Abstract
Colon cancer is the third significant reason for death related to cancers in the world. There are various treatments for colon cancer, which have several side effects. Polyphenol agents are a type of antioxidant in plants that have diverse biological properties, such as anti-cancer effects. Here, we investigate the effect of Trachyspermum ammi essential oil (TEO) and red light irradiation on the colorectal cancer cell line (SW 480). The colorectal cancer cell lines were irradiated at 660 nm for 90 s and then the cells were incubated with different TEO concentrations. In another study, cells initially were treated with various TEO concentrations and then irradiation for 90 s. Effect of TEO and the red light irradiation on viability of the cell, ROS generation, and cell cycle was assessed by MTT and flow cytometry, respectively. The findings demonstrated that early incubation with TEO and then irradiation decreased the SW 480 cells survival more than the early irradiation at 660 nm and then essential oil. In addition, TEO treatment at IC50 concentration in combination with low-level laser irradiation induces ROS generation in SW 480 cells as compared to the dark group. In addition, TEO treatment at IC50 in combination with low-level laser irradiation induces G0/G1 arrest of the cell cycle in SW 480 cells in comparison to the dark group. This study revealed that the Trachyspermum ammi essential oil in combination with low-level laser results in more reduction in survival which leads to ROS generation and cell cycle arrest in SW 480 colorectal cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colon or colorectal cancer (CRC) is the third greatest prevalent reason for cancer-related deaths in the world, which commonly occurs in the colon or rectum [1,2,3]. The walls of the colon and rectum are made of several layers. CRC starts in the lining of the mucosa and can spread to other layers or layers, so cancer cells that are in the wall can grow in blood vessels or lymphatic vessels and move to nearby or distant lymph nodes in the body [4,5,6]. It usually takes a long time for abnormal cells to grow and develop colorectal cancer. As a result, regular screening can prevent patients from death [7]. CRC is a serious health risk in most developed countries [8]. It has been suggested that regular exercise and physical activity could reduce the risk of colon cancer [9]. Also, It is recommended to eat foods rich in calcium and vitamin D3 and enough vegetables and fruits and should limit fat, red meat [10], plus processed meats for decreasing the risk of colorectal cancer [11,12,13,14,15]. Conventional treatments for colorectal cancer, like other cancers, include surgery, chemotherapy, and radiation therapy which have various side effects [16,17,18]. The application of medicinal plants in cancer treatment has shown that medicinal plants are an excellent and reliable source for the progress of new anti-cancer drugs [19, 20], around the world due to fewer side effects [21].
Traychspermum ammi is known as Anison Berry, Abyssinian Commune, Ajwan, Spray Kai, and Nankhah. Traychspermum ammi is an annual, hairless, and 30- to 90-cm-tall herbaceous plant that raises in the eastern regions of Iran, Egypt, and India [22, 23]. This plant is used in the treatment of gastrointestinal diseases, anorexia, and bronchial problems [24, 25]. Photobiomodulation from red to infrared (NIR) wavelength has low heat effects on cells. The therapeutic effects of this method appear to be due to photochemical reactions that alter the permeability of the cell membrane and subsequently increase the formation of mRNA and cell proliferation [26, 27]. Low-level laser cellular action is based on absorbing red and infrared light by chromophores or light receptors in mitochondria, producing nitric oxide (NO), reactive oxygen species (ROS), ultimately increasing ATP, and activating antioxidant enzymes such as catalase and peroxidase [28, 29]. Photobiomodulation can be used as a safe and effective multifunctional treatment that could potentially be used with other therapies or as an independent method in cancer treatment and other complex diseases [26, 30].
In this study for the first time, we studied the Traychspermum ammi essential oil (TEO) effects with red light irradiation on colon cancer cells (SW 480) and evaluated the ROS generation and cell cycle changes.
Methods
Materials
Acridine orange, trypan blue solution 0.4%, 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazoliumbromide (MTT), ethidium bromide, polysorbate 20, gallic acid, and dimethyl sulfoxide (DMSO) were received from Sigma-Aldrich (St Louis, MO, USA). Fetal bovine serum (FBS), phosphate buffer saline (PBS), and antibiotics (penicillin and streptomycin) were acquired from Gibco (Gibco BRL). Dulbecco’s Modified Eagle Medium (DMEM) was prepared from Invitrogen (Invitrogen, Carlsbad, CA, US). All the other materials were obtained from Merck.
