Abstract
This study assessed the effects of Astragalus polysaccharides (APS) on antioxidant abilities, non-specific immune responses, and immune protective efficacy (attacked by Aeromonas hydrophila) of Eriocheir sinensis, the most important Chinese freshwater crabs. A total of 720 crabs (initial mean weight 10.27 ± 1.58 g) were fed 60 days with six kinds of experimental diets containing graded dosages of APS (0, 300, 600, 900, 1200, 1500 mg/kg diets) in 18 outdoor cement tanks. The results showed that superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (T-AOC), lysozyme (LZM), and phenoloxidase (PO) activities of serum significantly increased (P < 0.05) with increasing APS dosages (0–900 mg/kg diets), but alkaline phosphatase (AKP) and acid phosphatase (ACP) activities of serum did not significantly changed (P > 0.05); SOD, CAT, T-AOC, LZM, AKP, and ACP activities of hepatopancreas significantly increased (P < 0.05) with increasing APS dosages (0–1200 mg/kg diets); the increased maximal multiples of LZM and PO activities were higher than SOD, CAT, and T-AOC which increased. The results of A. hydrophila attack test showed that mortality rates significantly decreased (P < 0.05) with increasing APS dosages (0–600 mg/kg diets), and the highest immune protective rate was 49.4%. In short, APS could help E. sinensis to improve immune responses and may reduce the risk of disease attacks as one kind of effective immunopotentiator in diets, and the best additive dosage was 1200 mg/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eriocheir sinensis is the most important freshwater crabs with tremendous economic value in China. It belongs to the phylum Arthropoda, class Crustacea, order Decapoda, family Grapsidae, and genus Eriocheir. The annual culture area and output of E. sinensis in China are more than 1 million hm2 and 600,000 t, respectively. However, there are many kinds of diseases that have attacked E. sinensis so that fishermen suffered losses with extensive farming in recent 20 years. Even though no any disease had happened, the survival rates were low (<50%) in the process of E. sinensis farming with high water temperature, low dissolved oxygen, river pollution, abuse of medical drugs in aquaculture farming and other stresses, and the low immunity and poor stress responses of E. sinensis may be the primary causes. In recent years, many kinds of immunopotentiators (such as Chinese herbal polysaccharides, vitamins, probiotics, and so on)were used to help aquatic animals to reduce the risk of bacterium and virus infections, since the significance of disease prevention is much larger than treatments in aquatic animals. Intensive researches had been conducted on the efficacy of multitudinous immunopotentiators on the antioxidant ability and non-specific immune responses of crabs and shrimps all over the world (Ai et al. 2008; Bidhan et al. 2014; Chen et al. 2014; Hou et al. 2015; Li et al. 2014; Li et al. 2015a, b; Ma and Chen 2013; Montero-Rocha et al. 2006; Song et al. 2005; Shen et al. 2004; Smith et al. 2003; Wei et al. 2015; Xu et al. 2005; Yuan et al. 2014; Yang et al. 2005; Zokaeifar et al. 2012; Zhao et al. 2016).
Astragalus polysaccharides (APS) are the most effective components of Astragalus which is a traditional herbal medicine in China. APS have many clinical efficacies, such as anti-tumor, antibacterium, anti-virus, regulating the body’s humoral immune, activating the immune cytokines, and so on(Chen and Huang 2008). In recent years, using the APS to carry on the antibacterium and anti-virus and regulating the body’s humoral immune treatments becomes one of focuses of increasing production in animal husbandry (Yao et al. 2009) and fishery (Liu et al. 2014) owing to significant effects and advantages (no side effects, rich resources, and low cost). However, to date, no investigations about APS had been conducted on crabs all over the world. This study investigated the use of APS to feed E. sinensis as a feed additive and assessed the effects on antioxidant abilities, non-specific immune responses and immune protective efficacy (attacked by Aeromonas hydrophila).
