YK, Guo ZX (2021) Oxidative stress DNA damage and cellular response in hydrogen peroxide-induced cell injury of mud crab (Scylla paramamosain). Fish Shellfish Immunol 114:82–89. https://doi.org/10.1016/j.fsi.2021.04.015
Chu L, Gao J, Song L, Sun Y, Shao N, Li Q, Nie Z, Xu G, Xu P (2021) Effects of effective microorganisms (EM) on the antioxidant capacity and non-specific immunity of Eriocheir sinensis. Freshwater Fisheries 51:91–99. https://doi.org/10.13721/j.cnki.dsyy.2021.05.012
Cifuentes Y, Vilcinskas A, Kämpfer P, Glaeser SP (2022) Isolation of Hermetia illucens larvae core gut microbiota by two different cultivation strategies. Antonie Van Leeuwenhoek 115:821–837. https://doi.org/10.1007/s10482-022-01735-7
Davis JA, Freeze HH (2001) Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta Gen Subj 1528:116–126. https://doi.org/10.1016/s0304-4165(01)00183-0
Dobrzynski J, Kulkova I, Wierzchowski PS, Wrobel B (2022) Response of physicochemical and microbiological properties to the application of effective microorganisms in the water of the Turawa Reservoir. Water 14:12. https://doi.org/10.3390/w14010012
Dong Z, Lv W, Zhang C, Chen S (2022) Correlation analysis of gut microbiota and serum metabolome with Porphyromonas gingivalis-induced metabolic disorders. Front Cell Infect Microbiol 12:858902. https://doi.org/10.3389/fcimb.2022.858902
Earley H, Lennon G, Balfe A, Kilcoyne M, Clyne M, Joshi L, Carrington S, Martin ST, Coffey JC, Winter DC, O’Connell PR (2015) A preliminary study examining the binding capacity of Akkermansia muciniphila and Desulfovibrio spp, to colonic mucin in health and ulcerative colitis. PLoS One 10:e0135280. https://doi.org/10.1371/journal.pone.0135280
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Fishery administration Bureau of the ministry of agriculture and villages, National aquatic products technology extension station, China Society of Fisheries (2022) 2022 China Fishery Statistical Yearbook. Chinese Agricultural Press, Beijing, p 24
Fu L, Zhou G, Pan J, Li Y, Lu Q, Zhou J, Li X (2017) Effects of Astragalus polysaccharides on antioxidant abilities and non-specific immune responses of Chinese mitten crab, Eriocheir sinensis. Aquac Int 25:1333–1343. https://doi.org/10.1007/s10499-017-0117-2
Gao F, Gan E, Liu W, Guo H, Wang Y, Wang R, Yan M, Dong P, Zhang D (2022a) Screening of carbon sources for enrichment and directional isolation of Rhodobacteraceae from the gut of Litopenaeus vannamei. Acta Microbiol Sin 62:1805–1818
Gao J, Shen L, Nie Z, Zhu H, Cao L, Du J, Dai F, Xu G (2022b) Microbial and planktonic community characteristics of Eriocheir sinensis culture ponds experiencing harmful algal blooms. Fishes 7:180. https://doi.org/10.3390/fishes7040180
Gillis TE, Ballantyne JS (1996) The effects of starvation on plasma free amino acid and glucose concentrations in lake sturgeon. J Fish Biol 49:1306–1316. https://doi.org/10.1111/j.1095-8649.1996.tb01797.x
Igarashi H, Ohno K, Horigome A, Fujiwara-Igarashi A, Kanemoto H, Fukushima K, Odamaki T, Tsujimoto H (2016) Fecal dysbiosis in miniature dachshunds with inflammatory colorectal polyps. Res Vet Sci 105:41–46. https://doi.org/10.1016/j.rvsc.2016.01.005
Interaminense JA, Vogeley JL, Gouveia CK, Portela RWS, Oliveira JP, Andrade HA, Peixoto SM, Soares RB, Buarque DS, Bezerra RS (2018) In vitro and in vivo potential probiotic activity of Bacillus subtilis and Shewanella algae for use in Litopenaeus vannamei rearing. Aquaculture 488:114–122. https://doi.org/10.1016/j.aquaculture.2018.01.027
Jia E, Zheng X, Cheng H, Liu J, Li X, Jiang G, Liu W, Zhang D (2019) Dietary fructooligosaccharide can mitigate the negative effects of immunity on Chinese mitten crab fed a high level of plant protein diet. Fish Shellfish Immunol 84:100–107. https://doi.org/10.1016/j.fsi.2018.09.074
Jia W, Fan Z, Shi Q, Zhang R, Wang X, Shi L (2021) LC-MS-based metabolomics reveals metabolite dynamic changes during irradiation of goat meat. Food Res Int 150:110721. https://doi.org/10.1016/j.foodres.2021.110721
Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Laskowska E, Jarosz Ł, Grądzki Z (2017) The effect of feed supplementation with effective microorganisms (EM) on pro- and anti-inflammatory cytokine concentrations in pigs. Res Vet Sci 115:244–249. https://doi.org/10.1016/j.rvsc.2017.03.008
Lee EJ, Lee SM, Lee GT, Kim IS, Kim YH (2008) Application of effective microorganisms for bioremediation of crude oil spill in Taean, Korea. J Environ Sci Int 17:795–799. https://doi.org/10.5322/JES.2008.17.7.795
Lei Y, Sun Y, Wang X, Lin Z, Bu X, Wang N, Du Z, Qin J, Chen L (2021) Effect of dietary phosphorus on growth performance, body composition, antioxidant activities and lipid metabolism of juvenile Chinese mitten crab (Eriocheir sinensis). Aquaculture 531:735856. https://doi.org/10.1016/j.aquaculture.2020.735856
Li X, Zhou L, Yu Y, Ni J, Xu W, Yan Q (2017) Composition of gut microbiota in the Gibel Carp (Carassius auratus gibelio) varies with host development. Microb Ecol 74:239–249. https://doi.org/10.1007/s00248-016-0924-4
Li X, Guo Q, Wang Y, Xu J, Wei Q, Chen L, Liao L (2020) Enhancing nitrogen and phosphorus removal by applying effective microorganisms to constructed wetlands. Water 12:2443. https://doi.org/10.3390/w12092443
Li S, Nie Z, Shen L, Shao N, Sun Y, Xu G, Xu P (2022) Effect of effective microorganisms (EM) on aquatic bacterial community structure in polyculture mode of Eriocheir sinensis and Micropterus salmoides. J Fish China 46:136–148
Ma QQ, Chen Q, Shen ZH, Li DL, Han T, Qin JG, Chen LQ, Du ZY (2017) The metabolomics responses of Chinese mitten-hand crab (Eriocheir sinensis) to different dietary oils. Aquaculture 479:188–199. https://doi.org/10.1016/j.aquaculture.2017.05.032
Mago T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Manan H, Rosland NA, Deris ZM, Hashim NFC, Kasan NA, Ikhwanuddin M, Fauzan F, Suloma A (2022) 16S rRNA sequences of Exiguobacterium spp. bacteria dominant in a biofloc pond cultured with whiteleg shrimp Penaeus vannamei. Aquac Res 53:2029–2041. https://doi.org/10.1111/are.15731
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. https://doi.org/10.14806/ej.17.1.200
Nikouli E, Kormas KA, Jin Y, Olsen Y, Bakke I, Vadstein O (2021) Dietary lipid effects on gut microbiota of first feeding Atlantic salmon (Salmo salar L.). Front Mar Sci 8:665576. https://doi.org/10.3389/fmars.2021.665576
Parrilla-Taylor DP, Zenteno-Savín T (2011) Antioxidant enzyme activities in Pacific white shrimp (Litopenaeus vannamei) in response to environmental hypoxia and reoxygenation. Aquaculture 318:379–383. https://doi.org/10.1016/j.aquaculture.2011.05.015
Peng F, Chen X, Meng T, Li E, Zhou Y, Zhang S (2018) Hematology and serum biochemistry parameters of captive Chinese alligators (Alligator sinensis) during the active and hibernating periods. Tissue Cell 51:8–13. https://doi.org/10.1016/j.tice.2018.02.002
Peng J, Zhao YL, Xu ZG, Liu BQ, Duan CC, Tang YC (2019) Effect of temperature stress on the survival of juvenile Chinese mitten crab (Eriocheir sinensis). Iran J Fish Sci 18:763–774. https://doi.org/10.22092/ijfs.2019.118594
Pujiastuti DR, Suwartha N (2017) Enhancing removal efficiency of ammonia and nitrate in shrimp farm wastewater using biofloc technology and effective microorganism S4 (EM4). Int J Technol 8:1021–1030. https://doi.org/10.14716/ijtech.v8i6.685
Qi C, Wang X, Han F, Chen X, Li E, Zhang M, Qin JG, Chen L (2021) Dietary arginine alleviates the oxidative stress, inflammation and immunosuppression of juvenile Chinese mitten crab Eriocheir sinensis under high pH stress. Aquacult Rep 19. https://doi.org/10.1016/j.aqrep.2021.100619
Quigley EMM (2017) Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep 17. https://doi.org/10.1007/s11910-017-0802-6
Ratsika A, Codagnone MC, O'Mahony S, Stanton C, Cryan JF (2021) Priming for life: early life nutrition and the microbiota-gut-brain axis. Nutrients 13. https://doi.org/10.3390/nu13020423