Traychspermum ammi essential oil preparation and analysis
Essential oil preparation
Trachyspermum ammi seeds were purchased from a medicinal plant market (Shiraz, Iran). The seeds were ground in a mechanical grinder and the essential oil was obtained from 100 g of ground powder by the hydro-distillation method using clevenger apparatus for 3 h. The essential oil usually floats on top of the aromatic water and was apart from aromatic water through a decanting funnel. The aromatic water and essential oil separately were collected in a glass container and kept in the refrigerator (4 ± 1 °C) and protected from light until used. Since the obtained essential oil is hydrophobic and fat-soluble, a water-soluble emulsion was prepared for ease of work. To make polysorbate-essential oil-aromatic water emulsion, 1 mL of essential oil included in 100 mL of aromatic water. Polysorbate-20 (100 µg/mL) included in the essential oil-aromatic water mixture and the mixture kept at 30 °C for 24 h and then at 4 °C for 48 h. At this stage, a milky emulsion created which applied for further experiments.
Essential oil analysis
The analysis of essential oil elements was taken by a gas chromatography-mass spectrometry (GC–MS) system (Agilent 5975, USA used a silica capillary HP-5 column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness). A mixture of homologous n-alkanes (C8–C25) as standard was injected on the GC column after essential oil through the same chromatographic terms. The constituents of the essential oils were detected by GC–MS apparatus software as compared to their retention indices and mass spectra with standard compounds of NIST library (software library, NIST05a and Wiley 7n Libraries) [31]. Quantitative analysis of each compound relied on the relative area under the curve of each peak in the spectrum.
Cell culture
Colon cancer (SW 480) and human dermal fibroblast (HDF) cell lines were gained from the Institute of Pasture, Tehran, Iran. The SW 480 cells were cultured in DMEM medium plus 10% FBS, 100 IU ml −1 penicillin, and 100 µg ml −1 of streptomycin and then kept in a humidified incubator including 5% CO2 at 37 ºC. For the tests, the cells were detached using trypsin 0.025%, EDTA 0.02%.
Effect of TEO on colon cancer cells
In short, the SW 480 colon cancerous and dermal fibroblast (HDF) cells were planted in plates covered by fresh culture medium and kept at the incubator for 24 h (5% CO2, at 37 °C). Afterward, the cells were incubated with various TEO concentrations including 0, 5, 10, 25, 50, and 100 μg/mL. Following another 24 h, the cells were rinsed using PBS then the MTT test was applied to identify the cell's survival. Every assay was done in triplicate.
In vitro red laser irradiation
Illumination was carried out using a red light source at 660 nm with 30 mW cm−2 power density. The plates were separated into a dark sample (without irradiation), and the cells that irradiated at 660 nm with 3 J/cm2 energy density for 90 s. Irradiation was done in a dark condition. The light source power-meter was undertaken as explained previously [32].
TEO and red light irradiation as pre and post-treatment
The SW 480 colon cancer and dermal fibroblast (HDF) cells (1 × 104 cells) were individually cultures in 96 well plates and kept in an incubator for 24 h as previously mentioned. For the pre-treatment test, first, the cells were illuminated and then treated with various TEO concentrations and kept incubator for 24 h. For the post-treatment test, first, the cells were incubated with various TEO concentrations and after 24 h, one of the plates mentioned as dark (without irradiation (control)) and the other plate under irradiation and kept in the incubator for 24 h. Eventually, the cells were rinsed with PBS and the cell survival was determined using the MTT test. Every assay was done in triplicate.
Cell proliferation assay
In brief, the culture medium was removed and cells were cover by a medium containing 0.5 mg/ml of thiazolyl blue tetrazolium bromide (MTT) for 3–4 h at 37 °C. The purple formazan crystals were solved using DMSO. The absorbance was read at 570 nm by an ELISA reader (Hyperion, Inc., FL, USA). Every assay was done in triplicate and data are shown as the mean ± SD.
Morphology changes determination
Morphological alternations of SW 480 colon cancer cells were determined after incubation in four different conditions: (1) dark (control), these cells were incubated only with culture medium; (2) TEO, these cells were treated by IC50 of TEO (25 µg/ml); (3) laser irradiation, these cells illuminated by red light irradiation for 90 s without TEO; and (4) combination therapy, these cells were treated firstly with IC50 of TEO and then irradiated at 660 nm for 90 s.. The morphology of SW 480 cells was investigated using light inverted microscopy at × 20 magnification.
