Abstract
We aimed at isolating and characterising microorganisms present in human breast milk with probiotic potential. In an 8-week postpartum sampling period, two strains of bifidobacteria (Bifidobacterium longum LM7a and Bifidobacterium dentium LM8a’) and four strains of lactobacilli were isolated, all during the first 4-week postpartum. B. longum LM7a and B. dentium LM8a’, together with four strains previously isolated from breast milk (Bifidobacterium lactis INL1, INL2, INL4 and INL5), were considered for further studies. Susceptibility of the strains to tetracycline, erythromycin, clindamycin, streptomycin, vancomycin and chloramphenicol was evaluated and the isolates exhibited, in general, the same properties as previously reported for bifidobacteria. All isolates showed low hydrophobicity and B. lactis and B. longum strains had satisfactory resistance to gastric digestion and bile shock, but not to pancreatin. B. lactis INL1, B. longum LM7a and B. dentium LM8a’ were selected for some comparative technological studies. In particular, B. lactis INL1 displayed technological potential, with satisfactory growth in cheese whey-based media in biofermentor and resistance to freeze-drying, accelerated storage conditions and simulated gastric digestion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional foods are described as those claimed to have positive effects on health beyond basic nutrition. Such products continue to gain widespread popularity and acceptance throughout the developed world and their global market has been in fast growth since the early 2000’s, particularly in the USA, Europe and Japan (Saad et al. 2013). Breast milk is a complex and complete food, and it may be considered as the best example of a natural functional food (Gotteland et al. 2011). In addition to nutrients, hormones, growth factors, immunoglobulins, cytokines and enzymes, which contribute to immune maturation and child welfare (Le Huërou-Luron et al. 2010), breast milk contains significant amounts of microorganisms (Boix-Amorós et al. 2016). So far, about 700 bacterial species have been detected in breast milk, although the number of cultivable species present in an individual is much lower, in a range of 2–18 different species (Cabrera-Rubio et al. 2012; Jeurink et al. 2013). These bacteria, including bifidobacteria and lactobacilli, are responsible in part for the gastrointestinal colonisation of the newborn and for the adequate maturation of the gut mucosal immune system. Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (Hill et al. 2014). Specific strains of lactobacilli and bifidobacteria are the most studied microorganisms worldwide and the most used in food and pharma as probiotics. In 2008, a Spanish company, Puleva Food S.L. (www.puleva.es), launched the first formula containing a probiotic lactobacilli isolated from breast milk, Lactobacillus fermentum CECT 5716. To the best of our knowledge, except in this case, so far commercial infant formulas containing probiotic microorganisms have been made with strains isolated from fermented food or faecal microbiota of children (Chassard et al. 2014). In this context, the use of strains isolated from human breast milk is attractive and the administration of bacteria from this source to infants could provide opportunities to help to establish a healthy intestinal microbiota more similar to that of breast milk fed ones, reducing the risk of intestinal disorders (Maldonado et al. 2012), when breastfeeding is impaired or limited. For a microorganism to be regarded as probiotic, safety and the capacity to induce a beneficial effect are required, but resistance to the technological processes found from production to consumption is also of importance (Heller 2001). Finally, in addition to providing added value to food, probiotics need to be cost-effectively produced, which implies maximising substrate-to-biomass yield and stability during processing and shelf life (Jankovic et al. 2010). The use of cheese whey, an abundant by-product of the dairy industry, for probiotic biomass production and drying has been evaluated previously as a lower cost alternative to synthetic media or protectants (Doleyres and Lacroix 2005; Lavari et al. 2014).
The aim of this work was to isolate and characterise microorganisms present in human breast milk with probiotic and technological potential, with emphasis on bifidobacteria, for their use in functional foods or supplements.
Materials and methods
Isolation of bacteria from breast milk
Human breast milk samples were voluntarily donated by mothers attending different health institutions in Santa Fe city (Argentina), with full knowledge and written consent about their use. This study was performed in conformity with the Declaration of Helsinki and was approved by the Advisory Committee on Research Ethics and Safety of the Faculty of Biochemistry and Biological Sciences, Universidad Nacional del Litoral, Santa Fe, Argentina. Mothers were surveyed about some characteristics of the delivery (vaginal or by C-section, full term or premature) and probiotics consumption habits. Mothers that received antibiotics or consumed probiotic products during pregnancy or postpartum were excluded. Ten mothers provided periodically samples of breast milk, from delivery to the eighth week postpartum (PP), in order to include in the sampling period the three stages of lactaction: colostrum (days 1–4 PP), transitional milk (day 5—week 2 PP), and mature milk (week 2 PP onwards). Sample collection and plating were performed according to Zacarías et al. (2011). Colonies presenting typical lactobacilli or bifidobacteria morphology (examined by phase-contrast microscopy, × 1000) were isolated and purified and assessed for Gram-staining, mobility and catalase activity. Presumptive lactobacilli or bifidobacteria isolates were stored frozen in MRS broth (Biokar, Beauvais, France) added with 20% (v/v) glycerol at − 70 °C for subsequent studies.
Identification of isolates
Total DNA of isolates was obtained from overnight cultures by using the GenElute-Bacterial Genomic DNA kit (Sigma, St Louis, MO, USA) according to the manufacturer’s instructions. Purified DNA samples were stored at − 20 °C until use. The identity of isolates was analysed by amplifying, sequencing and comparing their 16S rRNA gene (Edwards et al. 1989). A 1500 bp fragment of 16S rRNA gene was amplified using primers pA 5’-AGAGTTTGATCCTGGCTCAG-3′ and pH 5’-AAGGAGGTGATCCAGCCGCA-3′. All PCR reactions were performed using 2 μL of diluted (1:50) DNA as template, 2.5 U Taq DNA polymerase (GE Healthcare, Little Chalfont, UK), 200 nMdNTPs (GE Healthcare) and 400 nM each primer (Sigma-Genosys, The Woodlands, TX, USA) in a final volume of 50 μL. Amplifications were performed in a GeneAmp PCR System (Applied Biosystems, Foster City, CA, USA) under the following conditions: 3 min at 94 °C, 36 cycles of 1 min at 94 °C, 2 min at 51 °C and 2 min at 72 °C, and a final step of 7 min at 72 °C. The PCR products were separated on 1% (w/v) agarose gels in TBE buffer, stained with GelRed (Biotium, Hayward, CA, USA) and visualised under UV light (Sambrook and Russell 2001). Amplicons were purified with QIAquick Gel Extraction (QIAGEN, Hilden, Germany) and their nucleotide sequences were determined by primer extension at the DNA Sequencing Service of Macrogen (Seoul, Korea). The identity of isolates was checked by nucleotide-nucleotide BLAST of the NCBI database (www.ncbi.nlm.nhi.gov/blast) and 16S rRNA partial sequences from new isolates were deposited in GenBank (Table S1).