Material and methods
Materials
In March 2016, a total of 720 E. sinensis (initial mean weight 10.27 ± 1.58 g) were picked out at Chinese Mitten Crab Farm of Freshwater Fisheries Research Institute of Jiangsu Province, China.
APS was purchased from Nuoweikang Biology Science and Technology Co., Ltd., Anhui Province, China.
Fish meal, soybean meal, corn meal, wheat bran, Ca(H2PO4)2, NaH2PO4, and binder were used to make the basic diets. Five different dosages of APS were added to the basic diets to make the experimental diets: 300 mg/kg (group A1), 600 mg/kg (group A2), 900 mg/kg (group A3), 1200 mg/kg (group A4), and 1500 mg/kg (group A5). The control group (group A0) was treated with the basic diets. All the diets were made by the Haipurui Feed Co., Ltd., Jiangsu Province, China, and were stored in the refrigerator at −20 °C before using (Table 1).
Methods
A total of 720 E. sinensis were averagely divided into three parts at random, i.e., three repeats. Eath part was divided into six groups at random. Five different experimental diets and the basic diets were used to feed them. In eath group, 40 E. sinensis were placed in an outdoor cement tank (2.0 × 2.0 × 1.0 m) for farming, and four tiles were placed at the bottom of each tank for shelters. During the experiment, feeding amount (3% of the total crabs weight in a cement tank in accordance with normal farming methods in the ourdoor pond) and feeding time (7 pm each day; E. sinensis is a nocturnal aquatic animal.) of each group were the same, and the water was changed weekly in each pond. Continuous feeding was terminated after 60 days under the natural conditions: water temperature 15.8–27.2 °C, dissolved oxygen 4.3–7.8 mg/L, and pH 7.2–8.2.
Sample preparations and parameter determinations
A total of six E. sinensis were picked out at each group randomly, and their hemolymphs were respectively extracted from the root of the third paraeiopod which were broken off. After 24 h in the Eppendorf centrifugal tubes under the 4 °C condition, all the hemolymphs were centrifuged 10 mins under the conditions: 4 °C and 5000 r/min, then supernatant serum were sucked up for parameter determinations. At the same time, every crab was dissected to get the hepatopancreas which then were stored in a −80 °C refrigerator after quick-freezing of liquid nitrogen. When the parameters would be determinated, stroke-physiological saline solution (0.65%) was added in the hepatopancreas according to 1:9 volume. After being broken by the ultrasonic, all the hepatopancreas were centrifuged 10 mins under the conditions: 4 °C and 5000 r/min, then supernatant serum were sucked up for parameter determinations.
All the parameters, including superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (T-AOC), lysozyme (LZM), phenoloxidase (PO), alkaline phosphatase (AKP), and acid phosphatase (ACP) activities, were determinated with test kits which were produced by the Nanjing Jiancheng Bioengineering Institute, Jiangsu Province, China. The determination methods were carried out in accordance with all the test kit instructions (SOD: WST-1 method, reaction temperature 37 °C, wave length 450 nm; CAT: visible light method, reaction temperature 37 °C, wave length 405 nm; T-AOC: colorimetry method, reaction temperature 37 °C, wave length 520 nm; LZM: turbidimetry method, reaction temperature 37 °C, wave length 530 nm; PO: ELISA method, reaction temperature 37 °C, wave length 450 nm; AKP: visible light colorimetry method, reaction temperature 37 °C, wave length 520 nm; ACP: spectrophotometry method, reaction temperature 37 °C, wave length 520 nm).
Bacteria attack test
The strains of A. hydrophila were purchased from Shanghai Baili Biology Science and Technology Co., Ltd., China.
The strains were inoculated on agar culture medium and then rejuvenated for 48 h in a 30 °C biochemical incubator. The solution concentration of rejuvenated strains which were then put in stroke-physiological saline solution (0.65%) was 1.0 × 107 cfu/mL. The solution concentration, which was confirmed by a preliminary test, was the median lethal dose.
