Abstract
An in vitro study was designed to evaluate the effects of photobiomodulation (PBM) with 915-nm diode laser on human gingival fibroblast (HGF) cells under hyperglycemic condition. The HGF cells were cultured in Dulbecco’s modified eagle medium (DMEM) medium containing 30 mM glucose concentration for 48 h to mimic the hyperglycemic condition. Subsequently, the cells received three sessions of PBM (915 nm, continuous emission mode, 200 mW, energy density values of 3.2, 6, and 9.2 J/cm2). Twenty-four hours post-irradiation, cell proliferation, expression of interleukin 6 (IL-6), and vascular endothelial growth factor (VEGF) were assessed with MTT and real-time polymerase chain reaction (PCR) tests, respectively. Also, reactive oxygen species (ROS) production was measured using CM-H2DCFDA fluorimetry. No changes were detected in the cell proliferation rate between the high glucose control group and laser-treated cells, while VEGF and IL-6 gene expression levels increased significantly after PBM in the high glucose-treated cells group. ROS level was significantly decreased in the irradiated cells in high-glucose medium compared with the high glucose control group. Our study revealed the inductive role of 915-nm–mediated PBM on VEGF and the inflammatory response while concurrently reducing reactive oxygen species production in HGF cells in hyperglycemic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is an inflammatory disease of tooth-supporting tissues. The risk of developing periodontitis in adult diabetic patients type 2 is about 2.1–3 times higher than in the healthy adult population [1]. Individuals with diabetes often experience compromised immune function, rendering them more susceptible to infections, including periodontitis. Poorly controlled blood glucose levels create an environment conducive to bacterial growth in the oral cavity, increasing the risk of infections and exacerbating periodontal disease. Moreover, the chronic inflammation associated with diabetes can intensify the inflammatory response in the periodontal tissue, contributing to the severity of periodontal issues [2]. Increased inflammation in periodontal tissue, hinders new bone formation, and increases the expression of RANKL (receptor activator of nuclear factor kappa beta), the bone resorption factor. Additionally, diabetes-related factors such as delayed wound healing, altered collagen metabolism, and vascular changes can collectively negatively affect the structural integrity of periodontal tissues, making them more prone to periodontitis [3].
In the periodontal wound healing process, there are several interactions between different cells, including gingival fibroblasts, osteoblasts, cementoblasts, and periodontal ligament fibroblasts [4]. Among these cells, fibroblasts play a crucial role in releasing multiple growth factors during wound healing process [5].
The evidence shown that the development of periodontitis is associated with several molecular mechanisms [6]. Among them, angiogenesis has a vital role in the pathogenesis of inflammatory diseases such as periodontitis. There is a direct relationship between an increasing number of gingival blood vessels and the progression of chronic periodontitis [7]. Vascular endothelial growth factor (VEGF) is a pro-angiogenic factor that affects angiogenesis and the permeability of the blood vessels [8]. Several studies reported a correlation between the concentration of the VEGF in the gingival fluid and the clinical parameters in periodontitis; therefore, it was considered a diagnostic biomarker of periodontal disease progression [9]. It has been reported that diabetes mellitus (DM) and hyperglycemic conditions may be associated with increased VEGF gene expression [10].
In periodontitis, inflammatory cytokines regulate an inflammation’s stability and progression through crosstalk between the tissue and immune cells [11]. Interleukin 6 (IL-6) is one of the inflammatory cytokines with a destructive effect on the tissue cells that is mediated by increasing matrix-metalloproteinase-1 (MMP-1) in the periodontal inflamed tissues [12]. Additionally, an increase in the level of IL-6 was observed in patients with chronic periodontitis and type 2 DM compared with systemically healthy patients. Hence, this can be one of the reasons linked to the symptoms’ severity in the diabetic population [13].