Random amplification of polymorphic DNA (RAPD) analysis
Genotypic diversity among isolates was analysed by RAPD-PCR, using four single arbitrary primers, in independent reactions: (i) primer B08 with sequence 5’-GTCCACACGG-3′; (ii) primer B10 with sequence 5’-CTGCTGGGAC-3′; (iii) primer M13 with sequence 5’-GAGGGTGGCGGTTCT-3′, and (iv) primer 1254 with sequence 5’-CCGCAGCCAA-3′. Amplifications for the primers B08 and B10 were carried out according to Binetti et al. (2007) and for M13 and 1254, according to Giraffa et al. (2004). PCR reactions were performed in a total volume of 25 μL with 1-μL template-diluted DNA, 2.5 U Taq Polymerase (GE Healthcare) and 200 nM of each dNTP (GE Healthcare). The primer final concentrations were 500 nM for B08 and B10, 2000 nM for M13 and 800 nM for 1254. In all cases, a tube without template was included as negative control. Amplification products were analysed by electrophoresis on 1% (w/v) agarose gels on TBE buffer, following standard protocols.
Safety and functional characterisation of breast milk bifidobacteria
Strains
Two bifidobacteria strains isolated and identified during this work were used. Additionally, four breast milk derived-strains of Bifidobacterium animalis subsp. lactis, isolated previously (strains INL1, INL2, INL4 and INL5; Zacarías et al. 2011), were also studied in this work. Before use, the strains were sub-cultured two times in MRS broth supplemented with 0.1% (w/v) L-cysteine hydrochloride (Biopack, Buenos Aires, Argentina; MRSc) for 18 h under anaerobiosis (Anaeropack-Anaero, Mitsubishi Gas Chemical Co., Inc., Japan) at 37 °C.
Antibiotic resistance
Susceptibility to clindamycin, chloramphenicol, erythromycin, streptomycin, tetracycline and vancomycin was evaluated by using the E-test (AB Biodisk, Oxoid Inc., Ontario, Canada) method as previously described (Mättö et al. 2006). The inoculum was prepared by suspending colonies from cultures grown on LSM + cys agar for 2 days in LSM + cys broth to a cell density corresponding to 1 McFarland standard. The suspension was spread evenly on the pre-reduced agar plates by using a sterile cotton swab. The E-test strips were placed on the air-dried agar surface. The plates were incubated under anaerobic conditions at 37 °C for 48 h. The minimum inhibitory concentration (MIC) for each antibiotic was read as the lowest antibiotic concentration in which the growth was inhibited. Resistance to tetracycline was corroborated using a broth microdilution method, according to Cardamone et al. (2011). Briefly, the antibiotic stock solution was prepared in distilled water and then filter-sterilised (0.22-μm pore diameter) (Millipore, Sao Paulo, Brazil). The strains were grown overnight at 37 °C in MRSc and then diluted in LSM broth in order to reach a final inoculum of 105 CFU/ml in the microplates. After incubation, the MIC was determined as the lowest antibiotic concentration at which no growth was observed. Interpretation of results was based on the cut off values adopted by the updated technical guidance of FEEDAP (European Food Safety Authority 2012).
Hydrophobicity
The ability of the strains to adhere to hydrocarbons as a measure of their hydrophobicity was determined according to Vinderola and Reinheimer (2003). The partition of bacterial cells between organic (n-hexadecane) (Merck, Darmstadt, Germany) and aqueous phases was determined by measurement of optical density at 560 nm (OD560 nm). Assays were performed in triplicate and hydrophobicity (H%) was calculated as follows: H % = [(OD0–OD)/OD0] × 100, where OD0 and OD are the optical density before and after extraction with n-hexadecane, respectively.
Resistance to simulated gastrointestinal digestion (SGID)
Gastric digestion (step 1) was performed as described by Burns et al. (2014). Briefly, overnight (18 h) cultures (40 ml) of the strains under study in MRSc broth were centrifuged (2750 g, 10 min, 5 °C), washed twice with PBS (pH 7.2) and resuspended in 20 ml of 10% (w/v) sterile skim milk, and 20 ml of a simulated gastric juice containing 0.6% (w/v) porcin pepsin (Merck) and 0.5% (w/v) sodium chloride were added. The cell suspensions were incubated in a thermal bath at 37 °C and pH was gradually decreased by the addition of HCl (1 and 0.1 N), until pH 2.0 in a time period of 90 min. Cultures were then centrifuged and resuspended in a 1% (w/v) bovine bile (Sigma) PBS (0.1 M, pH 8.0) solution and incubated at 37 °C for 10 min (step 2: bile shock). The cultures were finally centrifuged and resuspended in a 0.3% (w/v) bovine bile (Sigma) + 0.1% (w/v) pancreatin from porcine pancreas (Sigma) PBS (0.1 M, pH 8.0) solution and kept 90 min at 37 °C (step 3: intestinal digestion). At the beginning of the experiment and at the end of each step, colony counts on MRSc agar were performed.
Technological characterisation
Growth in in-house and whey-based media
B. lactis INL1, B. longum LM7a and B. dentium LM8a’ were used in this assay. MRS broth was prepared in-house using ingredients manufactured by local providers (Microquin S.A. and Cicarelli, both from Santa Fe, Argentina). Also, two whey-based culture media were prepared by supplementing 5% (w/v) reconstituted cheese whey powder (Verónica, Lehmann, Argentina) with either 1% (YE1) or 5% (YE5) (w/v) yeast extract (Microquín). The aim of assessing formulated growth media using ingredients from local providers (less expensive than imported commercial media) or a dairy by-product as cheese whey, was to determine their capacity to promote the growth of the strains under study in lower cost culture media, compared to commercial MRS. In all cases, growth media were supplemented with 0.1% (w/v) L-cysteine hydrochloride. Overnight (18 h) cultures of the strains (MRSc, 37 °C, 18 h, anaerobiosis) were centrifuged (2750 g, 10 min, 5 °C), washed twice and resuspended in PBS solution (pH 7.2). Cell suspensions were inoculated (2% v/v) in in-house formulated MRS and in YE1 and YE5 and incubated overnight (37 °C, 18 h, anaerobiosis). Commercial MRS (Biokar) was used as reference medium for assessing bifidobacteria growth capacity. Cell counts were performed on MRSc agar (37 °C, 72 h, anaerobiosis). The usefulness of successive previous transfers was assessed to improve growth in formulated culture media, when necessary.