According to the original design of experiment, 30 E. sinensis were picked out at each group randomly and then placed in 18 plastic cases (0.8 × 0.5 × 0.5 m), respectively. Each crab was injected with A. hydrophila solution 0.1 mL at the root of the third paraeiopod and cultured tentatively in corresponding plastic cases. The numbers of dead crabs were recorded in each plastic case every 24 h, and the mortality rates and immune protective rates were calculated respectively.
Data processing and statistical analysis
SPSS19.0 was used to construct the data processing for a comparison of means and variance analysis. Two multiple comparison methods, least significant difference and least significant ranges, were used to compare the results (P < 0.05).
Results
Effects of APS on SOD, CAT, T-AOC, LZM, PO, AKP, ACP activities of serum and hepatopancreas of E. sinensis
SOD, CAT, T-AOC, LZM, PO, AKP, and ACP activities of serum and hepatopancreas of E. sinensis of different groups after APS feeding were shown in Figs. 1, 2, 3, 4, 5, 6, and 7.
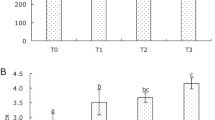
Figure 1 showed that SOD activities of serum and hepatopancreas gradually increased after APS feeding with increasing dosages. SOD activities of serum of groups A0, A1, A2, A3, A4, and A5 were 86.36 ± 4.13, 87.72 ± 4.51, 115.64 ± 7.57, 138.22 ± 6.93, 145.28 ± 10.97, and 141.28 ± 9.37 U/mL, respectively; SOD activities of hepatopancreas of the abovementioned groups were 116.78 ± 6.21, 120.40 ± 8.57, 133.56 ± 10.57, 168.97 ± 11.86, 175.76 ± 8.94, and 169.37 ± 10.56 U/mL, respectively.
Figure 2 showed that CAT activities of serum and hepatopancreas gradually increased after APS feeding with increasing dosages. CAT activities of serum of the abovementioned groups were 1.46 ± 0.12, 1.52 ± 0.15, 1.84 ± 0.17, 2.32 ± 0.29, 2.27 ± 0.27, and 2.41 ± 0.37 U/mL, respectively; CAT activities of hepatopancreas of the abovementioned groups were 1.86 ± 0.09, 1.92 ± 0.19, 2.34 ± 0.31, 2.78 ± 0.40, 2.71 ± 0.42, and 2.86 ± 0.57 U/mL, respectively.
Figure 3 showed that T-AOC activities of serum and hepatopancreas gradually increased after APS feeding with increasing dosages. T-AOC activities of serum of the above-mentioned groups were 8.16 ± 0.82, 8.02 ± 0.55, 9.76 ± 0.97, 11.58 ± 1.09, 11.34 ± 0.87, and 11.48 ± 0.69 U/mL, respectively; T-AOC activities of hepatopancreas of the abovementioned groups were 8.56 ± 0.76, 8.72 ± 0.59, 10.54 ± 0.71, 12.82 ± 1.02, 13.08 ± 0.86, and 12.92 ± 1.17 U/mL, respectively.
Figure 4 showed that LZM activities of serum and hepatopancreas gradually increased after APS feeding with increasing dosages. LZM activities of serum of the above-mentioned Groups were 0.16 ± 0.02, 0.25 ± 0.03, 0.30 ± 0.02, 0.33 ± 0.03, 0.42 ± 0.03, 0.43 ± 0.02 U/mL, respectively; LZM activities of hepatopancreas of the above-mentioned Groups were 0.13 ± 0.01, 0.19 ± 0.02, 0.25 ± 0.03, 0.27 ± 0.02, 0.35 ± 0.02, 0.35 ± 0.02 U/mL, respectively.
Figure 5 showed that PO activities of serum gradually increased after APS feeding with increasing dosages. PO activities of serum of the above-mentioned Groups were 11.13 ± 1.22, 16.23 ± 1.14, 23.30 ± 2.26, 28.61 ± 3.02, 34.63 ± 3.26, 35.50 ± 1.93 U/mL, respectively.