Apoptosis detection using fluorescence microscopy
The SW 480 cells were seeded and then treated in four different conditions as described previously. Following 24 h after incubation time, the cells were studied using s fluorescent staining by Acridine Orange/Ethidium Bromide (AO/EB) as described in previous work [33]. Morphological alternations as a result of apoptosis death were determined via fluorescence microscopy (BEL, Italy).
ROS production after TEO and irradiation in SW 480 colon cancerous cells
The intracellular generation of ROS was detected by the 7.2-dichlorofluorosine diacetate (DCFH2-DA) test. Therefore, the SW 480 colon cancer cells were seeded in about 106 cells in a petri dish then were incubated by TEO at concentrations of 0 and IC50 for 24 h then were irradiated at 660 nm for 90 s. After that, the cells were treated with 2 mM DCFH2-DA for 45 min in the dark. Later the cells were rinsed with PBS and shifted to a flow cytometer for detecting produced ROS. The results were examined by FlowJo 7.6.1.
Cell cycle determination after treatment with TEO and then irradiation
Around 1 × 106 SW 48 cells/cm2 were incubated with TEO then irradiation after that the cells were rinsed with PBS twice using centrifugation (200 g, 5 min, 4 °C) and were fixed by cold 70% ethanol (24,102; Sigma). The fixed cells were remaining at a minimum at − 20 °C for 2 h. Then, the SW 480 cells centrifuged and resuspended in 300 μL of PBS containing 10 mg/mL PI (P4170; Sigma),100 mg/mL RNAse (PR891628C; SinaClon BioScience, Tehran, Iran), and 10 mL of 0.1% (v/v) Triton X‐100 (108,643; Merck, Germany) in the dark for 15 min. Obtained graphs were assayed by Becton–Dickinson FACS Calibur Flow Cytometer using FlowJo 7.6.1 program.
Statistical analysis
Data were stated as mean of standard deviation (± SD) of at least three independent experiments and analyzed with the Student’s t-test. P values less than 0.05 were used as statistically significant.
Results
TEO chemical composition
TEO chemical composition was analyzed by GC–MS. As shown in Table 1, TEO mainly composed of monoterpenes (58%), and monoterpenoids (42%). Among them, the principal constituents of TEO were identified p-cymene (15.84%), γ-terpinene (38.68%), and thymol (40.25%) (Fig. 1) and the lower elements were hexadecane (1.1%), β-pinene (1.9%) and ethylene methacrylate (6.9%).
Effect of after TEO on SW 480 colon cancer: dark cytotoxicity
The TEO cytotoxicity effect in dark conditions was analyzed by considering the cell viability after 24 h treatment with various TEO including 0, 5, 10, 25, 50, and 100 μg/mL in the dark condition. The results represented that the viability of colon cancer cells reduced in the existence of essential oil and the cell survival was 31.3% at 100 μg /mL of TEO (Fig. 2). As it is shown in Fig. 2 without irradiation at 0, 5, 10, 25, 50, and 100 μg/mL of TEO, the cell survival of SW 480 cell lines was 100%, 95%, 80%, 46.3%, 33%, and 31%, accordingly. Considering the findings, the TEO IC50 on colon SW 480 cell was around 25 µg/mL at dark.
Effect of pre- and post-red light irradiation on TEO effect against SW 480 colon cancer cells
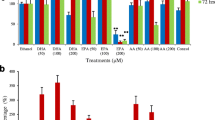
The TEO cytotoxicity impact in the existence of pre and post illumination was determined in SW 480 cells. As illustrated in Fig. 3, in control groups incubated with PBS, there were no considerable alternations as compared to the dark sample which suggests the used light dose did not has any photo-toxicity on human SW 480 colon cancer cells.
The cell viability of SW 480 colon cancer treated with different concentrations of TEO in pre (A), and post (B) red light irradiation. (C) The cell viability of HDF human dermal fibroblast cells treated with different concentrations of TEO in dark, pre and post red light irradiation. Low-level laser irradiation was used at 3 J/cm2 energy density for 90 s. The results are expressed as the mean ± SD (n = 3), *P < 0.05 compared with control (untreated) group
The impact of TEO on colon cancerous cells using pre-irradiation at 660 nm with 3 J/cm2 energy density represented that pretreatment with low-level laser decreased SW 480 colon cancer cells viability. The cell survival in pre- (L–T) and post-irradiation (T-L) was 26% and 25% at 100 μg/mL of TEO, respectively (Fig. 3A and B).