Reshma KJ, Sumithra TG, Nair AV, Stefi Raju V, Kishor TG, Sreenath KR, Sanil NK (2018) An insight into the gut microbiology of wild-caught Mangrove Red Snapper Lutjanus argentimaculatus (Forsskal, 1775). Aquaculture 497:320–330. https://doi.org/10.1016/j.aquaculture.2018.08.008
Schmidt V, Gomez-Chiarri M, Roy C, Smith K, Amaral-Zettler L (2017) Subtle microbiome manipulation using probiotics reduces antibiotic-associated mortality in fish. mSystems 2. https://doi.org/10.1128/mSystems.00133-17
Schock TB, Stancyk DA, Thibodeaux L, Burnett KG, Burnett LE, Boroujerdi AFB, Bearden DW (2010) Metabolomic analysis of Atlantic blue crab, Callinectes sapidus, hemolymph following oxidative stress. Metabolomics 6:250–262. https://doi.org/10.1007/s11306-009-0194-y
Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF (2012) Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12:277–288. https://doi.org/10.1016/j.chom.2012.08.003
Sharma V, Smolin J, Nayak J, Ayala JE, Scott DA, Peterson SN, Freeze HH (2018) Mannose alters gut microbiome, prevents diet-induced obesity, and improves host metabolism. Cell Rep 24:3087–3098. https://doi.org/10.1016/j.celrep.2018.08.064
Sombatjinda S, Wantawin C, Techkarnjanaruk S, Withyachumnarnkul B, Ruengjitchatchawalya M (2014) Water quality control in a closed re-circulating system of Pacific white shrimp (Penaeus vannamei) postlarvae co-cultured with immobilized Spirulina mat. Aquac Int 22:1181–1195. https://doi.org/10.1007/s10499-013-9738-2
Song DH, Chun BH, Lee S, Reddy CK, Jeon CO, Lee CH (2020) Metabolite profiling and microbial community of traditional Meju show primary and secondary metabolite differences correlated with antioxidant activities. J Microbiol Biotechnol 30:1697–1705. https://doi.org/10.4014/jmb.2007.07026
Sontowski R, van Dam NM (2020) Functional variation in dipteran gut bacterial communities in relation to their diet life cycle stage and habitat. Insects 11:543. https://doi.org/10.3390/insects11080543
Sun H, Wang N, Cang Z, Zhu C, Zhao L, Nie X, Cheng J, Xia F, Thai H, Lu Y (2016) Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes Facts 9:365–378. https://doi.org/10.1159/000449507
Sun S, Guo Z, Fu H, Ge X, Zhu J, Gu Z (2018) Based on the metabolomic approach the energy metabolism responses of oriental river prawn Macrobrachium nipponense hepatopancreas to acute hypoxia and reoxygenation. Front Physiol 9. https://doi.org/10.3389/fphys.2018.00076
Talpur AD, Ikhwanuddin M, Abdullah MDD, Bolong A-MA (2013) Indigenous Lactobacillus plantarum as probiotic for larviculture of blue swimming crab Portunus pelagicus (Linnaeus, 1758): Effects on survival, digestive enzyme activities and water quality. Aquaculture 416:173–178. https://doi.org/10.1016/j.aquaculture.2013.09.018
Wan JJ, Pan JL, Shen MF, Xue H, Sun ML, Zhang MQ, Zhu XH, Ma XK (2022) Changes in the growth performance, antioxidant enzymes and stress resistance caused by dietary administration of synbiotic (fructooligosaccharide and probiotics) in juvenile Chinese Mitten Crab, Eriocheir sinensis. Aquac Int 30:467–481. https://doi.org/10.1007/s10499-021-00811-5
Wang P, Wu X, Li K, Hu Y (2004) Study of prelimnary test with utilization of effective microorganisms (EM)in algae-type eutrophical water treatment. Res Environ Sci 17:39–43. https://doi.org/10.13198/j.res.2004.03.41.wangp.011
Wang SP, Blachier F, Zhao F, Yin YL (2011) Intestinal microbiota: development metabolism and functions. J Food Agric Environ 9:121–129
Wang AR, Ran C, Ringo E, Zhou ZG (2018) Progress in fish gastrointestinal microbiota research. Rev Aquac 10:626–640. https://doi.org/10.1111/raq.12191
Wang T, Yang C, Zhang S, Rong L, Yang X, Wu Z, Sun W (2021) Metabolic changes and stress damage induced by ammonia exposure in juvenile Eriocheir sinensis. Ecotoxicol Environ Saf 223:112608. https://doi.org/10.1016/j.ecoenv.2021.112608