Figure 6 showed that AKP activities of hepatopancreas gradually increased after APS feeding with increasing dosages, but AKP activities of serum had not significantly changed. AKP activities of serum of the above-mentioned Groups were 4.26 ± 0.72, 4.35 ± 0.93, 4.46 ± 1.32, 4.33 ± 1.11, 4.22 ± 0.98, 4.63 ± 1.41 U/mg, respectively; AKP activities of hepatopancreas of the above-mentioned Groups were 9.45 ± 1.75, 10.15 ± 2.02, 14.58 ± 2.93, 16.27 ± 3.33, 24.87 ± 4.21, 25.66 ± 3.47 U/mg, respectively.
Figure 7 showed that ACP activities of hepatopancreas gradually increased after APS feeding with increasing dosages, but ACP activities of serum had not significantly changed. ACP activities of serum of the abovementioned groups were 4.29 ± 0.62, 4.40 ± 0.98, 4.69 ± 1.08, 4.55 ± 1.17, 4.48 ± 1.06, and 4.31 ± 0.89 U/mg; ACP activities of hepatopancreas of the abovementioned groups were 9.52 ± 1.66, 10.21 ± 2.43, 15.98 ± 2.82, 17.48 ± 3.09, 25.55 ± 3.63, and 25.27 ± 3.28 U/mg, respectively.
With regard to SOD, CAT, T-AOC, LZM, PO, AKP, and ACP activities, significant differences of the abovementioned groups were all shown in the figures by sharing the different letter (P < 0.05).
Evaluation of APS on immune protective efficacy of E. sinensis which were injected A. hydrophila
The mortality rates and immune protective rates of different groups which were injected A. hydrophila were shown in Fig. 8. Figure 8 showed that the mortality rates gradually decreased with increasing APS dosages after A. hydrophila attack. The mortality rates of groups A0, A1, A2, A3, A4, and A5 were 87.8 ± 3.8, 71.1 ± 3.0, 47.8 ± 2.1, 44.4 ± 3.1, 52.2 ± 2.8, and 47.8 ± 1.9%, respectively; the immune protective rates of groups A0, A1, A2, A3, A4, and A5 were 0, 19.0, 45.6, 49.4, 40.5, and 45.6%, respectively.
Discussion
Effects of APS on antioxidant abilities and non-specific immune responses of E. sinensis
The results of this study showed that the antioxidant abilities and non-specific immune responses of E. sinensis had been significantly improved after APS feeding. That APS could improve the antioxidant abilities and non-specific immune responses of fishes was in conformity with this study. After being perfused, different concentrations of APS decoction (0.5, 1.0, 2.0%, respectively), SOD activities of serum and livers of Acipenser schrencki Brandt had been significantly improved compared to the control group, and the malondialdehyde content gradually decreased(Liu et al. 2006). When the additive dosages of APS in formula feeds were 1000–2000 mg/kg, SOD and CAT activities of the tilapia had been significantly improved (Zhang et al. 2010). That the yellow catfish were fed formula feeds which contained appropriate levels (300–1500 mg/kg) of APS could significantly improve the SOD and CAT activities and decreased the malondialdehyde content, and the highest immune protective rate after Edwardsiella tarda attack was 50.0% (group 1200, 1500 mg/kg) (Bai et al. 2011). In addition, that other Chinese herbal medicines or immunopotentiators could improve the antioxidant abilities and non-specific immune responses of E. sinensis were in conformity with this study. The antioxidant abilities and non-specific immune responses were significantly promoted after E. sinensis were fed the test feeds which contained six kinds of Chinese herbal medicines (dosage, 2.4%): Folium Isatidis, Cordate Houttuynia, Epimedium brevicornu, Rheum officinale, Andrographis paniculata and Radix Scutellariae, and the immune protective rate after A. hydrophila attack was 62.5% (Liu et al. 2008). The antioxidant abilities and non-specific immune responses of E. sinensis showed the largest increase when the feeds Asparagus officinalis, Menthahaplocalyx, Crataegus pinnatifida, Astragalus membranaceus and Epimedium brevicornu (dosage: 1.0%) were added (Li et al. 2015a, b). Vc could significantly promote non-specific immune function including SOD, LZM, PO, AKP, and ACP activities in E.sinensis, and the optimum dosages were 500–1000 mg/100 g diets (Ai et al. 2008). Bamboo shoots polysaccharide could significantly promote non-specific immune function including SOD, LZM, respiratory burst activities, total hemocyte count, phagocytic percentage, and phagocytic index in E.sinensis, and the optimum dosage was 10 mg/kg body weight (Li et al. 2014).