Cheese whey as lyoprotectant
Overnight cultures (18 h, 37 °C, anaerobiosis) of B. lactis INL1, B. longum LM7a and B. dentium LM8a’ were centrifuged, washed twice in PBS and resuspended in 10% (w/v) cheese whey (Verónica) or 10% (w/v) lactose (Merck) solution. Cell suspensions were aliquoted in glass vials (1 ml/vial), frozen at a freezing rate of approx. 1 °C/min and stored at − 70 °C until freeze-drying. Samples were freeze-dried over 24 h in a single-chamber freeze drier (beta 2–16, Christ, Osterode, Germany). The drying vacuum applied was 37 Pa assuring product temperatures of − 15 °C during primary drying and 25 °C in the secondary drying phase. At the end of the process, vials were sealed under vacuum. Moisture content was assessed gravimetrically in triplicate (101 °C, 20 h). Resistance to simulated gastric digestion (SGD) was assessed prior and immediately after freeze-drying and after 3 weeks of frozen (− 20 °C) or an accelerated (25 °C) storage stability test. Freeze-dried cells were reconstituted with distilled water (1 ml/vial) and were allowed to stand for 15 min at room temperature for complete rehydration. Cell suspensions were brought to pH 2.0 with 1 N and 0.1 N HCl in the presence of 0.5% (w/v) NaCl and 0.5% (w/v) porcine pepsin (Merck) and incubated at 37 °C in a water bath for 90 min. Cell counts were performed before and after SGD.
Growth in biofermentor in cheese whey-based medium and resistance to SGD
B. lactis INL1 was used in this part of the study. In a preliminary series of fermentations, the strain was grown in a 1.5-l biofermentor (Biostat B, Sartorius Stedim Systems, Melsungen, Germany) in 5% (w/v) cheese whey supplemented with some of the ingredients found in MRS broth (yeast extract, glucose, and salts, in the concentrations they are found on MRS) and different pH values (6.5, 5.8 or 5.0) and modes (free or constant pH) were evaluated. Furthermore, the usefulness of cheese whey protein hydrolysis was also assessed. For enzymatic hydrolysis, 5% (w/v) cheese whey was prepared, transferred to the biofermentor, and 0.3% (w/w) pancreatic trypsin (Novo 6.0S, Type Saltfree, Novozymes, Bagsvaerd, Denmark) was added. Cheese whey was incubated at 37 °C and pH 7.5 (adjusted with 8N NaOH) for 90 min to allow hydrolysis, prior to sterilisation. Finally, a formulation based on the composition of MRS broth, called W-MRS, was selected: 5% (w/v) cheese whey, hydrolysed with pancreatic trypsin, was supplemented with all MRS ingredients except for meat extract and polypeptone (protein sources), and glucose content was decreased by half (from 2% (w/v) in MRS to 1% (w/v) in W-MRS). B. lactis INL1 was grown in W-MRS under two conditions: (a) pH 6.5 (15 h) followed by a moderate acid stress induced with lactic acid (Merck) (pH 5.0, 4 h) and (b) pH free (initial pH 5.8) (15 h) followed by addition of 8N NaOH (Merck) to keep pH at 5.0 for 4 h. The biofermentor was inoculated (2% v/v) with an overnight culture of the strain grown in MRSc, washed twice and resuspended in PBS. The initial concentration in the biofermentor was similar for both conditions (7.00 and 7.04 log CFU/ml, for condition a) and b), respectively. Biomass production was conducted at 37 °C with a CO2 influx of 0.2 l/min and a stirring rate of 150 rpm. Samples of the fermentation medium (500 ml) were taken at 15 and 19 h of culture, centrifuged (2750 g, 10 min., 5 °C), washed twice with PBS and suspended in 10% (w/v) lactose solution. Cell suspensions were frozen at − 70 °C until further processing. Samples were freeze-dried and the resistance to SGD was assessed as described in the previous item.
Statistical analysis
One-way ANOVA and Tukey’s post hoc comparison were used to evaluate the resistance to SGID and data from growth capacity and resistance to freeze-drying, storage and SGD in cheese whey. Student’s t test was used to analyse the growth in biofermentor. Data were analysed using GraphPad Prism software version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Statistical significance was set at p < 0.05.
Results
Isolation and identification of bacteria from breast milk
Ten mothers (identified as LM1 to LM10) provided breast milk samples between day 1 and week 8PP. Information about donors and isolates is summarised in Table 1. RAPD analysis allowed us to evaluate the genetic diversity among isolates, prior to the molecular identification. In particular, primer B10 (Fig. 1) showed at least 2 RAPD types for LM1, LM4 and LM7, and at least 4 RAPD types for LM5 and LM8. Two bifidobacterial strains, B. longum LM7a (isolated on day 9 PP) and B. dentium LM8a’ (isolated on day 20 PP), and four lactobacilli strains (L. gasseri LM8b, L. salivarius LM8a, L. salivarius LM8h and L. vaginalis LM8f, isolated between week 3 and 4 PP) were identified based on the nucleotidic sequence of their 16S rRNA gene. The differences between the two L. salivarius strains from mother LM8 could be corroborated through their RAPD profiles. The rest of the isolates belonged to the genera Propionibacterium and Actinomyces (Table 1).
RAPD profile obtained with primer B10 for 23 breast milk isolates (named with letters) from five donating mothers (LM1, LM4, LM5, LM7 and LM8). N, negative control; M, Tracklt 1Kb plus DNA Ladder (Invitrogen). Isolates were subsequently identified by 16S rRNA sequencing as members of the genera Bifidobacterium (LM7a and LM8a’) and Lactobacillus (LM8a, LM8b, LM8f and LM8h) or as Propionibacterium or Actinomyces
Safety and functional characterization of breast milk bifidobacteria
In this work, the E-test® method was used to analyse the susceptibility of the bifidobacteria strains to clindamycin, chloramphenicol, erythromycin, streptomycin, tetracycline and vancomycin. MIC values are shown in Table 2. TC resistance was also analysed by the microdilutions (MD) broth method (Table 2). The TC resistance of B. lactis INL1 was corroborated and was detected also for B. lactis INL2 (not detected before by the E-test®). When hydrophobicity was studied, all isolates showed low values (20.1 ± 2.0% for B. lactis strains and 18.4 ± 0.4 and 3.7 ± 0.2% for B. longum LM7a and B. dentium LM8a’, respectively). As shown in Table 3, all strains of B. lactis were able to resist the simulated gastric digestion and bile shock steps. However, the exposure to pancreatin combined with bile salts caused a reduction of 3 to 5 log orders in the cell counts of the strains of B. lactis, while the B. longum strain showed more resistance (0.4 log orders).
Technological characterization of the isolates
Growth in in-house and whey-based media
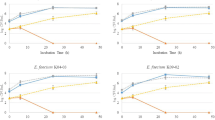
Figure 2 shows the levels of viable cells achieved by the strains in in-house MRS and in whey-based YE1 and YE5 formulated media, compared to commercial MRS (Biokar; reference medium). Overall, B. lactis INL1 was the strain with most satisfactory growth in all tested media.
