Abstract
The objective of this study was to demonstrate the oncologic efficacy of awake endoscopic laryngeal surgery in the treatment of T1-T2 glottic carcinoma. This is a retrospective study. Seventy-one patients with early glottic carcinoma (T1a- 26, T1b- 18, T2- 27) who underwent awake flexible endoscopic laryngeal surgery under local anesthesia and mild intravenous sedation were included in the study. In 64 cases (90.1%) only endoscopic tumor ablation by Nd:YAG laser (in 32.4% of cases being preceded by diathermy snare excision) was performed, and in 7 T2 cases postoperative radiotherapy was also offered. There were no complications during or after the endoscopic surgery. Ultimate control of disease, including salvage treatment, was obtained in 67 patients (94.4%). Cure without recurrence was achieved in 60 cases (84.5%). Local control without salvage radiotherapy or/and open surgery was achieved in 64 (90.1%) patients. Larynx preservation was obtained in 66 (93.0%) cases. At 5 years from the beginning of endoscopic treatment, 74.6% of the patients were alive and free of disease. The best results were obtained in the T1a group of treated patients, all the patients being free of disease with the preserved larynx. Awake endoscopic laryngeal surgery is a safe and oncologically efficient method of treatment of early glottic carcinoma that can be considered as an alternative to the traditional approach, primarily, for patients with risks/contraindications for radiotherapy, general anesthesia, and transoral microsurgery, and also for the patients who prefer to avoid general anesthesia with its related risks and would rather choose office-based laryngeal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinoma of the larynx represents about 30–40% of head and neck malignancies [1]. The glottic part of the larynx is affected in approximately 60% of cases [1, 2]. The definition of early glottic carcinoma is not unanimous. Some experts define it as early glottic cancer only Tis, T1a, and T1b stages [3]. Other authors also consider the T2 stage as early carcinoma [4]. The regional lymph node involvement in T1 glottic cancer is rare (under 1%) [3].
Radiotherapy, open surgery, and transoral CO2 laser microsurgery have comparable oncological efficacy in the treatment of early glottic carcinoma, but all the above-mentioned methods have shortcomings. Some of the known drawbacks of radiotherapy are the impossibility of reusing radiation for a recurrent or another primary tumor; long treatment duration with associated increased costs; related complications (xerostomia, accelerated carotid artery narrowing, hypothyroidism, laryngeal chondronecrosis, etc.); induction of second malignancy in the head and neck region [5,6,7,8]. Reported open surgery disadvantages are: cutting/resection of normal anatomic structures; temporary tracheostomy; swallowing impairment in the postoperative period; common development of cicatricial airway stenosis after the surgery; long hospitalization period [9, 10]. Nowadays, transoral CO2 laser microsurgery is the mainstay in the surgical treatment of glottic carcinoma, nevertheless, this method also has limitations, mostly, due to anatomic particularities (temporomandibular joint ankylosis, short mandible, large tongue base, insufficient neck extension, etc.) that cause inadequate lesion exposure, and contraindications for general anesthesia [4, 11, 12].
Office-based laryngeal surgery is an evolving trend in modern laryngology that is successfully used in the management of benign and premalignant laryngeal lesions, and is preferred by the majority of patients [13], but is rarely practiced for the treatment of glottic carcinoma. Awake flexible endoscopic laryngeal surgery has the potential to overcome the limitations of transoral CO2 laser microsurgery, but it is still unclear if this method is oncologically effective and safe for the patients. The objective of this study was to demonstrate the safety and oncologic efficacy of awake endoscopic laryngeal surgery (AELS) in the treatment of T1-T2 glottic carcinoma.
Materials and methods
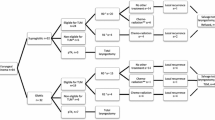
This manuscript was approved by the Institutional Ethics Committee of “Timofei Moșneaga” Republican Clinical Hospital. This is a retrospective study. We performed flexible endoscopic laryngeal surgery (FELS) on 124 patients with T1-T2 glottic carcinoma in the period from 1993 to 2022. All surgical procedures were performed by a single surgeon who has skills in interventional bronchoscopy. In 77 cases (62.1%) interventions were performed as awake procedures under local anesthesia and mild intravenous sedation. Written informed consent was obtained from the patients. Selection criteria for the study were the following: (1) consecutive patients with T1-T2, N0, M0 glottic carcinoma without vocal cord mobility impairment, (2) time interval of at least 5 years after the endoscopic treatment, and (3) at least 2 years of the follow-up period (i.e. patients who met the first two criteria, but were not lost to follow-up and did not die from unrelated reasons before 2 years of the follow-up period). For this reason, 6 patients were excluded from the study (1 patient was under observation for 18 months, 2 patients died from unrelated reasons before 2 years of follow-up, and 3 patients were lost to follow-up before 2 years from the surgery (Fig. 1).