Differential effects of APS on antioxidant abilities and non-specific immune responses of serum and hepatopancreas of E. sinensis
The results of this study showed that SOD, CAT, T-AOC, AKP, and ACP activities of hepatopancreas were higher than serum, but LZM activity was lower than serum. Hepatopancreas, the nutrient regulatory center of E. sinensis plays the most important role in the process of metabolism. In this process, some harmful products which contain peroxy radical, superoxide, hydroxyl radical, hydrogen peroxide, singlet oxygen, and so on appear in tissues. That the antioxidant system which was constituted by SOD, CAT, T-AOC, and so on was in charge of clearing them protects some biological membranes from damages (Ortuno et al. 1999).
The results showed that AKP and ACP activities of serum of E. sinensis did not change significantly, this was because AKP and ACP were released to serum when the hepatopancreas were damaged or taken some diseases. AKP and ACP, mainly existing in hepatopancreas of E. sinensis, catalyze the transfer reactions of all the phosphate monoesters and groups and play an important role in absorbing of calcium, calcium phosphate deposition, chitin secretion, and so on, since crabs molt many times in the process of growth(Chen et al. 1996; Kobayashi et al. 1983). In addition, ACP is one kind of lysosomal enzyme produced by activated macrophages as an important indicator in the assessment of immune state, as it can kill some pathogenic microorganisms (Li et al. 2015a, b).
The results showed that LZM activities of serum and hepatopancreas and PO activities of serum were increased significantly after APS feeding, and the maximums which increased contrasting with the control group were 168.9, 169.2, and 219.0%, respectively. These were higher than SOD, CAT, and T-AOC activities which increased, only 50.5, 53.8, and 52.8%, respectively. The main factor for this phenomenon was likely that APS could stimulate to produce LZM and PO or take part in producing something which was closely related to LZM and PO, and it was consistent with the significant clinical efficacy of APS: antibacterium and anti-virus. LZM, one kind of hydrolytic enzymes which specially act on cell walls of microorganisms, is an important non-specific immune factor in body fluids of crustaceans. Animals can enhance their disease resistances through much stronger LZM activities (Ma et al. 2006). PO which is produced from prPO system in blood cells of crustaceans plays an important role in foreign matter recognitions, opsonin releases, improving phagocytosis of cells, producing agglutinins and LZM and so on, and it has a close connection with immune functions of body (Soderhall 1999; Wang 1993). Since the body fluids of crustaceans have not immunoglobulins, so LZM and PO play the leading roles in humoral immunity.
When the additive dosage of APS in the basic diets was more than 900 mg/kg, SOD, CAT, and T-AOC activities did not change significantly; when the additive dosage of APS in the basic diets was more than 1200 mg/kg, LZM, PO, AKP, and ACP activities did not change significantly. This fact showed that the antibacterium and anti-virus efficacy of APS needed higher additive dosages for E. sinensis.
Evaluation of APS on immune protective efficacy of E. sinensis which were injected A. hydrophila
The results of bacteria attack test showed that the mortality rates gradually decreased with increasing APS dosages. When the additive dosage of APS in the basic diets was more than 600 mg/kg, mortality rates did not change significantly. This phenomenon was not consistent with the change rules of the abovementioned parameters, and this was likely related to experimental errors and precision. In short, APS could help E.sinensis to improve immune responses and may reduce the risk of disease attacks as one kind of effective immunopotentiator in diets, and the best additive dosage was 1200 mg/kg.