The polymicrobial complex within the sub-gingival biofilm stimulates the production of various cytokines, increasing the number and activity of polymorphonuclear leukocytes (PMN). These PMNs produce reactive oxygen species (ROS) as a kind of defense response to the local infection and contribute to the oxidative killing of the pathogens [14]. Noteworthy, any imbalance between ROS and antioxidant defense systems can trigger oxidative stress (OS) response and act as upstream modulators of the autophagy involved in the development of periodontitis by promoting cell death or blocking apoptosis in the infected cells [15].
In the recent two decades, laser technology has gained increasing attention from scholars in oral and dental clinical applications. Lasers in a high-power mode can be utilized for soft tissue surgeries [16]. Whereas a low power mode, recently known as photobiomodulation (PBM), can exert several beneficial effects in favor of alleviation of pain [17], reduction of inflammation, immunomodulation, and promotion of wound healing and tissue regeneration [18, 19] PBM has been used as an adjuvant therapy in periodontitis following non-surgical periodontal treatment [20]. As a non-thermal light therapy, PBM can initiate and trigger many cellular responses depending on the chosen wavelength and dosimetry [21].
Previous studies have indicated the in vitro efficacy of PBM in modifying the associated signaling pathways and underlying factors linked to periodontitis and diabetes, including oxidative stress [22,23,24]. In an in vitro study, it was shown that PBM with 660-nm diode laser on cultured HGFs in a high-glucose medium (35 mM) reduced the expression of pro-inflammatory cytokines with no significant effect on ROS [22]. However, in an investigation on the effects of a 635/808-nm dual-wavelength semiconductor laser PBM on human embryonic skin fibroblasts (CCC-ESFs) in a high glucose environment, it was found that PBM can enhance the proliferation of fibroblasts and increase intracellular ROS production [23]. Also, the antioxidant effect of PBM was highlighted in a study on cells derived from a diabetic rat source [24]. Even with extensive promising evidence of PBM effectiveness on human gingival fibroblast (HGF) cells, it is still seeking evidence due to insufficient data on PBM efficacy in challenging conditions such as hyperglycemic medium [23] Hence, we aimed to design the present in vitro study to investigate the effects of 915-nm–mediated PBM on HGF cells in a hyperglycemic medium and explore its effects on cell viability, ROS production, and VEGF and IL-6 expressions.
Materials and methods
Study design
An in vitro study was conducted to evaluate the effects of 915-nm laser PBM on HGF cells in a hyperglycemic medium. Ethical approval was obtained from the Ethical Committee of Islamic Azad University (NO: IR.IAU.DENTAL.REC.1400.104).
Cell culture
The HGF cells were obtained from pasture institute in Iran and cultured in DMEM (Gibco, Germany) containing 10% fetal bovine serum (FBS), 0.1-mg/ml streptomycin, and 100-U/ml penicillin at 37 °C and 5% CO2. Cells at 3–5 passages were used for all experiments, and the culture medium was changed every 2 days [22]. For the induction of hyperglycemic condition, the HGF cells were treated with 30 mM glucose for 48 h, followed by PBM laser irradiation [23].
Interventional and control groups
The HGF cells were utilized in eight groups in which six of them were interventional irradiated with 915-nm PBM at different laser dosimetry and treatment protocols, whereas the remaining two groups that received no PBM irradiation were in control (standard and high-glucose culture mediums).
Photobiomodulation therapy protocol of interventional and control groups
The near infra-red (NIR) PBM laser wavelength that was employed in our study was 915-nm diode laser (PL-ADV-PLD6W Pocket Laser Dental Diode Laser 88Dent Advance Kit, Pero (MI), Italy). The cells in all the interventional groups were irradiated with output power of 200mw in a continuous emission mode, whereas the irradiation protocols of the eight study groups were defined as follows:
-
Group 1: Without laser irradiation in standard culture medium (control group).
-
Group 2: PBM with the irradiation time of 8 s corresponding to 3.2 J/cm2 energy density in standard culture medium.
-
Group 3: PBM with the irradiation time of 15 s corresponding to 6 J/cm2 energy density in standard culture medium.