The first awake intervention for glottic carcinoma was performed on 10.03.1993. At that time, the clinic did not have either a CO2 laser or surgical microscope. The only available options for patients with glottic cancer were either radiotherapy or open surgery. Having some experience in using Nd:YAG laser through the flexible bronchoscope for endoscopic ablation of tracheobronchial tumors, we proposed these tools for the ablation of glottic tumors. The patients were informed about awake endoscopic surgery as an alternative to radiotherapy and open surgery, and the preserved availability of both above-mentioned methods in case of endoscopic approach failure. Many patients readily accepted the proposed alternative. Patient cooperation is critical during awake endoscopic procedures. The grade of patient compliance is estimated during the diagnostic flexible laryngoscopy with tumor biopsy by flexible forceps. Selection criteria for the awake endoscopic surgery were: contraindications or major risk for general anesthesia and/or transoral microsurgery, predominantly due to anatomical particularities (short neck, insufficient head extension, temporomandibular joint ankylosis, poor anterior commissure exposure, etc.), the patient desire to undergo an awake endoscopic surgery, and patient compliance and ability to tolerate the procedure. The cases with many treatment sessions are related to the above-mentioned period of lack of equipment for transoral laser surgery.
For AELS we used a therapeutic flexible bronchoscope with a 2.6-3.0 mm working channel (models: BF-1T10, BF-TE2, BF-1T60 (“Olympus”, Japan)), flexible guide-based Nd:YAG laser (wavelength: 1064 nm, model: LTN-102 (Russia)), a diathermy snare (oval-shaped, size 10–15 mm (“Micro-Tech”, China)), and flexible biopsy forceps (4.5 mm cup opening (“Micro-Tech”, China)). The accessories (laser guide, forceps, diathermy snare) were delivered to the target lesion through the working channel of the bronchoscope. Patients were treated in sitting up-right position. After mild intravenous sedation and local anesthesia of the nasal and pharyngeal mucosa with Lidocaine spray 10% (“Egis Pharmaceuticals”, Hungary), the bronchoscope was introduced transnasally to the laryngeal aditus. Laryngeal mucosa was anesthetized with Lidocaine 2% instilled through a catheter (model: PR-2B (“Olympus”, Japan)), inserted in the working channel of the bronchoscope, during phonation, producing a laryngeal gargle. Flat and infiltrative lesions were ablated by laser in continuous near non-contact mode, the power being set up to 40 W. In the presence of a bulky exophytic component of the tumor, diathermy snare excision was performed in some cases, and the laser was applied as a second-line tool for the ablation of the residual lesion (Fig. 2). When necessary, repeated sessions of AELS were performed with a time interval of 2–7 days. Control biopsy from the tumor bed was not performed routinely immediately after the intervention, but was performed at follow-up flexible laryngoscopic exams only in case of suspicion of persistent/ recurrent tumor.
The data were recorded and analyzed using Microsoft Office Excel 2007 (Redmond, WA, USA) and SPSS version 20.0 (IBM, Armonk, NY, USA).
Results
According to the selection criteria, 71 patients were eligible for the study (males- 65, females- 6). The age of the patients varied from 18 to 83 years (mean – 56.8 ± 11.6 years). The histological structure of the tumors was as follows: squamous cell carcinoma-54, verrucous carcinoma-13, hybrid verrucous carcinoma-1, and spindle cell carcinoma-3. In 67 (94.4%) cases we dealt with primary tumors and in 4 cases (5.6%) patients presented with recurrent (2) or persistent (2) tumors after radiotherapy. According to the tumor extent, 26 patients (36.6%) had T1a stage, 18 patients (25.4%) - T1b stage (vocal fold carcinoma with anterior commissure involvement was considered as T1b stage), and 27 patients (38.0%) – T2 stage of disease (due to supraglottic or/and subglottic extension of the tumor without impairment of vocal cord mobility). The anterior commissure involvement was in 42 cases (59.2%). In 64 cases (90.1%) only endoscopic treatment was applied, and in 7 cases (all of them - T2) postoperative radiotherapy was also offered. The following endoscopic techniques were applied: Nd:YAG laser ablation as a single technique – in 48 (67.6%) cases, and laser ablation preceded by diathermy snare excision of the tumor – in 23 (32.4%) cases. The number of treatment sessions varied from 1 to 9 (mean – 1.9). In 34 (47.9%) cases treatment was realized in one session.