It could be noted that using red light irradiation leads to phototoxic reactions which can sensitize cancer cells to TEO lead to cell survival reduction. It could be due to the red light illumination function in ROS generation in the cells. As TEO as an anticancer agent could also produce ROS in cancer cells, applying it prior than light could enhance the impact of red light illumination in ROS production the cancer cells. To evaluate the toxic effect of this combination treatment on normal healthy cells, HDF cells were used and treated as described for SW 480 colon cancer cell. The effect of TEO and red irradiation on HDF cells is shown in Fig. 3C. As it can be seen the TEO at dark or in combination with irradiation had effect on cell viability of HDF cells but this reduction was not significant (P > 0.05). It can be noted that combination of TEO and red irradiation has not toxic effect on normal HDF cells as the cell viability of HDF in IC50 of TEO (25 µg/mL) was around 80% (Fig. 3C).
Cytotoxicity of TEO in presence of different irradiation energy densities on
The impact of various laser irradiation energy densities at 660 nm at 0 and 25 μg/ml (IC50) of TEO on SW 480 cancer cells was studied. The finding represented that 25 μg/ml of TEO without irradiation decreased the cell viability up to 46% in the SW 480 cell line. The cell survival was reduced to 46% (L–T) and 45% (T-L) at red light illumination for 30 s and 1 J/cm2 energy density. At 60 s and 2 J/cm2 energy density irradiation, the cell survival was 45% (L–T) and 44% (T-L) and at 90-s irradiation with an energy density of 3 J/cm2, cell viability was 44% (L–T) and 42.5% ( T-L). The cell survival was declined to 30% (L–T) and 36% (T-L) at 180 s of radiation with 6 J/cm2 energy density (Fig. 4).
These findings implied that at higher energy density the cell viability was more decreased. Moreover, at 180 s with 6 J/cm2 energy density, the cell viability of the control also reduced to 85%. Regarding findings, the optimum radiation time and energy for the next experiments were planned to 90 s at 3 J/cm2 energy density.
Analysis of cellular morphology by invert light microscopy and apoptosis induction by AO/EB double staining
To notice the TEO effect and irradiation, the SW 480 cells treated with (0 0 μg/mL and IC50 of TEO) for 24 h then were illuminated using red light illumination at 660 nm and energy density of 3 J/cm2. The morphology of cells was investigated by light inverted microscopy (× 20) and apoptosis induction was investigated by AO/EB double staining.
As shown in Fig. 5 by adding TEO at IC50 (25 μg/mL) and then using red light, the cell number particularly reduced also the shape of the cells altered from spindle to circular.
The lower panel displays the cell shape alternation of SW 480 cells by fluorescent dual staining. As illustrated in the control sample (0 μg/mL of TEO), the cells show the form of live cells with green color. By the addition of TEO at 25 μg/ml, the nuclei of cells alter to orange/red cells represent early or late apoptosis. After illumination and adding TEO, the colon cancer cells display the features of the apoptotic cells including nuclear fragmentation and condensation of chromatin. It implies that using TEO and red light illumination leads to SW 48 cell death.
ROS generation in SW 480 colon cancer cells after TEO treatment then irradiation
As represented in Fig. 6, the SW 480 colon cancer cells displayed the ROS generation after incubation with 25 μg/mL TEO in comparison to the control sample (0 µg/mL). By irradiation, the SW 480 cells denote more accumulation of ROS as compared to TEO alone cells or control (irradiation- 0 µg/mL) samples. It suggests that ROS generation could consider as primary agents in cancer cells death mechanism under TEO and irradiation incubation.
Cell cycle changes in SW 480 colon cancer cells after TEO treatment then irradiation
To assay the anti-proliferative mechanism of TEO then irradiation, cell cycle progression in treated SW 480 colon cancer cells was determined. The percentages of the cell cycle were determined using flow cytometry. As seen in Fig. 7, treatment with TEO caused to a potent arrest of G0/G1 phase in dose-dependent manner. Whereas the SW 480 cells were treated with 25 μg/mL of TEO for 24 h and then irradiation, mass of cells in a G0/G1 phase decreased in comparison to alone TEO treatment group.