Cell counts of B. lactis INL1, B. longum LM7a and B. dentium LM8a’ in in-house (double dagger; white square) and commercial (black square) MRS broth (18 h; 37 °C, anaerobiosis), and in 5% (w/v) cheese whey + 1% (w/v) yeast extract (white triangle) or 5% (w/v) yeast extract (black triangle) (YE1 e YE5, respectively). Values are the mean ± SD. Superscript lowercase letters indicate values significantly different (ANOVA + Tukey’s multiple comparative test; p < 0.05) for the same strain. A single asterisk indicates at least two successive subcultures needed. A double dagger indicates same composition of commercial MRS broth but prepared with ingredients from local companies
Cheese whey as lyoprotectant
The usefulness of cheese whey as lyoprotectant, as compared to lactose, was assessed. Freeze-dried cultures were subjected to an accelerated storage test (storage at 25 °C, 3 weeks) and to SGD. B. lactis INL1 reached ca. 10 log orders after freeze-drying (FD), and these levels of viable cells were maintained after storage and SGD, regardless the lyoprotectant used (Fig. 3a). B. longum LM7a did not show any significant decay after FD, but a significant 2 log-order reduction was observed during the SGD of FD cultures (Fig. 3b), for both lyoprotectants. When stored at 25 °C, a significant higher loss in viability was observed for lactose (2.7 log orders) compared to cheese whey (1.2 log order). However, when these cultures were subjected to SGD, the reduction in viability was higher for cheese whey so, similar counts were obtained for both protectants after SGD (inactivation of 3.5 log orders). Finally, B. dentium LM8a’ showed a higher sensitivity to freeze drying with viability losses of 2.8 and 2.1 log orders for lactose and cheese whey, respectively) and accelerated storage test (Fig. 3c).
Cell counts of a concentrated culture (C) of B. lactis INL1 (a), B. longum LM7a (b) and B. dentium LM8a’ (c), after freeze-drying (FD) in 10% (w/v) cheese whey (gray square) or 10% (w/v) lactose (dark-gray square), after simulated gastric digestion (SGD) and storage for 3 weeks at − 20 °C (S-20) or 25 °C (S25). Each column named SGD indicates cell counts after simulated digestion of the culture on the left. Values are the mean ± SD (n = 3). A single asterisk indicates significant differences for a condition and its respective DGS. A double dagger significant differences within the same condition between different lyoprotectants. A single section sign indicates significant differences between successive technological steps (concentration/freeze-drying; freeze-drying/storage) (ANOVA + Tukey’s multiple comparative test; p < 0.05)
Growth in biofermentor in cheese whey-based medium and resistance to SGD
B. lactis INL1 was grown in a 1.5-l biofermentor in the in-house-prepared W-MRS broth under two different conditions. After 19 h of fermentation, similar cell counts (ca. 109 CFU/ml) were obtained under both fermentation conditions (Fig. 4). However, in the first 15 h of fermentation at pH 6.5, cell counts were significantly lower (p = 0.043) compared to growth in non-controlled pH conditions and, when pH was switched from 6.5 to 5.0 (and maintained for 4 h), B. lactis INL1 reached 9.0 log orders. For fermentation without pH control, 2 log orders of growth, from the inoculation level, were obtained in the first 15 h of culture and, when pH was brought to pH 5.0, the level of viable cells remained constant.
Cell counts of B. lactis INL1 grown in a cheese whey-based culture medium (W-MRS) at pH 6.5 constant (15 h) + pH 5.0 (4 h) (gray square) and at pH free (initial pH 5.8, 15 h) + pH 5.0 (4 h) (black square). Values expressed as mean ± SD (n = 2). A double dagger indicates significant differences for the same time, between different fermentation conditions. A single asterisk indicates significant differences within the same fermentation condition with respect to harvesting time (p < 0.05; Student’s t test)
When freeze-dried cultures were subjected to SGD, cells grown without pH control + moderate acid stress (pH 5.0, 4 h), were the most resistant ones to SGD (Table 4). Cells from this fermentation but harvested after 15 h (prior to exposure to pH 5.0) were more sensitive to SGD, although losses of cell viability were of ca. 1 log order. In contrast, cells grown at pH 6.5 (15 h) were the most sensitive ones to SGD with a cell decay of ca. 4 log orders, and this sensitivity to acidity could not be overcame when cells underwent a moderate acid stress during growth.
Discussion
In the last years, the studies on breast milk microbiota have increased, focusing particularly on its composition and effects on the infant health (Fernández et al. 2013; Pannaraj et al. 2017; Williams et al. 2017). In a lesser extent, the isolation and functional characterisation of potential new probiotics from this source has been performed, being the main features studied the antibiotic resistance, the adhesion capacity to epithelial cell lines, survival to simulated gastrointestinal passage and antagonistic activity against enteric pathogens (Arboleya et al. 2011; Kozak et al. 2015; Martín et al. 2005; Reis et al. 2016). Less attention has been paid to the characterisation of breast milk isolates from a technological point of view. In this study, bifidobacteria and lactobacilli strains with probiotic potential were isolated and diversity was evaluated by RAPD analysis. Whereas, primer B10 was useful to differentiate some of the isolates, primers M13 and 1254 were not as effective. As for primer B08, none of the presumptive bifidobacterial isolates in this trial showed a profile for this primer, whereas in our previous sampling trial (Zacarías et al. 2011) 4 out of 6 isolates, identified later as B. lactis (strains INL1, INL2, INL4 and INL5), were differentiated with it and from the commercial strain B. lactis Bb12. Following RAPD analysis, all the potential candidates were identified by partial sequencing of the 16S rRNA gene. No Bifidobacterium or Lactobacillus strains were found on the colostrum stage, whereas positive isolates were found on the transitional milk and early mature milk stages. All strains isolated in this work belonged to species already described as part of breast milk microbiota (Jeurink et al. 2013).
In this study, B. longum LM7a and B. dentium LM8a’ were isolated from different mothers at days 9 and 20 PP, and they were present in breast milk in levels of 1.48 and 2.51 log CFU/ml, respectively. These values are in the range of those found in our previous sampling trial (Zacarías et al. 2011) and of qPCR estimates reported by Grönlund et al. (2007), Gueimonde et al. (2007) and Martín et al. (2009). Several factors influence the composition of breast milk microbiota including the mode of delivery. Differences in the abundances of specific bacterial genera and a higher diversity have been described in the colostrum (Toscano et al. 2017) and breast milk (Cabrera-Rubio et al. 2015) from mothers with vaginal deliveries compared to C-section ones. An interesting observation that emerged from both trials performed by our group is the fact that all bifidobacteria or lactobacilli isolates came from mothers who had pregnancies in term and vaginal delivery. To further study the factors influencing microbiota composition in breast milk, a larger sample size and more complex tools as qPCR or metagenomics should be used. However, this kind of study goes beyond the scope of the present work.