-
Group 4: PBM with the irradiation time of 23 s corresponding to 9.2 J/cm2 energy density in standard culture medium.
-
Group 5: Without laser irradiation in high-glucose culture medium (control group).
-
Group 6: PBM with the irradiation time of 8 s corresponding to 3.2 J/cm2 energy density in high-glucose culture medium.
-
Group 7: PBM with the irradiation time of 15 s corresponding to 6 J/cm2 in energy density high-glucose culture medium.
-
Group 8: PBM with the irradiation time of 23 s corresponding to 9.2 J/cm2 energy density in high-glucose culture medium.
All the irradiation experiments were performed under a laboratory hood delivered with a bio-stimulation hand piece (intraoral biostimulation tip code PL-B670-8A) of 8 mm (0.5 cm−2 spot size) at a fixed distance of 1 cm from the cells. To avoid light transmission to adjacent wells, one well was left empty between each experimental one. The PBM irradiation was repeated in three sessions at a time interval of 24 h [25]. It should be noted that the output power of the laser was checked and calibrated with a power meter (laser point. s.r.1, Milano, Italy) at each session before its application. Finally, 24 h after the last laser irradiation session, the cell proliferation and gene expression rate were assessed [26].
Methods of assessment
Cell proliferation
To assess cell proliferation, HGF cells were cultured in a 96-well plate at a concentration of 104 cells and 100 μl of cell culture medium per well. The confluence was about 70% at the time of irradiation. The cells were seeded so that there was only one empty well between the wells under laser irradiation. On the day of the MTT assay, Tetrazolium salt (3–4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma, Germany) was prepared at a ratio of 5 mg/ml in PBS (phosphate-buffered saline) (Sigma Germany), and 100 μL solutions were replaced with the culture medium. The plate was incubated for 3–4 h at 37 °C and 5% CO2. Finally, the MTT solution was removed and replaced with 60 μL of DMSO (Merck. Germany). After shaking for 15 min, 50 μL of solution was transferred to a clean plate, and the optical density was measured by spectrophotometer (BioTek, USA) at of 570 nm wavelength [23]. Six replicates were applied for the MTT assay, and cell proliferation was reported as a percentage.
Real-time polymerase chain reaction
Real-time PCR was performed in 24 well- plates using 700 μl of cell culture medium in each well. RNA was extracted by phenol chloroform method, using RNA XPlus solution (Cynogen). The concentration and quality of extracted RNA were assessed using a Thermo Scientific Nanodrop 2000c UV–Vis spectrometer (BioTek, USA). DNase-I and RNase kits were used to remove genomic DNA. A total of 1000 ng of RNA was converted to cDNA by the cDNA synthesis kit according to the manufacturer’s instructions (Biofact, China). In the next step, using specific primers (VEGF: F-CTTCTGGGCTGTTCTCGCTTC, R-CCGCCTCACCCGTCCAT; IL-6: F-TTCTGCCAGTGCCTCTTTGCTG, R-AGACAGCCACTCACCTCTTCAG; beta actin: F-GAGACCTTCAACACCCCAGCC, R- AATGTCACGCACGATTTCCC. Beta actin was used as an internal control. Light Cycler 96 system (Roche) qRT-PCR was performed based on SYBR master mix. The relative expression was calculated based on the following formula: 2−∆∆C formula [27]. Three replicates were applied for real-time PCR.
Reactive oxygen species measurement
DA-DCFH kit (Digibonyan, Iran) was used to measure the level of ROS according to the manufacturer’s protocol. In brief, the cells in 96-well plates were washed with a buffer solution containing EDTA, HEPES, KCl, and sucrose. Then, the DCFH-DA solution was added to each well, followed by 30 min of incubation at 37 °C. Finally, DCFH-DA solution was removed, and the cells were washed with buffer. The related fluorescence was measured using an ELISA fluorimeter (BioTek, USA) (excitation 488 nm; emission 525 nm). The Values of each sample were normalized for total protein concentration using the Bradford method [27].