There were no complications during or after the endoscopic surgery. No patients had swallowing problems. Ultimate control of disease, including salvage treatment, was obtained in 67 patients (94.4%). Cure without recurrence was achieved in 60 cases (84.5%). Recurrent disease with successful salvage treatment was registered in 7 patients. In 6 (85.7%) cases disease recurrence occurred during 2 years after surgery and in 1 case – after 4 years. From 7 patients with recurrent disease, 4 patients underwent repeated FELS, salvage radiotherapy (RT) was offered to 1 patient, open partial laryngectomy was performed in 1 case, and 1 patient underwent total laryngectomy. Local control (LC) without salvage RT or/and open surgery was achieved in 64 (90.1%) patients. Larynx preservation (LP) was obtained in 66 (93.0%) cases. In the time interval from 2 up to 5 years after endoscopic surgery, 12 patients died of unrelated reasons and 2 patients were lost to follow-up. Death of disease was registered in 4 cases (5.6%). Primary tumor progression was observed in 2 of these cases and regional metastatic disease without recurrence of the primary tumor- in the other 2 cases. At 5 years from the beginning of endoscopic treatment, 53 patients (74.6%) were alive and free of disease (Table 1). The best results were obtained in the T1a group of treated patients, all the patients being free of disease with the preserved larynx (Table 2). In the group of cured patients (67), 53 (79.1%) patients were followed up for more than 5 years (Table 3). The mean follow-up period in this group was 120 months. In the group of patients that were cured with larynx preservation (66), satisfactory long-term voice outcome/ mild dysphonia was registered in 27 cases (40.9%), moderate dysphonia – in 18 cases (27.3%), and severe dysphonia – in 21 cases (31.8%). Mild dysphonia was predominant in the T1a subgroup (88.5%), moderate dysphonia – in the T1b subgroup (62.5%), and severe dysphonia – in the T2 subgroup (58.3%) (Table 4).
Discussion
The treatment approach to early glottic carcinoma is still debatable. Radiation therapy, open surgery, and endoscopic surgery demonstrate similar oncological results and the treatment approach is largely dependent on institutional traditions, specialists preferences, and equipment availability.
Endoscopic laser surgery as primary treatment is attractive, particularly, because it leaves more salvage options in case of initial treatment failure and the future appearance of a new malignancy in the head and neck region [3, 14, 15]. Nowadays, transoral CO2 laser microsurgery is the most used surgical method for glottic carcinoma management, being considered the gold standard [14, 16]. In the recent 2 decades, due to the development of fiber-based laser technology, other types of lasers, such as PDL, KTP, Thulium, Nd:YAG, and diode, have been also used in laryngology. Karkos et al., in a review article, report that 980 nm diode laser microsurgery has similar oncologic results, compared to CO2 laser, mentioning improved access to difficult-to-reach areas, such as the anterior commissure [14].
Laser ablation, as an alternative to laser resection, has been gaining popularity in recent years. There are publications related to glottic carcinoma ablation by transoral microsurgery using fiber-based lasers (mainly, KTP laser) [15,16,17,18,19,20,21,22]. Zeitels et al., using KTP laser as an ablation tool, demonstrated comparable oncological results to CO2 laser resection in the treatment of early glottic carcinoma [15]. Suppah et al., in a systematic review, also mention similar oncological outcomes of KTP laser ablation to CO2 laser resection, mentioning improved voice outcomes [22].
Office-based awake laryngeal surgery is a developing trend in modern laryngology that has gained popularity in recent years, offering such advantages as avoidance of general anesthesia risks, cost-effectiveness, and time economy, but available publications include, mostly, small series related to benign and premalignant lesions management [23,24,25,26,27,28,29]. Office-based awake laryngeal surgery seems to be preferred by the majority of patients. In the study by Rees et al. (2006), the authors mention that the vast majority (87%) of 54 patients with aerodigestive tract lesions who underwent both, operating room laser surgery under general anesthesia and office-based awake laser surgery for the same pathosis, preferred awake procedure, and 83% of these patients found the in-office unsedated treatment more comfortable than the surgery in the operating room [13].