Discussion
Nowadays, herbal medicines have received a lot of attention due to their lack of side effects compared to chemical medicines [34, 35]. Trachyspermum ammi is an important medicinal plant that has properties such as reducing cholesterol level, affecting the activity of pancreatic and small intestinal digestive enzymes, fungal infections, and cleanses the bloodstream [36].
The phytochemical studies of trachyspermum ammi have shown the existence of flavonoids, alkaloids, glycosides, steroids, carbohydrates, phenols, tannins, and terpenes.
The most important compounds in tannins include thymol, para-cymen, alpha-pinene, dipenten, gamma terpenine, betapinin, myrsin, and carvacrol. Other chemical compounds include protein, fat, and cations, including sodium, potassium, iron, calcium, magnesium, zinc, copper, and cobalt.
The T. ammi essential oil GC–MS analysis in the present study shows that the principal elements are p-cymene (15.84%), γ-terpinene (38.68%), and thymol (40.25%). This data is in agreement with previous results, Mahboubi and Kazempour noted that the great compounds of T. ammi oil are p-cymene (19%), γ-terpinene (20.6%), and thymol (45.9%) [37]. The GC–MS analysis of T. ammi essential oil by Ahmed M. Abdallah et al. indicated that TEO consists about 20 compositions, in particular the major elements are aromatic and monocyclic terpenes [38]. Thymol was existed in the highest level (36%) then m-cymene (21.44%) and γ-terpinene (26.01%). The T. ammi essential oil includes around 6.70% of β-pinene and 9.77% bicyclic terpenes [39].
Vitali et al. examined the diverse biological activies of T. ammi essential oil and showed that γ-terpinene (11.3%), p-cymene (17.9%) and thymol (67.4%) are the main parts of TEO. TEO has antimicrobial and antioxidant activity. Also, MTT test results after treatment with varoius concentrations of TEO (0.78 to 200 μg/ml) for 72 h in melanoma cancer cells (A375), breast adenocarcinoma (MDA-MB 231) and colon cancer cells (HCT116) showed that TEO has anti-cancer effects and its IC50 is 16.93, 66.52, and 9.6 μg/mL, respectively [40].
As it noted the main active ingredient of this essential oil is thymol [41] that is a potent antiseptic and antifungal compound. Ramya et al. studied the anti-cancer activity of T. ammi ethanolic extract against the MCF7 breast cancer cell category. The results of the MTT assay from their study showed that there is more cell toxicity at a dose of 25 µg/mL [42].
Our study also uncovered that the colorectal cancer SW 480 cells survival was reduced by using various essential oils concentrations in a dose-dependent behavior. It was also found that the IC50 of TEO was 25 µg/mL.
On the other hand, previous researches have shown that low-level laser can be used as a safe and effective multifunctional treatment, potentially for use with other treatments or be used as an independent method in the cancer treatment and other complex diseases.
The findings of this study noted that pre-treatment of colorectal cancer cells with different TEO concentrations and then red light illumination has better effect than TEO alone on reducing the survival of colorectal cancer cells. Therefore, this study can be suggested as an effective way to combat colorectal cancer cells. This study has shown that laser irradiation alone could not kill colorectal cancer cells which were the same as our previous finding on melanoma and breast cancer cell lines [32, 43].
Morphological study of cells treated with TEO, along with irradiation, confirms the findings of cell survival study. As a result, the death in cells after TEO along with irradiation was higher in compare to dark groups. Our experiment also revealed that the SW 480 colorectal cancer cells treatment with TEO and then the irradiation increases the ROS generation in cancer cells more than the alone TEO treated groups.
In addition, the results of the cell cycle study demonstrated that treatment with TEO and then irradiation leads to more cell arrest in G0 phase. All of these results confirm more deaths in cells exposed to TEO and the laser irradiation as compared to treated cells with alone TEO.
Conclusion
This study showed that TEO, in addition to several medicinal properties mentioned in other studies, also has anti-cancer effects in SW 480 colorectal cancer cells. Besides, here we showed that the first treatment with TEO then irradiation caused more cell death in comparison to cells kept in dark. This is could be due to the fact that TEO could produce ROS, which using irradiation could enhance the previous generated ROS and lead to a series of molecular reactions that cause free radicals production in the cells and consequently more cell death. The combination of TEO with low-level laser irradiation could be considered as an effective way for colon cancer treatment. Investigation of anti-cancer effects of TEO and its active ingredients alone and along with low-level laser irradiation in other cancer cells as well as in the tumor-bearing animal models are suggested in future studies as results have shown here are preliminary.