Statistical analysis
To test the assumption of normality, we used the Shapiro–Wilks test. Then, the data were analyzed by one-way ANOVA followed by the post hoc Turkey test to detect the differences between the groups. IBM SPSS statistics 20 software (IBM Corp., Armonk, NY, USA). The statistical significance level was defined as p value < 0.05.
Results
Cell proliferation
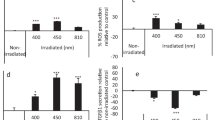
The results showed that PBM laser irradiation did not significantly affect cell proliferation with different energy density values (3.2, 6, and 9.2 J/cm2) in a hyperglycemic medium (p = 0.06). Whereas in the control group containing standard glucose concentration, all three energy density values (3.2, 6, and 9.2 J/cm2) showed a significant increase in the rates of the cell (P < 0.001) with the highest proliferation rate in 9.2 J/cm2 laser group compared with 3.2 J/cm2 and 6 J/cm2 groups at p = 0.029 and p = 0.049 respectively (Fig. 1).
Real-time PCR
The gene expression of VEGF and IL-6 were evaluated 24 h after the last laser irradiation session. The results revealed that PBM increased the VEGF expression in the hyperglycemic medium compared with the control group (p < 0.001) and with no significant difference among the three PBM laser groups (p > 0.5). VEGF was significantly upregulated in the standard medium in all PBM groups (p < 0.001). No significant difference (p = 0.936) existed between the energy density values of 6 and 9.2 J/cm2 (Fig. 2).
The results of real-time PCR test regarding the expression of vascular endothelial growth factor (VEGF) (* represents statistically significant differences at a p-value of < 0.05: comparison between PBM laser irradiation at various energy density values groups with no PBM laser irradiation group in standard medium. Whereas # represents a statistically significant difference at p value < 0.05: comparison between PBM laser irradiation at various energy density values groups with no PBM laser irradiation group in high-glucose medium)
The expression of IL-6 was increased in all three PBM laser groups following 24 h post-irradiation compared with the control group in hyperglycemic medium (p < 0.001). Among the three PBM laser groups, only the group with the energy density of 9.2 J/cm2 exhibited a significantly higher effect on IL-6 expression compared with 3.2 J/cm2 energy density group (p = 0.016). PBM laser irradiation with all three energy density values in the standard medium effectively induced IL-6 expression (p < 0.001). There were significant differences among the three laser groups in a dose-dependent manner (p < 0.001) (Fig. 3).
The results of real-time PCR test regarding the expression of interleukin-6 (IL-6) (* represents a statistically significant difference at p value < 0.05: comparison between PBM laser of various energy density with no PBM laser irradiation group in standard medium. Whereas # represents a statistically significant difference at a p value of < 0.05: comparison between PBM laser of various energy density values groups with no PBM laser group in high-glucose medium)
Reactive oxygen species
Twenty-four hours after the last PBM laser irradiation session, the level of ROS in the hyperglycemic medium was higher compared to the standard medium. Also, all PBM groups in both hyperglycemic and standard culture medium showed a significant reduction in ROS level compared with the control groups (p < 0.001), while there was no significant difference among the three PBM laser groups (p > 0.5) (Fig. 4).
Reactive oxygen species (ROS) production (* represents a statistically significant difference at p < 0.05: comparison between PBM laser irradiation at various energy density values groups with no PBM laser irradiation group in standard medium. Whereas # represents a statistically significant difference at p value < 0.05: comparison between PBM laser of various energy density values groups with no PBM laser group in a high-glucose medium)
Discussion
Our study explored the effects of 915-nm laser PBM delivered at different energy density values on HGF proliferation, IL-6, and VEGF gene expression, and ROS production under hyperglycemic conditions. This wavelength is recently used in dental practice, and there is some limited but encouraging data on the PBM effect of 915 nm on different cells, such as HGF [28] and osteoblast [29].