Awake flexible endoscopic laryngeal surgery permits overcoming such limitations of traditional transoral CO2 laser microsurgical approach as difficult anatomy and a major risk for general anesthesia, but there is a lack of publications relating to awake laryngeal surgery for glottic cancer. Lai et al. (2001) for the first time in English literature reported performing awake fiberoptic laryngoscopic laser treatment for early glottic carcinoma. The authors presented their experience of using Nd:YAG laser under local anesthesia in 34 patients with Tis and T1 glottic carcinoma, with a cure rate of 85.2% [30]. Wellenstein et al. (2018), in a review article, state the absence of studies related to office-based laser surgery for early glottic carcinoma [31]. Lechien et al. (2021), in a systematic review article, mention that the use of KTP laser in the office for malignant lesions is controversial [21]. We have not found in accessible English literature any other studies related to AELS for glottic carcinoma. To our knowledge, this manuscript presents the largest series of glottic carcinoma patients, treated by awake laryngeal surgery using Nd:YAG laser, with long-term follow-up. The obtained results (overall LC rate of 94.4%, LC without salvage RT or/and open surgery of 90.1%, and LP rate of 93.0%) are comparable with those obtained by radiotherapy [32,33,34,35], open surgery [36], and transoral CO2 laser microsurgery [37, 38]. The results, obtained in the T1a subgroup of patients (overall LC rate of 100%, LC without salvage RT or/and open surgery of 96.2%, and LP rate of 100%) are particularly encouraging. The limitations of this study are the lack of a thorough voice outcome assessment and a comparison of pre and post-treatment voice outcomes that would be useful for a follow-up study. Future studies could also compare the relative voice improvements after different oncologic treatments. Concerning the voice outcome, it mostly depended on the tumor extent and, particularly, on the anterior commissure involvement that worsened the voice quality. None of the follow-up patients had regrets about their treatment choice regardless of the voice quality, being grateful for their disease-free survival and organ preservation. The cases with tumor affection of the anterior commissure are challenging for endoscopic treatment. The thyroid cartilage involvement is often missed, and, as a result, these tumors are often understaged, with T3 and T4 tumors being treated as T1b and T2. Taking this into account, a part of specialists do not recommend endoscopic laser surgery for tumors involving the anterior commissure [36]. As the anterior commissure involvement was registered in the majority of the patients, included in our study (59.2%), we did not expect a great voice outcome in this subgroup of patients. We consider sparing the anterior commissure during surgery for obtaining a better voice outcome not relevant in patients with glottic carcinoma because it is related to an increased risk of a persistent tumor, so oncological outcome should be a priority. The limitation of ablative techniques is the absence of tumor resection margins that could be examined histologically. This drawback can be compensated by a control biopsy from the tumor bed [16, 19] and a “wait and see” strategy, taking into consideration the accessibility of the glottic region for visual examination [39]. Patient cooperation and closely scheduled follow-up flexible laryngoscopy exams can ensure early detection of potential tumor recurrence. According to our follow-up protocol, we recommend office flexible laryngoscopy once a month during the first year after surgery, once in 2 months during the second year, once in 3 months during the third year, once in 6 months during the fourth and fifth year, and once a year after 5 years. In T2 disease, we also recommend neck ultrasonography once in 3 months during the first two years after the surgery.