References
Mármol I, Sánchez-de-Diego C, Pradilla Dieste A et al (2017) Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci 18:197. https://doi.org/10.3390/ijms18010197
Inamura K (2018) Colorectal cancers: an update on their molecular pathology. Cancers (Basel) 10:26. https://doi.org/10.3390/cancers10010026
Tudyka V, Madoff R, Wale A et al (2018) Session 1: colon cancer - 10 years behind the rectum. Color Dis 20:28–33. https://doi.org/10.1111/codi.14074
Nguyen H, Duong H (2018) The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy (Review). Oncol Lett. https://doi.org/10.3892/ol.2018.8679
Dekker E, Tanis PJ, Vleugels JLA et al (2019) Colorectal cancer. Lancet 394:1467–1480. https://doi.org/10.1016/S0140-6736(19)32319-0
Goldstein DA, Zeichner SB, Bartnik CM et al (2016) Metastatic colorectal cancer: a systematic review of the value of current therapies. Clin Colorectal Cancer 15:1–6. https://doi.org/10.1016/j.clcc.2015.10.002
Miles A, van Duijnhoven F, McQueen A, Oliphant R (2015) Colorectal cancer: advances in prevention and early detection. Biomed Res Int 2015:1–2. https://doi.org/10.1155/2015/518068
Peluso G, Incollingo P, Calogero A et al (2017) Current tissue molecular markers in colorectal cancer: a literature review. Biomed Res Int 2017:1–8. https://doi.org/10.1155/2017/2605628
Oruç Z, Kaplan MA (2019) Effect of exercise on colorectal cancer prevention and treatment. World J Gastrointest Oncol 11:348–366. https://doi.org/10.4251/wjgo.v11.i5.348
Kruger C, Zhou Y (2018) Red meat and colon cancer: a review of mechanistic evidence for heme in the context of risk assessment methodology. Food Chem Toxicol 118:131–153. https://doi.org/10.1016/j.fct.2018.04.048
Song M, Garrett WS, Chan AT (2015) Nutrients, foods, and colorectal cancer prevention. Gastroenterology 148:1244-1260.e16. https://doi.org/10.1053/j.gastro.2014.12.035
Ishihara J, Inoue M, Iwasaki M et al (2008) Dietary calcium, vitamin D, and the risk of colorectal cancer. Am J Clin Nutr 88:1576–1583. https://doi.org/10.3945/ajcn.2008.26195
Thanikachalam K, Khan G (2019) Colorectal cancer and nutrition. Nutrients 11:164. https://doi.org/10.3390/nu11010164
Afshari K, Haddadi N, Haj-Mirzaian A et al (2019) Natural flavonoids for the prevention of colon cancer: a comprehensive review of preclinical and clinical studies. J Cell Physiol 234:21519–21546. https://doi.org/10.1002/jcp.28777
Pan P, Yu J, Wang L-S (2018) Colon cancer. Surg Oncol Clin N Am 27:243–267. https://doi.org/10.1016/j.soc.2017.11.002
Crosara Teixeira M (2014) Primary prevention of colorectal cancer: myth or reality? World J Gastroenterol 20:15060. https://doi.org/10.3748/wjg.v20.i41.15060
Hobday TJ, Erlichman C (2002) Adjuvant Therapy of colon cancer: a review. Clin Colorectal Cancer 1:230–236. https://doi.org/10.3816/CCC.2002.n.004
Mano MS, Duhoux F (2008) Colon Cancer: update on adjuvant therapy. Clin Colorectal Cancer 7:178–183. https://doi.org/10.3816/CCC.2008.n.023
Kooti W, Servatyari K, Behzadifar M et al (2017) Effective medicinal plant in cancer treatment, part 2: review study. J Evid Based Complementary Altern Med 22:982–995. https://doi.org/10.1177/2156587217696927
Demain AL, Vaishnav P (2011) Natural products for cancer chemotherapy. Microb Biotechnol 4:687–699. https://doi.org/10.1111/j.1751-7915.2010.00221.x
Lichota A, Gwozdzinski K (2018) Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci 19:3533. https://doi.org/10.3390/ijms19113533
Rajeshwari CU, Vinay Kumar AV, Andallu B (2011) Therapeutic potential of Ajwain (Tracyspermum ammi L.) Seeds. In: Nuts and Seeds in Health and Disease Prevention. Elsevier pp 153–159