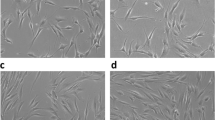
HGFs are the most abundant cells in the gingival connective tissues, which play a pivotal functional role in the health of the gingival tissues by contributing to the immune and inflammatory cascades in periodontal diseases [30]. Our findings showed that PBM effectively increased the HGF cell proliferation rate in a standard medium, whereas, in hyperglycemic conditions, no statistically significant increase was detected compared with the control. A recent similar study conducted by Chen et al. [23] investigated the effects of PBM laser dual-wavelengths (635 nm/808 nm) and each wavelength alone on human embryonic skin fibroblasts (HESF) under 33.3 mM glucose medium. Their results showed that all the wavelengths except 635 nm laser (3 J/cm2 and 12 J/cm2) were ineffective in inducing cell proliferation, which coincided with our findings. Contrarily, the results of a study conducted by Esmaeelinejad et al. [31] showed stimulatory effects on the proliferation of human skin fibroblasts were reported following PBM irradiation with HeNe laser delivered at the following energy density values: 0.5, 1, and 2 J/cm2 in a high glucose medium of 15 mM, which had different PBM protocol with much lower glucose concentration compared with our study. Notably, the reported PBM parameters in the current study were calculated at the end of the laser handpiece. As we irradiated the cells from the top of each well and considering the used wavelength and type and thickness of culture medium, we had an effective transmission of about 73%, according to the study of Silva et al. [32].
Glucose is a vital component in commercial cell culture media, serving as the primary energy source for cells. Its concentration plays a pivotal role in influencing cell growth and metabolism. The range of glucose levels in cell culture formulations varies widely, typically spanning from 1 g/L (5.5 mM) to as high as 10 g/L (55 mM) [33]. In the context of in vitro models for diabetes, the choice of glucose concentration can vary considerably based on the specific objectives and design of the study. Indeed, various research investigations have employed distinct glucose concentrations to replicate the hyperglycemic conditions associated with diabetes. Commonly utilized concentrations for modeling prediabetic conditions typically hover around 10 mM, while for diabetic conditions, concentrations of 25 mM (equivalent to 450 mg/dL) and even higher are often employed [33]. However, including antioxidants in commercial cell culture formulations can potentially influence glucose levels and their effects in an in vitro model of hyperglycemia. Some in vitro studies aim to replicate the physiological range of glucose levels in individuals with diabetes. Since antioxidants present in cell culture formulations can shield cells from oxidative stress induced by elevated glucose levels, there may be an inclination to utilize higher glucose concentrations in such scenarios [34]. Therefore, in the present study, we used a 30 mM glucose concentration as a diabetic model [35]. It is noteworthy that this concentration, in our study, did not inhibit cell proliferation, which coincided with the findings of studies conducted by Xuan et al. [36] and Lee et al. [22] on human foreskin primary fibroblast and HGF cells respectively. Interestingly, the study conducted by Lee et al. [22] showed no difference in the morphological changes of HGF cells in a high-glucose medium and no short-term reduction in the cells’ viability or cytotoxicity.
High glucose concentrations can have varying effects on cell proliferation in cell culture, depending on the cell type and the time of exposure [22, 36]. In some cell types, proliferation particularly in those with a high glucose such as fibroblasts, elevated glucose levels may provide increased energy substrates for cellular processes, potentially promoting cell metabolism rate. Conversely, high glucose concentrations can induce cellular stress and causing oxidative stress. This oxidative stress may negatively impact cell proliferation. In general, persistent exposure to high glucose, as seen in conditions like diabetes, can influence cellular behavior [22]. In our study, fibroblast cells were exposed to a high glucose condition for a brief period of 48 h. It is conceivable that prolonging this exposure time would hinder cell proliferation. Overall, our in vitro study utilizing photobiomodulation revealed interference of PBM in the cell cycle and proliferation of cells exposed to high glucose.