Conclusion
Awake endoscopic laryngeal surgery is a safe and oncologically efficient method of treatment of early glottic carcinoma that can be considered as an alternative to the traditional approach, primarily, for patients with risks/contraindications for radiotherapy, general anesthesia, and transoral microsurgery, and also for the patients who prefer to avoid general anesthesia with its related risks and would rather choose office-based laryngeal surgery.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The author has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Markou K, Christoforidou A, Karasmanis I, Tsiropoulos G, Triaridis S, Constantinidis I, Vital V, Nikolaou A (2013) Laryngeal cancer: epidemiological data from Νorthern Greece and review of the literature. Hippokratia 17:313–318
Seyed Resuli A, Cansiz H (2020) Advantages of transoral endoscopic diode laser microsurgery for the treatment of early-stage glottic laryngeal cancers. Int J Cancer Manag 13:e97928
Gallo A, de Vincentiis M, Manciocco V, Simonelli M, Fiorella ML, Shah JP (2002) CO2 laser cordectomy for early-stage glottic carcinoma: a long-term follow-up of 156 cases. Laryngoscope 112:370–374
Sjögren EV (2017) Transoral laser microsurgery in early glottic lesions. Curr Otorhinolaryngol Rep 5:56–68
Remijn EE, Marres HA, van den Hoogen FJ (2002) Endoscopic laser treatment in pre-malignant and malignant vocal fold epithelial lesions. J Laryngol Otol 116:1019–1024
Zeitels SM, Hillman RE, Franco RA, Bunting GW (2002) Voice and treatment outcome from phonosurgical management of early glottic cancer. Ann Otol Rhinol Laryngol Suppl 190:3–20
August M, Wang J, Plante D, Wang CC (1996) Complications associated with therapeutic neck radiation. J Oral Maxillofac Surg 54:1409–1415
Young JR (1983) Laser surgery for T1 glottic carcinoma - the argument against. J Laryngol Otol 97:243–246
Rubinstein M, Armstrong WB (2011) Transoral laser microsurgery for laryngeal cancer: a primer and review of laser dosimetry. Lasers Med Sci 26:113–124
Motta G, Esposito E, Motta S, Tartaro G, Testa D (2005) CO2 laser surgery in the treatment of glottic cancer. Head Neck 27:566–573
Quer M, León X, Orús C, Venegas P, López M, Burgués J (2000) Endoscopic laser surgery in the treatment of radiation failure of early laryngeal carcinoma. Head Neck 22:520–523
Guimarães AV, Dedivitis RA, Matos LL, Aires FT, Cernea CR (2018) Comparison between transoral laser surgery and radiotherapy in the treatment of early glottic cancer: a systematic review and meta-analysis. Sci Rep 8:11900
Rees CJ, Halum SL, Wijewickrama RC, Koufman JA, Postma GN (2006) Patient tolerance of in-office pulsed dye laser treatments to the upper aerodigestive tract. Otolaryngol Head Neck Surg 134:1023–1027
Karkos PD, Koskinas I, Stavrakas M, Triaridis S, Constantinidis J (2021) Diode laser for laryngeal cancer: 980 nm and beyond the classic CO2. Ear Nose Throat J 100:19S–23S
Zeitels SM, Burns JA (2014) Oncologic efficacy of angiolytic KTP laser treatment of early glottic cancer. Ann Otol Rhinol Laryngol 123:840–846
Strieth S, Ernst BP, Both I, Hirth D, Pfisterer LN, Künzel J, Eder K (2019) Randomized controlled single-blinded clinical trial of functional voice outcome after vascular targeting KTP laser microsurgery of early laryngeal cancer. Head Neck 41:899–907
Zeitels SM, Burns JA, Lopez-Guerra G, Anderson RR, Hillman RE (2008) Photoangiolytic laser treatment of early glottic cancer: a new management strategy. Ann Otol Rhinol Laryngol Suppl 199:3–24
Murono S, Endo K, Kondo S, Wakisaka N, Yoshizaki T (2013) Oncological and functional outcome after transoral 532-nm pulsed potassium-titanyl-phosphate laser surgery for T1a glottic carcinoma. Lasers Med Sci 28:615–619
Lahav Y, Cohen O, Shapira-Galitz Y, Halperin D, Shoffel-Havakuk H (2020) CO2 laser cordectomy versus KTP laser tumor ablation for early glottic cancer: a randomized controlled trial. Lasers Surg Med 52:612–620
Parker NP, Weidenbecher MS, Friedman AD, Walker BA, Lott DG (2021) KTP laser treatment of early glottic cancer: a multi-institutional retrospective study. Ann Otol Rhinol Laryngol 130:47–55
Lechien JR, Burns JA, Akst LM (2021) The use of 532-nanometer-pulsed potassium-titanyl-phosphate (KTP) laser in laryngology: a systematic review of current indications, safety, and voice outcomes. Ear Nose Throat J 100:4S–13S