Wang CH, Zhou YF, Lv DW, Ge Y, Li H, You Y (2019) Change in the intestinal bacterial community structure associated with environmental microorganisms during the growth of Eriocheir sinensis. Microbiologyopen 8. https://doi.org/10.1002/mbo3.727
Wen C, Wei S, Zong X, Wang Y, Jin M (2021) Microbiota-gut-brain axis and nutritional strategy under heat stress. ANIM NUTR 7:1329–1336. https://doi.org/10.1016/j.aninu.2021.09.008
White JR, Nagarajan N, Pop M, Ouzounis CA (2009) Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLOS Computational Biology 5. https://doi.org/10.1371/journal.pcbi.1000352
Wong S, Rawls JF (2012) Intestinal microbiota composition in fishes is influenced by host ecology and environment. Mol Ecol 21:3100–3102. https://doi.org/10.1111/j.1365-294X.2012.05646.x
Wu P, Liu Y, Li C, Xiao Y, Wang T, Lin L, Xie Y (2021) The composition of intestinal microbiota from Collichthys lucidus and its interaction with microbiota from waters along the pearl river estuary in China. Front Environ Sci 9. https://doi.org/10.3389/fenvs.2021.675856
Xie MX, Zhou W, Xie YD, Li Y, Zhang Z, Yang YL, Olsen RE, Ran C, Zhou ZG (2021) Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture 543. https://doi.org/10.1016/j.aquaculture.2021.736943
Xing CH, Cai MZ, Yu HB (2007) The application of EM (effective microorganisms) technology in environment protection. J Mirobio 27:93–97
Yoshii K, Hosomi K, Sawane K, Kunisawa J (2019) Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr 6. https://doi.org/10.3389/fnut.2019.00048
Yu D, Richardson NE, Green CL, Spicer AB, Murphy ME, Flores V, Jang C, Kasza I, Nikodemova M, Wakai MH, Tomasiewicz JL, Yang SE, Miller BR, Pak HH, Brinkman JA, Rojas JM, Quinn WJ, Cheng EP, Konon EN, Haider LR, Finke M, Sonsalla M, Alexander CM, Rabinowitz JD, Baur JA, Malecki KC, Lamming DW (2021) The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab 33:905-922.e906. https://doi.org/10.1016/j.cmet.2021.03.025
Zarina Z, Yaacob ND, Al Bakri AMM, Ruzaidi CM (2013) Halal based sourced EM for Turbidity Reducing in wastewater treatment, International Conference on Advanced Materials Engineering and Technology (ICAMET 2013), Bandung, INDONESIA, 191–195. https://doi.org/10.4028/www.scientific.net/KEM.594-595.191
Zhang W, Cheng H, Gui Y, Zhan Q, Li S, Qiao W, Tong A (2021) Mannose treatment: a promising novel strategy to suppress inflammation. Front Immunol 12:756920. https://doi.org/10.3389/fimmu.2021.756920
Zhang ZD, Wu YP, Zhang Y, Cao Y, Chen SH, Tian Z, Li QJ, Sun XF, Chen AH (2022) Effects of adding EM bacteria and mechanical aeration on water quality, growth and antioxidant status of Meretrix meretrix and Exopalaemon carinicauda farmed in the clam-shrimp polyculture system. Aquac Res 53:1823–1832. https://doi.org/10.1111/are.15710
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This work was supported by National Key R&D Program of China (2022YFD2400700) and the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2021XT0701).
Author information
Authors and Affiliations
Contributions
Gangchun Xu designed the study and was involved in supervision. Material preparation, data collection, and analysis were performed by Quanjie Li, Yi Sun, Jinliang Du, and Le Li. The first draft of the manuscript was written by Xiangyu Yi. Jiancao Gao and Liping Cao commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The experimental protocol was performed following the guidelines approved by the Institutional Animal Care and Use Committee of the Ministry of Freshwater Fisheries Research Center of the Chinese Academy of Fishery Sciences (authorization number CZ202050400).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Yi, X., Li, L. et al. Effects of effective microorganisms on the physiological status, intestinal microbiome, and serum metabolites of Eriocheir sinensis. Int Microbiol 27, 167–178 (2024). https://doi.org/10.1007/s10123-023-00375-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00375-9