References
Ai CX, Chen LQ, Liu XL, Gao LJ, Wen XB (2008) Effects of vitamin C on non-specific immune responses of Chinese mitten crab, Eriocheir sinensis. J Fish China 32(2):249–256
Bai DQ, Wu X, Guo YJ, Zhu GX, Xing KZ, Ning B (2011) Effects of Astragalus polysaccharides on antioxidant and non-specific immune indices of yellow catfish (PeReobagrus fulvidraco) over long-term feeding. Chinese Journal of Animal Nutrition 23(9):1622–1630
Bidhan CD, Meena DK, Behera BK, Pronob D, Das Mohapatra PK, Sharma AP (2014) Probiotics in fish and shellfish culture: immunomodulatory and ecophysiological responses. Fish Physiol Biochem 40:921–971
Chen GH, Huang WF (2008) Advances in studies Astragalus on chemical constituents and pharmacological effects. Chinese Journal of New Drugs 17(17):1482–1485
Chen QX, Chen SL, Shi Y, Zhu LX, Yan SX (1996) Characterization of alkaline phosphatase from Penaeus penicillatus. Journal of Xiamen University(Natural Science Edition) 35(2):257–261
Chen YL, Li EC, Yu N, Tian WJ, Jiang X, Sun LM, Qi J, Chen LQ (2014) Effect of replacing dietary fish oil with soybean oil on growth, non-specific immune response, and resistance to Aeromonas hydrophila challenge in Chinese mitten crab, Eriocheir sinensis. Journal of Fishery Sciences of China 3:511–521
Hou YM, Jin M, Zhang W, Huo YW, Zhou QC (2015) Dietary vitamin C requirement of juvenile swimming crab (Portunus trituberculatus). Acta Zoonutrimenta Sinica 12:3772–3781
Kobayashi K, Matsui M, Haraguchi H, Fuwa K (1983) Indentification of alkaline phosphatase in sea water. J Inorg Biochem 18:41–47
Liu HB, Lu TY, Zhang CY, Sun DJ, Bai XJ (2006) Effects of Astragalus on antioxidant abilities and immune responses of Acipenser schrencki Brandt. Journal of Dalian Fisheries University 21(3):231–235
Liu LP, Xue H, Zhou G (2008) Effects of Chinese herbal medicine on immune function of Chinese mitten crab, Eriocheir sinensis. Journal of Nanjing Normal University (Natural Science Edition) 31(1):109–113
Liu MZ, Yu H, Yin FQ, Shang XG (2014) Advances in studies Astragalus polysaccharides on immune performance of fishes. Hubei Agricultural Sciences 53(9):1985–1989
Li SM, Ding HJ, Guo XZ, Fu H, Tang YQ, Zhong JY (2015a) Effects of a Chinese herbal compound on growth performance, non-specific immune responses and disease resistances of Chinese mitten crab, Eriocheir sinensis. Fish Sci 34(4):201–207
Li Y, Wang YF, He YB, Chen YJ, Xu L (2014) Effects of bamboo shoots polysaccharides on immune function of immunosuppressed Chinese mitten crab (Eriocheir sinensis). China Feed 22:33–36
Li Y, Zhang W, Jin M, Huo YW, Qiu H, Hou YM, Zhou QC (2015b) Effects of dietary vitamin E level on growth performance, non-specific immune and anti-oxidant indices of juvenile swimming crab (Portunus trituberculatus). Acta Zoonutrimenta Sinica 5:1431–1439
Ma GH, Chen DY, Zhong Q, Liu LY, Li LL (2006) Current situations and prospects of immunological research of Eriocheir sinensis. Acta Agriculture Jiangxi 18(4):141–144
Montero-Rocha A, Mcintosh D, Sanchez-Merino R, Flores I (2006) Immunostimulation of white shrimp (Litopenaeus vannamei) following dietary administration of Ergosan. J Invertebr Pathol 91(3):188–194
Ma SQ, Chen HJ (2013) Effects of Chinese herbal medicine on non-specific immune factors of juvenile Eriocheir sinensis under high water temperature stress. Freshwater Fisheries 6:62–66