Suppah M, Kamal A, Karle WE, Saadoun R, Lott DG (2023) Outcomes of KTP laser ablation in glottic neoplasms: a systematic review and meta-analysis. Laryngoscope 133:1806–1814
Zeitels SM, Akst LM, Burns JA, Hillman RE, Broadhurst MS, Anderson RR (2006) Office-based 532-nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann Otol Rhinol Laryngol 115:679–685
Zeitels SM, Burns JA, Akst LM, Hillman RE, Broadhurst MS, Anderson RR (2006) Office-based and microlaryngeal applications of a fiber-based thulium laser. Ann Otol Rhinol Laryngol 115:891–896
Koufman JA, Rees CJ, Frazier WD, Kilpatrick LA, Wright SC, Halum SL, Postma GN (2007) Office-based laryngeal laser surgery: a review of 443 cases using three wavelengths. Otolaryngol Head Neck Surg 137:146–151
Hu H-C, Lin S-Y, Hung Y-T, Chang S-Y (2017) Feasibility and associated limitations of office-based laryngeal surgery using carbon dioxide lasers. JAMA Otolaryngol Head Neck Surg 143:485–491
Wellenstein DJ, Honings J, Schimberg AS, Schutte HW, Herruer JM, van den Hoogen FJA, Takes RP, van den Broek GB (2020) Office-based CO2 laser surgery for benign and premalignant laryngeal lesions. Laryngoscope 130:1503–1507
Filauro M, Vallin A, Fragale M, Sampieri C, Guastini L, Mora F, Peretti G (2021) Office-based procedures in laryngology. Acta Otorhinolaryngol Ital 41:243–247
Miller BJ, Abdelhamid A, Karagama Y (2021) Applications of office-based 445 nm blue laser transnasal flexible laser surgery: a case series and review of practice. Ear Nose Throat J 100:105S–112S
Lai JP, Tao ZD, Xiao JY, Chen XH, Zhao SP, Tian YQ, Betz CS (2001) Microinvasive Nd:YAG laser therapy of early glottic carcinoma and its effect on soluble interleukin-2 receptor, interleukin-2, and natural killer cells. Laryngoscope 111:1585–1588
Wellenstein DJ, Schutte HW, Takes RP, Honings J, Marres HAM, Burns JA, van den Broek GB (2018) Office-based procedures for the diagnosis and treatment of laryngeal pathology. J Voice 32:502–513
Pellitteri PK, Kennedy TL, Vrabec DP, Beiler D, Hellstrom M (1991) Radiotherapy: the mainstay in the treatment of early glottis carcinoma. Arch Otolaryngol Head Neck Surg 117:297–301
Khan MK, Koyfman SA, Hunter GK, Reddy CA, Saxton JP (2012) Definitive radiotherapy for early (T1-T2) glottic squamous cell carcinoma: a 20 year Cleveland clinic experience. Radiat Oncol 7:193
Jørgensen K, Godballe C, Hansen O, Bastholt L (2002) Cancer of the larynx–treatment results after primary radiotherapy with salvage surgery in a series of 1005 patients. Acta Oncol 41:69–76
Johansen LV, Grau C, Overgaard J (2002) Glottic carcinoma- patterns of failure and salvage treatment after curative radiotherapy in 861 consecutive patients. Radiother Oncol 63:257–267
Pradhan SA, Pai PS, Neeli SI, D’Cruz AK (2003) Transoral laser surgery for early glottic cancers. Arch Otolaryngol Head Neck Surg 129:623–625
Lee HS, Chun B-G, Kim SW, Kim ST, Oh JH, Hong JC, Lee KD (2013) Transoral laser microsurgery for early glottic cancer as one-stage single-modality therapy. Laryngoscope 123:2670–2674
Canis M, Ihler F, Martin A, Matthias C, Steiner W (2015) Transoral laser microsurgery for T1a glottic cancer: review of 404 cases. Head Neck 37:889–895
Sigston E, de Mones E, Babin E, Hans S, Hartl DM, Clement P, Brasnu DF (2006) Early-stage glottic cancer: oncological results and margins in laser cordectomy. Arch Otolaryngol Head Neck Surg 132:147–152
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The author (PG) contributed to the study conception and design, material preparation, data collection and analysis, the first draft of the manuscript and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no relevant financial or non-financial interests to disclose.
Ethical approval
This manuscript was approved by the Institutional Ethics Committee of “Timofei Mo?neaga” Republican Clinical Hospital (Ref. No 3/23). The research was conducted ethically, with all study procedures performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Informed consent
This study has been granted an exemption from requiring written informed consent from participants in the study by the Institutional Ethics Committee of “Timofei Mo?neaga” Republican Clinical Hospital in view of the retrospective nature of the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gurău, P. Awake endoscopic laser surgery for early glottic carcinoma. Lasers Med Sci 39, 77 (2024). https://doi.org/10.1007/s10103-024-04027-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-024-04027-w