Dubey S, Kashyap P (2015) Trachyspermum ammi: a review on its multidimensional uses in Indian folklore medicines. Res J Med Plant 9:368–374. https://doi.org/10.3923/rjmp.2015.368.374
Anwar S, Ahmed N, Habibatni S, Abusamra Y (2016) Ajwain (Trachyspermum ammi L.) Oils. In: Essential Oils in Food Preservation, Flavor and Safety. Elsevier pp 181–192
Bairwa R, Rajawat B, Sodha R (2012) Trachyspermum ammi. Pharmacogn Rev 6:56. https://doi.org/10.4103/0973-7847.95871
Cotler HB, Chow RT, Hamblin MR, Carroll J (2015) The use of low level laser therapy (LLLT) For musculoskeletal pain. MOJ Orthop Rheumatol 2:68. https://doi.org/10.15406/mojor.2015.02.00068
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22:348–364. https://doi.org/10.1109/JSTQE.2016.2561201
Hamblin MR (2016) Photobiomodulation or low-level laser therapy. J Biophotonics 9:1122–1124. https://doi.org/10.1002/jbio.201670113
Karu TI (2015) Cellular mechanisms of photobiomodulation. In: Lasers in Dentistry. John Wiley & Sons, Inc, Hoboken pp 23–26
Hamblin MR, Nelson ST, Strahan JR (2018) Photobiomodulation and cancer: what is the truth? Photomed Laser Surg 36:241–245. https://doi.org/10.1089/pho.2017.4401
Sparkman OD (2007) Review. J Am Soc Mass Spectrom 18:803–806. https://doi.org/10.1016/j.jasms.2007.01.001
Khorsandi K, Kianmehr Z, Hosseinmardi Z, Hosseinzadeh R (2020) Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int 20:18. https://doi.org/10.1186/s12935-020-1100-y
Kasibhatla S (2006) Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb Protoc 2006:pdb.prot4493-pdb.prot4493. https://doi.org/10.1101/pdb.prot4493
Dutta S, Mahalanobish S, Saha S et al (2019) Natural products: an upcoming therapeutic approach to cancer. Food Chem Toxicol 128:240–255. https://doi.org/10.1016/j.fct.2019.04.012
Costa C, Tsatsakis A, Mamoulakis C et al (2017) Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem Toxicol 110:286–299. https://doi.org/10.1016/j.fct.2017.10.023
Asif HM, Sultana S, Akhtar N (2014) A panoramic view on phytochemical, nutritional, ethanobotanical uses and pharmacological values of Trachyspermum ammi Linn. Asian Pac J Trop Biomed 4:S545–S553. https://doi.org/10.12980/APJTB.4.2014APJTB-2014-0242
Mahboubi M, Kazempour N (2011) Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran J Microbiol 3:194
Ali AMH, Abdallah EM, Mujawah AAH, Avdeeva EY (2019) Chemical composition, larvicidal and antibacterial activity of the essential oil of trachyspermum ammi fruit. J Nat Remedies 19:64–73. https://doi.org/10.18311/jnr/2019/23310
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Vitali LA, Beghelli D, Biapa Nya PC et al (2016) Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab J Chem 9:775–786. https://doi.org/10.1016/j.arabjc.2015.06.002
Eftekhari M, Hoseinsalari A, Mansourian M et al (2019) Trachyspermum ammi (L.) Sprague, superb essential oil and its major components on peptic ulcers: in vivo combined in silico studies. DARU J Pharm Sci 27:317–327. https://doi.org/10.1007/s40199-019-00279-y
Ramya N, Priyadharshini XX, Prakash RDR (2017) Anti-cancer activity of Trachyspermum ammi against MCF7 cell lines mediates by p53 and Bcl-2 mRNA levels. J Phytopharm 6:78–83
Kianmehr Z, Khorsandi K, Mohammadi M, Hosseinzadeh R (2020) Low-level laser irradiation potentiates anticancer activity of p-coumaric acid against human malignant melanoma cells. Melanoma Res 30:136–146. https://doi.org/10.1097/CMR.0000000000000603
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khorsandi, K., Kianmehr, Z. & Ghelichkhani, E. Combination effect of red light irradiation and Traychspermum ammi essential oil on colorectal cancer cells (SW480). Lasers Med Sci 37, 1031–1040 (2022). https://doi.org/10.1007/s10103-021-03350-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03350-w