Altered synthesis and secretion of the proteins of the VEGF family are the typical findings in hyperglycemia [37]. In this context, a study by Tsai et al. [38] utilized human synovial fibroblasts and showed increased VEGF levels in a high-glucose medium.
This finding was suggested to be associated with ROS, PI3K, Akt, c-Jun, and AP-1 signaling pathways. In our study, an increase in the levels of VEGF was detected in the hyperglycemic condition compared with the standard medium. Comparing the PBM groups with the control, PBM groups showed a higher VEGF expression with no priority among the three energy density values. It is essential to report that the findings of a study conducted by Chen et al. [23], where an increase in the level of VEGF expression at an energy density of 3 J/cm2 in all laser groups coincided with our study’s findings, except at energy density values of 6 and 12 J/cm2 where a significant decrease in the level of VEGF expression in all the samples was initiated. Although the wavelength and origin of fibroblast cells differed from our study, PBM at higher energy density may exert an inhibitory effect on VEGF expression.
Interleukin 6 (IL-6), as an inflammatory factor, plays a significant role in the progression of periodontitis and bone loss [39]. Our study observed an increase in IL-6 in the hyperglycemic medium compared with the control group of standard glucose. This finding was also consistent with previous studies [40, 41]. In both high and low glucose mediums, PBM irradiation increased the level of IL-6 expression with the highest effect in the group of energy density of 9.2 J/cm2. In this context, however, there is conflicting data in the literature. A study conducted by Chen et al. [23] showed a similar pattern to our study in detecting IL-6 expression at 3 J/cm2 with 808 nm and dual wavelengths of 635 nm/808 nm. However, an inhibitory effect on IL-6 expression was detected when PBM irradiation at higher energy densities of 12 and 24 J/cm2 was employed.
A study conducted by Esmaeelinejad et al. [25] showed that human skin fibroblast irradiation with PBM delivered with helium–neon laser at an energy density of 0.5 and 2 J/cm2 stimulated the release of IL-6 in a high glucose medium compared with those unirradiated with PBM. Moreover, a single irradiation with 660 nm at 8 J/cm2 on HGF cells in 35 mM glucose concentration showed a significant decrease in the level of IL-6 expression [22]. Contravetioanlly, a study by Góralczyk [42] showed no significant effect of PBM (630 nm and 830 nm, 2 J/cm2) on IL-6 expression compared with unirradiated cells. These differences may be linked to using different wavelengths, PBM dosimetry, treatment protocols, or assessment of different time points. IL-6 is essential in regulating the host response to bacterial infection in periodontitis [11]. Concurrently, IL-6 can trigger tissue destruction by increasing MMP-1 levels in an inflamed periodontal tissue [12].
ROS production was another outcome measure in our study. Our findings showed a significant ROS elevation in a high glucose medium compared with a standard medium, which coincided with a study conducted by Chen et al. [35]. ROS is a double-edged sword in periodontal diseases. Low ROS concentration has a stimulatory effect on cellular proliferation and differentiation. Whereas at higher concentrations, they may have cytotoxic effects [43].
In our study, following 915 nm PBM irradiation of the HGF cells with all three energy density values (3.2, 6, and 9.2 J/cm2), a notable decrease in ROS production was detected in both high and normal glucose mediums. Contravertionally, a study by Chen et al. [35] showed a dose-dependent increase in the level of ROS production in both low and high glucose mediums was reported on HESF irradiated with PBM of the following diode laser wavelengths: 635 nm, 808 nm, and 635 nm/808 nm.
After reviewing the evidence in the literature, there are conflicting data and contradictory effects of PBM on ROS production concerning different wavelengths. In a study conducted by George et al. [44], the quantities of ROS generated by 636-nm laser irradiation on human primary dermal fibroblasts at energy density values of 5, 10, 15, 20, and 25 J/cm2 were much lower than those of unirradiated cells. However, after 825-nm laser irradiation, ROS production was significantly increased compared with the control group. They concluded that ROS generated within the biological systems depends more on laser wavelength than energy density. PBM irradiation with 808 nm on human endothelial cells showed an increase in the level of ROS production [45], which coincided with the findings of a study conducted by George et al. [44], but in contrast with our findings, which ultimately confirmed “wavelength-dependent results”.