Currently, the determination of antibiotic resistance is a safety criterion that must be studied for potential probiotic strains (EFSA 2008; FAO / WHO 2002). In 2012, the FEEDAP Panel (EFSA) issued a guide recommending the determination of MIC for some antimicrobial substances. As previously reported for Bifidobacterium (Ammor et al. 2008), in this study, the strains showed in general a high resistance to streptomycin and a marked sensitivity to clindamycin, chloramphenicol, erythromycin and vancomycin. MIC values detected were similar to those reported by Mättö et al. (2007) for strains of B. lactis. In particular, for B. lactis INL1 a marked resistance to tetracycline was observed, both by E test and the MD method, indicating some diversity among the isolates. Tetracycline (TC) resistance has been reported as the most common resistance within the genus Bifidobacterium, showing all strains of B. lactis studied so far an average degree of resistance to it (Gueimonde et al. 2010). As for B. lactis INL2, its resistance to TC was only detected by MD method. Although in general a good correlation between both methods has been observed for different antibiotics (Huys et al. 2010; Kushiro et al. 2009), MD is the method recommended by the EFSA guidance. Particularly, lower MICs were reported by the E test compared to the MD method for TC (Mayrhofer et al. 2008; Tosi et al. 2007). The most abundant genetic determinants responsible for resistance to this antibiotic in bifidobacteria are the tet genes encoding ribosomal protection proteins. In order to ensure the safety of these strains for potential inclusion in food formulations, the presence of resistance genetic determinants and the nature of resistance, whether intrinsic or acquired, should be determined (EFSA 2012). The industrial implications of tet resistance genes in B. lactis strains has been approached, in particular considering the safety of commercial strains as B. lactis Bb12 and HNO19 (FDA GRAS Notices No. GRN 000049 (2005), and GRN 000445 (2013), respectively), but so far, the presence of these resistance genes does not change the GRAS status of B. lactis by FDA or the QPS by EFSA.
Adhesion of probiotics to the gastrointestinal surface is associated with the competitive exclusion of pathogens, persistence in the gut and modulation of the immune response (Lee and Puong 2002), and the measure of hydrophobicity has been used as a first indicator of adhesion capacity (Vinderola et al. 2004). The low values observed for all isolates in this study are in coincidence with previous reports for strains of B. lactis and B. longum (Mättö et al. 2004; Souza et al. 2013). The presence of reduced values of cell surface hydrophobicity in some probiotic strains as B. lactis Bb12 has been correlated with a defined balance of cell components, which apparently helps to ensure the resistance to adverse conditions, improves viability, and hence the overall probiotic properties of bacteria (Shakirova et al. 2013). Considering this, the low values found for the bifidobacteria isolates should not discourage their further study as probiotic candidates.
The resistance to gastrointestinal transit is another in vitro selection parameter for potential probiotics. Currently, the available tests usually involve measuring the survival in a medium acidified with HCl (pH 2–3) containing NaCl and pepsin, the subsequent use of bile salts (Mainville et al. 2005) and less frequently, the resistance to pancreatic digestion. All strains of B. lactis were found resistant to simulated gastric digestion and to exposure to bile salts. Resistance to acidity has been reported in previous studies for B. lactis, this tolerance may be due to an increase in the activity of H+-ATPase pump (Matsumoto et al. 2004). In a comparative test, Mättö et al. (2006) also observed reductions ≤ 1 log order for fresh cultures of two strains of B. lactis after 2 h at pH 2.0 + pepsin, whereas in the absence of pepsin the cultures showed a great susceptibility, so that pepsin would fulfil a protective role as a protein not yet activated by low pH. However, for other species of the genus Bifidobacterium, including a strain of B. longum, the protective effect of pepsin was not observed and the strains were highly sensitive to acidic pH, although in our study B. longum LM7a was highly resistant. Since bile salts have considerable antimicrobial activity at physiological concentrations, resistance to bile is important for the colonisation and persistence of intestinal microorganisms and is therefore one of the criteria for the selection of new probiotic bacteria (Grimm et al. 2014). When exposed to pancreatin and bile salts, all B. lactis strains were affected, whereas B. longum LM7a showed more resistance. This susceptibility to pancreatin in B. lactis is consistent with the results obtained by Masco et al. (2007), where a comparative study between members of the genus Bifidobacterium showed that strains belonging to the species B. lactis showed less tolerance towards the pancreatic enzyme, while a strain-dependent resistance was observed in case of B. longum.
The usefulness of cheese whey for biomass production of specific strains of lactobacilli (Lavari et al. 2014; Pérez Guerra et al. 2007) and bifidobacteria (Balciunas et al. 2016; Corre et al. 1992) was reported. When the growth in in-house and whey-based media was evaluated, no significant differences in the final cell counts of B. lactis INL1 were observed in all formulated media, although to be able to grow in YE1, at least one subculture (2% (v/v)) in this medium was needed. B. longum LM7a also showed similar growth in formulated and reference media but, while B. lactis INL1 showed satisfactory growth (ca. 2 log orders), B. longum LM7a only increased cell counts in ca. 1.5 log orders, irrespective of the culture medium. Also, B. longum LM7a had difficulties to grow satisfactorily in whey-based media when inoculated as washed cells obtained in MRS, and the strain required 48 h of incubation plus at least one subculture in YE1 and YE5 to reach the same levels than in commercial MRS, suggesting perhaps the need of adaptation to this medium. Finally, for B. dentium LM8a’, significant differences between in-house and commercial MRS were observed, and the strain showed difficulties to grow in the formulated medium. However, when grown in whey-based media, a satisfactory growth was observed although in YE1 two successive subcultures in this medium were needed in order to reach similar levels of viable cells compared to MRS and YE5. From the three bifidobacteria strains studied, B. lactis INL1 showed the most satisfactory growth in all formulated media, and only one previous subculture was needed for YE1 medium.