Ortuno J, Esteban MA, Meseguer J (1999) Effect of high dietary intake of vitamin C on non-specific immune response of gilthead seabream (Sparus aurata L.). Fish & Shellfish Immunology 9:429–443
Shen JY, Liu W, Cao Z, Yin WL, Shen ZH, Qian D, Wu YL (2004) Effects of immunity-stimulants on immune function of Eriocheir sinensis. Acta Agriculturae Zhejiangensis 16(1):25–29
Soderhall K (1999) Invertebrate immunity. Dev Comp Immunol 23:263–266
Song LP, Huang XX, Zhou HQ, Liu XG (2005) Effects of Vc, Beta-glucan and Algae powder on growth, survival rate and immune enzyme activities of Penaeus chinensis juvenile. J Shanghai Fish Univ 14(3):276–281
Smith VJ, Brown JH, Hauton C (2003) Immunostimulation in crustaceans: does it really protect against infection. Fish & Shellfish Immunology 15(1):71–90
Wei JJ, Zhang F, Tian WJ, Li EC, Wu QQ, Chen LQ (2015) Effects of dietary folic acid and vitamin B12 on growth performance, non-specific immunity and disease resistance of juvenile Chinese mitten crab (Eriocheir sinensis). Acta Hydrobiologica Sinica 6:1069–1075
Wang L(1993)Researches on disease preventions and treatments of Penaeus chinensis[D]. Institute of Oceanology of Chinese Academy of Sciences
Xu Z, Yao J, Chen CF, Xiao ZM, Tan B, Tan CJ, Wang SH (2005) Enhancements of disease resistances in Eriocheir sinensis by oral administration of immune polysaccharide (yeast cell wall). Journal of Huazhong Agricultural University 24(4):383–386
Yuan CY, Zhang Y, Cui QM (2014) Effects of chi-tooligosaccharide on immune function of Chinese mitten crab (Eriocheir sinensis). China Feed 6:27–30
Yang FG, Zhou HQ, Huang XX (2005) Effects of beta-glucans on growth and non-specific immune responses of Litopenaeus vannamei. J Shanghai Fish Univ 14(3):264–269
Yao XJ, Wang M, Jiang SX, Xue FQ (2009) Advances in studies Astragalus polysaccharides on pharmacological effects and application in animals producing. Feed Industry 30(18):1–3
Zokaeifar H, Balcázar JL, Saad CR, Kamarudin MS, Sijam K, Arshad A, Nejat N (2012) Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp (Litopenaeus vannamei). Fish & Shellfish Immunology 33:683–689
Zhao L, Long XW, Wu XG, Liu ZH, He J, Cheng YX (2016) Effects of fish oil replacement by blending vegetable oils in fattening diets on gonadal development, lipid metabolism, antioxidant and immune function of adult male Chinese mitten crab (Eriocheir sinensis). Acta Zoonutrimenta Sinica 2:455–467
Zhang WN, Lin X, Wang SK, Zhang XL, Huang YZ, Wang QX, Chen JM, Zhao J (2010) Effects of Astragalus polysaccharides on non-specific immune responses and endocrine function in stomach and foregut of tilapia. Acta Zoonutrimenta Sinica 22(2):401–409
Acknowledgments
This work was supported by grants from the Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF)(CX(15)1011)and the Aquatic Sanxin Project of Jiangsu Province (D2015-5, D2016-1, Y2014-24).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, L., Zhou, G., Pan, J. et al. Effects of Astragalus polysaccharides on antioxidant abilities and non-specific immune responses of Chinese mitten crab, Eriocheir sinensis . Aquacult Int 25, 1333–1343 (2017). https://doi.org/10.1007/s10499-017-0117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0117-2