Cheese whey seemed to be a slightly better protectant than lactose, although statistical differences were only detected during the SGD of the freeze-dried cultures. The fact that cheese whey showed better results than lactose for B. longum LM7a in the accelerated storage test and during some SGD stages for both B. longum LM7a and B. dentium LM8a’, could be explained by its composition. The protectant material composed of constituents of the same nature (carbohydrates or proteins), may not be as effective as when a mixture of carbohydrates and proteins is used (Young et al. 1993; Choi et al. 2010). The presence of molecules of different size, as occur in cheese whey, may contribute to a better protection compared to ingredients constituted by a single type of molecule (Carvalho et al. 2004). It can be hypothesised that all breast milk strains have functional relevance in the gut (and then they could all be potential probiotics) as they are naturally transferred from the mother to the child for early colonisation (Vael and Desager 2009) and maturation of the gut mucosal immune system (Romano-Keeler and Weitkamp 2015). However, from our results, it can be observed that not all breast milk isolates may display also technological potential to be manipulated in the laboratory or in the food industry. In this preliminary technological characterisation, the most satisfactory results were observed for B. lactis INL1. Some strains of B. longum have industrial importance not only for their function as probiotics (B. longum BB536 Morinaga, B. longum ES1) but also for their potential for the biosynthesis of products with added value for the food industry such as ß-galactosidase (Hsu et al. 2007; Han et al. 2014). However, the results obtained for B. longum LM7a in this study were not satisfactory and a first challenge would be to optimise the culture and dehydration conditions and then further evaluate its potential as probiotic. B. dentium was described as an opportunistic pathogen, since it is part of the ecology of the oral cavity (Ventura et al. 2009), and it was included in this study more for academic reasons rather than for a potential application, as safety issues were raised for this species.
The probiotic potential of B. lactis INL1 has been previously evaluated, showing immunomodulatory and protective properties in salmonellosis and acute and chronic colitis models (Burns et al. 2017; Zacarías et al. 2011, 2014, 2017). Based on this fact together with the results obtained in this study, the strain was selected for further technological characterisation. Results from biomass production in biofermentor showed and effect of the culture conditions particularly on the survival of the freeze-dried cultures to SGD. Cells grown at pH close to neutrality were more sensitive to SGD and this effect could not be overcame even when a mild acid stress was applied. The influence of growth conditions and other processing parameters on cell functionality has been previously described for probiotic bacteria. Vinderola et al. (2012) showed higher sensitivity to gastric acidity in B. lactis INL1 grown in MRS at pH 6.5 compared to cells grown at pH 5.0, for the same harvesting time. Deepika et al. (2009, 2012) found that other functionality parameters such as hydrophobicity and adhesion to Caco-2 cells were not only dependent on the pH at which the biomass of L. rhamnosus GG was produced, with a significant increase in this parameters when fermentations were carried out at pH 5.0, but also on the growth phase and temperature of fermentation. Moreover, Grześkowiak et al. (2011) demonstrated that the same probiotic strain, isolated from different food or pharma matrices, displayed different inhibitory capacity towards food pathogens, indicating that the manufacturing processes might have permanently altered the functional properties of the strain. In this regard, even if the use of different types of sub-lethal stress is a widely extended strategy to enhance the resistance to environmental stresses occurring during production, storage or digestion (Santos et al. 2016), it is necessary to evaluate the stability also in terms of functional properties to ensure that probiotics confer the expected health benefit (Gueimonde and Sánchez 2012).
In this study, the presence of bifidobacteria and lactobacilli in breast milk was observed only until week 4 postpartum. The isolates corresponding to the genus Bifidobacterium were studied and some safety, functional and technological characteristics were determined in order to detect potential probiotic candidates. Resistance to antibiotics was consistent with those reported by other authors, and in particular, resistance to tetracycline for strains B. lactis INL1 and INL2 should be further studied. Low hydrophobicity and satisfactory resistance to gastrointestinal digestion were observed in general for all strains of B. lactis (INL1, INL2, INL4 and INL5) and B. longum LM7a. The use of cheese whey as ingredient for the biomass production of bifidobacteria is promising, although the composition of the media should be optimised on a strain basis. Furthermore, cheese whey was effective in protecting some strains during freeze-drying and simulated gastrointestinal digestion. Whereas B. lactis INL1 showed technological potential, with satisfactory growth in different culture media and resistance to freeze-drying, accelerated storage and acidity, B. longum LM7a and B. dentium LM8a’ were more sensitive to these treatments. These results are encouraging to further study the potential of B. lactis INL1 and support the idea that not all isolates from breast milk may be potential probiotics, as many technological difficulties might arise for managing some of these strains outside their natural ecological niche.
References
Ammor MS, Flórez AB, van Hoek AH, de Los Reyes-Gavilán CG, Aarts HJ, Margolles A, Mayo B (2008) Molecular characterization of intrinsic and acquired antibiotic resistance in lactic acid bacteria and bifidobacteria. J Mol Microbiol Biotechnol 14:6–15
Arboleya S, Ruas-Madiedo P, Margolles A, Solís G, Salminen S, de Los Reyes-Gavilán CG, Gueimonde M (2011) Characterization and in vitro properties of potentially probiotic Bifidobacterium strains isolated from breast-milk. Int J Food Microbiol 149(1):28–36
Balciunas EM, Al Arni S, Converti A, Leblanc JG, Oliveira RP dS (2016) Production of bacteriocin-like inhibitory substances (BLIS) by Bifidobacterium lactis using whey as a substrate. Int J Dairy Technol 69:236–242
Binetti A, Suárez V, Tailliez P, Reinheimer J (2007) Characterization of spontaneous phage-resistant variants of Streptococcus thermophilus by randomly amplified polymorphic DNA analysis and identification of phage-resistance mechanisms. Int Dairy J 17:1115–1122
Boix-Amorós A, Collado MC, Mira A (2016) Relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Front Microbiol 7:492
Burns P, Lafferriere L, Vinderola G, Reinheimer J (2014) Influence of dairy practices on the capacity of probiotic bacteria to overcome simulated gastric digestion. Int J Dairy Technol 67:448–457
Burns P, Alard J, Hrdy J, Boutillier D, Páez R, Reinheimer J, Pot B, Vinderola G, Grangette C (2017) Spray-drying effect on the protective capacity of the breast milk-derived Bifidobacterium lactis INL1 on acute and chronic colitis in mice. Sci Rep 7:43211
Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A (2012) The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 6(3):544–551
Cabrera-Rubio R, Mira-Pascual L, Mira A, Collado MC (2015) Impact of mode of delivery on the milk microbiota composition of healthy women. J Dev Orig Health Dis 7(1):54–60
Cardamone L, Quiberoni A, Mercanti DJ, Fornasari ME, Reinheimer JA, Guglielmotti DM (2011) Adventitious dairy Leuconostoc strains with interesting technological and biological properties useful for adjunct starters. Dairy Sci Technol 91(4):457–470
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P (2004) Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int Dairy J 14:835–847
Chassard C, de Wouters T, Lacroix C (2014) Probiotics tailored to the infant: a window of opportunity. Curr Opin Biotechnol 26:141–147
Choi K, Ryu J, Kwak H (2010) Spray-dried conjugated linoleic acid encapsulated with maillard reaction products of whey proteins and maltodextrin. Food Sci Biotechnol 19:957–965
Corre C, Madec MN, Boyaval P (1992) Production of concentrated Bifidobacterium bifidum. J Chem Technol Biotechnol 53:189–194
Deepika G, Green RJ, Frazier RA, Charalampopoulos D (2009) Effect of growth time on the surface and adhesion properties of Lactobacillus rhamnosus GG. J Appl Microbiol 107(4):1230–1240
Deepika G, Karunakaran E, Hurley CR, Biggs CA, Charalampopoulos D (2012) Influence of fermentation conditions on the surface properties and adhesion of Lactobacillus rhamnosus GG. Microb Cell Factories 11:116
Doleyres Y, Lacroix C (2005) Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int Dairy J 15:973–988
Edwards U, Rogall T, Blockerl H, Emde M, Bottger E (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853
European Food Safety Authority (EFSA) Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (2008) Technical guidance - update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance. EFSA J 732:1–15
European Food Safety Authority (EFSA) Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10:2740
FAO/WHO (2002) Guidelines for the evaluation of probiotics in food. Food and Agriculture Organization of the United Nations and World Health Organization Working Group report. Available at ftp://ftp.fao.org/es/esn/food/wgreport2.pdf. Accessed 17/09/2017
FDA (2005) Additional correspondence, GRAS notice n° GRN 000049. Available at http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154391.htm. Accessed 17/09/2017
FDA (2013) GRAS notice n° GRN 000445. Available at http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm352951.htm. Accessed 17/09/2017
Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM (2013) The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 69(1):1–10
Giraffa G, Andrighetto G, Antonello C, Gatti M, Lazzi C, Marcazzan G, Lombardi A, Neviani E (2004) Genotypic and phenotypic diversity of Lactobacillus delbrueckii subsp. lactis strains of dairy origin. Int J Food Microbiol 91:129–139
Gotteland M, Cruchet S, Brunser O (2011) Functional food in child nutrition. In: Functional food product development. Wiley, Chicester, pp 440–458
Grimm V, Westermann C, Riedel CU (2014) Bifidobacteria-host interactions: an update on colonisation factors. Biomed Res Int 2014:1–10. https://doi.org/10.1155/2014/960826
Grönlund MM, Gueimonde M, Laitinen K, Kociubinski G, Grönroos T, Salminen S, Isolauri E (2007) Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin Exp Allergy 37(12):1764–1772
Grześkowiak Ł, Isolauri E, Salminen S, Gueimonde M (2011) Manufacturing process influences properties of probiotic bacteria. Br J Nutr 105(6):887–894
Gueimonde M, Sánchez B (2012) Enhancing probiotic stability in industrial processes. Microb Ecol Health Dis 23. https://doi.org/10.3402/mehd.v23i0.18562
Gueimonde M, Laitinen K, Salminen S, Isolauri E (2007) Breast milk: a source of bifidobacteria for infant gut development and maturation? Neonatol 92(1):64–66
Gueimonde M, Flórez AB, van Hoek AH, Stuer-Lauridsen B, Strøman P, de los Reyes-Gavilán CG, Margolles A (2010) Genetic basis of tetracycline resistance in Bifidobacterium animalis subsp. lactis. Appl Environ Microbiol 76:3364–3369
Han YR, Youn SY, Ji GE, Park MS (2014) Production of α- and β-galactosidases from Bifidobacterium longum subsp. longum RD47. J Microbiol Biotechnol 24(5):675–682
Heller KJ (2001) Probiotic bacteria in fermented foods: product characteristics and starter organisms. Am J Clin Nutr 73:374S–379S
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514
Hsu CA, Yu RC, Lee SL, Chou CC (2007) Cultural condition affecting the growth and production of beta-galactosidase by Bifidobacterium longum CCRC 15708 in a jar fermenter. Int J Food Microbiol 116(1):186–189
Huys G, D'Haene K, Cnockaert M, Tosi L, Danielsen M, Flórez AB, Mättö J, Axelsson L, Korhonen J, Mayrhofer S, Egervärn M, Giacomini M, Vandamme P (2010) Intra- and interlaboratory performances of two commercial antimicrobial susceptibility testing methods for bifidobacteria and nonenterococcal lactic acid bacteria. Antimicrob Agents Chemother 54(6):2567–2574
Jankovic I, Sybesma W, Phothirath P, Ananta E, Mercenier A (2010) Application of probiotics in food products—challenges and new approaches. Curr Opin Biotechnol 21:175–181
Jeurink PV, van Bergenhenegouwen J, Jiménez E, Knippels LM, Fernández L, Garssen J, Knol J, Rodríguez JM, Martín R (2013) Human milk: a source of more life than we imagine. Benefic Microbes 4:17–30
Kozak K, Charbonneau D, Sanozky-Dawes R, Klaenhammer T (2015) Characterization of bacterial isolates from the microbiota of mothers’ breast milk and their infants. Gut Microbes 6(6):341–351
Kushiro A, Chervaux C, Cools-Portier S, Perony A, Legrain-Raspaud S, Obis D, Onoue M, van de Moer A (2009) Antimicrobial susceptibility testing of lactic acid bacteria and bifidobacteria by broth microdilution method and Etest. Int J Food Microbiol 132(1):54–58
Lavari L, Páez R, Cuatrin A, Reinheimer J, Vinderola G (2014) Use of cheese whey for biomass production and spray drying of probiotic lactobacilli. J Dairy Res 81(3):267–274
Le Huërou-Luron I, Blat S, Boudry G (2010) Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 23:3–36
Lee YK, Puong KY (2002) Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Br J Nutr 88(1):S101–S108
Mainville I, Arcand Y, Farnworth ER (2005) A dynamic model that simulates the human upper gastrointestinal tract for the study of probiotics. Int J Food Microbiol 99(3):287–296
Maldonado J, Cañabate F, Sempere L, Vela F, Sánchez AR, Narbona E, López-Huertas E, Geerlings A, Valero AD, Olivares M, Lara-Villoslada F (2012) Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr 54(4):55–61
Martín R, Olivares M, Marín ML, Fernández L, Xaus J, Rodríguez JM (2005) Probiotic potential of 3 Lactobacilli strains isolated from breast milk. J Hum Lact 21(1):8–17
Martín R, Jiménez E, Heilig H, Fernández L, Marín ML, Zoetendal EG, Rodríguez JM (2009) Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol 75:965–969
Masco L, Crockaert C, Van Hoorde K, Swings J, Huys G (2007) In vitro assessment of the gastrointestinal transit tolerance of taxonomic reference strains from human origin and probiotic product isolates of Bifidobacterium. J Dairy Sci 90:3572–3578
Matsumoto M, Ohishi H, Benno Y (2004) H+-ATPase activity in Bifidobacterium with special reference to acid tolerance. Int J Food Microbiol 93:109–113
Mättö J, Malinen E, Suihko ML, Alander M, Palva A, Saarela M (2004) Genetic heterogeneity and functional properties of intestinal bifidobacteria. J Appl Microbiol 97(3):459–470
Mättö J, Alakomi HL, Vaari A, Virkajarvi I, Saarela M (2006) Influence of processing conditions on Bifidobacterium animalis subsp. lactis functionality with a special focus on acid tolerance and factors affecting it. Int Dairy J 16:1029–1037
Mättö J, van Hoek AH, Domig KJ, Saarela M, Flórez AB, Brockmann E, Amtmann E, Mayo B, Aarts HJ, Danielsen M (2007) Susceptibility of human and probiotic Bifidobacterium spp. to selected antibiotics as determined by the Etest method. Int Dairy J 17:1123–1131
Mayrhofer S, Domig KJ, Mair C, Zitz U, Huys G, Kneifel W (2008) Comparison of broth microdilution, Etest, and agar disk diffusion methods for antimicrobial susceptibility testing of Lactobacillus acidophilus group members. Appl Environ Microbiol 74(12):3745–3748
Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, Bailey A, Bushman FD, Sleasman JW, Aldrovandi GM (2017) Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr 171(7):647–654
Pérez Guerra N, Fajardo Bernárdez P, Méndez J, Cachaldor P, Pastrana Castro L (2007) Production of four potentially probiotic lactic acid bacteria and their evaluation as feed additives for weaned piglets. Anim Feed Sci Technol 134:89–107
Reis NA, Saraiva MA, Duarte EA, de Carvalho EA, Vieira BB, Evangelista-Barreto NS (2016) Probiotic properties of lactic acid bacteria isolated from human milk. J Appl Microbiol 121(3):811–820
Romano-Keeler J, Weitkamp JH (2015) Maternal influences on fetal microbial colonization and immune development. Pediatr Res 77(1–2):189–195
Saad N, Delattre C, Urdaci M, Schmitter JM, Bressollier P (2013) An overview of the last advances in probiotic and prebiotic field. LWT Food Sci Technol 50:1–16
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Santos M, Tymczyszyn E, Golowczyc M, Mobili P, Gomez-Zavaglia A (2016) Probiotic cell cultivation. In: Foerst P, Santivarangkna C (eds) Advances in probiotic technology. CRC Press, Boca Ratón, pp 45–76
Shakirova L, Grube M, Gavare M, Auzina L, Zikmanis P (2013) Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 cell surface hydrophobicity and survival of the cells under adverse environmental conditions. J Ind Microbiol Biotechnol 40(1):85–93
Souza TC, Silva AM, Drews JRP, Gomes DA, Vinderola CG, Nicoli JR (2013) In vitro evaluation of Bifidobacterium strains of human origin for potential use in probiotic functional foods. Benefic Microbes 4(2):179–186
Toscano M, De Grandi R, Peroni DG, Grossi E, Facchin V, Comberiati P, Drago L (2017) Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol 17(1):205
Tosi L, Berruti G, Danielsen M, Wind A, Huys G, Morelli L (2007) Susceptibility of Streptococcus thermophilus to antibiotics. Antonie Van Leeuwenhoek 92(1):21–28
Vael C, Desager K (2009) The importance of the development of the intestinal microbiota in infancy. Curr Opin Pediatr 21(6):794–800
Ventura M, Turroni F, Zomer A, Foroni E, Giubellini V, Bottacini F, Canchaya C, Claesson MJ, He F, Mantzourani M, Mulas L, Ferrarini A, Gao B, Delledonne M, Henrissat B, Coutinho P, Oggioni M, Gupta RS, Zhang Z, Beighton D, Fitzgerald GF, O'Toole PW, van Sinderen D (2009) The Bifidobacterium dentium Bd1 genome sequence reflects its genetic adaptation to the human oral cavity. PLoS Genet 5(12):e1000785
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative in vitro study of probiotic characteristics and biological barrier resistance. Food Res Int 36(9–10):895–904
Vinderola CG, Medici M, Perdigón G (2004) Relationships between interaction sites in the gut, hydrophobicity, mucosal immunomodulating capacities and cell-wall protein profiles in lactic acid bacteria. J Appl Microbiol 96(2):230–243
Vinderola G, Zacarías MF, Bockelmann W, Neve H, Reinheimer J, Heller KJ (2012) Preservation of functionality of Bifidobacterium animalis subsp. lactis INL1 after incorporation of freeze-dried cells into different food matrices. Food Microbiol 30(1):274–280
Williams JE, Carrothers JM, Lackey KA, Beatty NF, York MA, Brooker SL, Shafii B, Price WJ, Settles ML, McGuire MA, McGuire MK (2017) Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. J Nutr 147(9):1739–1748
Young SL, Sarda X, Rosenberg M (1993) Microencapsulating properties of whey proteins. Combination of whey proteins with carbohydrates. J Dairy Sci 76:2878–2885
Zacarías MF, Binetti A, Laco M, Reinheimer J, Vinderola G (2011) Preliminary technological and probiotic characterization of bifidobacteria isolated from breast milk for use in dairy products. Int Dairy J 21:548–555
Zacarías MF, Reinheimer J, Forzani L, Grangette C, Vinderola G (2014) Mortality and translocation assay to study the protective capacity of Bifidobacterium lactis INL1 against Salmonella Typhimurium infection in mice. Benefic Microbes 5(4):427–436
Zacarías MF, Souza TC, Zaburlín N, Carmona Cara D, Reinheimer J, Nicoli J, Vinderola G (2017) Influence of technological treatments on the functionality of Bifidobacterium lactis INL1, a breast milk-derived probiotic. J Food Sci 82(10):2462–2470
Acknowledgements
Authors are very grateful to the mothers and medical institutions that participated during the breast milk sampling trial.
Funding
María Florencia Zacarías received a research grant from DAAD (Deutscher Akademischer Austausch Dienst) and a CONICET (Consejo Nacional de Investigaciones Científicas y Tecnológicas) doctoral scholarship. The present work was supported by the following projects: “Desarrollo de cultivos probióticos nacionales a partir de cepas autóctonas de lactobacilos y bifidobacterias,” Proyecto CAI + D Convocatoria 2011, Código 501 201101 00136 LI; “Cultivos microbianos autóctonos para la producción de alimentos funcionales para humanos y animales utilizando secado spray,” Proyecto PICT-2013-0260, ANPCyT Resolución N° 214/14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed in conformity with the Declaration of Helsinki and was approved by the Advisory Committee on Research Ethics and Safety of the Faculty of Biochemistry and Biological Sciences, Universidad Nacional del Litoral, Santa Fe, Argentina.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(XLSX 12 kb)
Rights and permissions
About this article
Cite this article
Zacarías, M.F., Binetti, A., Bockelmann, W. et al. Safety, functional properties and technological performance in whey-based media of probiotic candidates from human breast milk. Int Microbiol 22, 265–277 (2019). https://doi.org/10.1007/s10123-018-00046-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-018-00046-0