Finally, the comparison between our results that demonstrated the efficacy of 915 nm PBM irradiation at different energy density values on HGF cells under hyperglycemic conditions with the findings of the current evidence in the literature revealed that our applied PBM protocol showed to have beneficial effects on controlling OS, which was measured by ROS production in hyperglycemic condition. Moreover, IL-6, an inflammatory cytokine, and VEGF were upregulated in the irradiation groups at the measured timepoints and were well-defined in our study.
It is important to note that the induction of interleukin-6 (IL-6) and other inflammatory responses in periodontitis can be both a natural defense mechanism and, if prolonged or excessive, detrimental. It is essential to strike a balance between the necessary inflammatory response for infection control and preventing excessive, chronic inflammation that could contribute to the progression of periodontal disease [11]. Our data based on in vitro study revealed the inductive role of PBM on VEGF and the inflammatory response while concurrently reducing reactive oxygen species production. As a result of different signaling pathway interactions, various cytokines and growth factors regulate the cellular response in challenging conditions such as hyperglycemia. Hence, the overall interpretation of the results and the efficacy of PBM should be taken with caution, and it remains challenging to recommend PBM for the treatment of diabetes-related periodontitis in clinical settings. In this context, future studies exploring the effect of PBM delivered with 915-nm wavelength on other cytokines and signaling pathways associated with hyperglycemia and comparing the results with other PBM protocols are highly recommended to identify the optimal PBM protocol.
Conclusion
Our results, for the first time, demonstrated the efficacy of PBM delivered with a 915-nm diode laser in inhibiting ROS production in favor of controlling OS response in HGF cells under hyperglycemic conditions. Moreover, our PBM protocol did not significantly affect cell viability and survival in a high-glucose medium. However, upregulation of IL-6 and VEGF was observed in PBM groups in both standard and hyperglycemic conditions. Hence, further studies are warranted to validate our work.
Data Availability
The authors confirm that the data supporting the finding of the study are available within the article. Raw data is also available upon reasonable request from corresponding author.
References
Dhir S, Lalwani R, Sharma JK, Kolte A, Bansal S, Gupta A (2019) “The Perio-Diabetes Symposium”: consensus report of the Indian Society of Periodontology and Research Society for the Study of Diabetes in India-A joint event on Periodontitis and Diabetes. J Indian Soc Periodontol 23:593–594
Polak D, Sanui T (2000) Nishimura F and Shapira L (2020) Diabetes as a risk factor for periodontal disease—plausible mechanisms. Periodonto 83:46–58
Graves DT (2000) Ding Z and Yang Y (2020) The impact of diabetes on periodontal diseases. Periodontol 82:214–224
Choi E-J, Yim J-Y, Koo K-T, Seol Y-J, Lee Y-M, Ku Y, Rhyu I-C, Chung C-P, Kim T-I (2010) Biological effects of a semiconductor diode laser on human periodontal ligament fibroblasts. J Periodontal Implant Sci 40:105–110
Basso FG, Pansani TN, Turrioni APS, Bagnato VS, Hebling J, de Souza Costa CA (2012) In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int J Dent 2012:719452. https://doi.org/10.1155/2012/719452
Li S, Liu X, Zhou Y, Acharya A, Savkovic V, Xu C, Wu N, Deng Y, Hu X, Li H (2018) Shared genetic and epigenetic mechanisms between chronic periodontitis and oral squamous cell carcinoma. Oral Oncol 86:216–224
Artese L, Piattelli A, de Gouveia Cardoso LA, Ferrari DS, Onuma T, Piccirilli M, Faveri M, Perrotti V, Simon M, Shibli JA (2010) Immunoexpression of angiogenesis, nitric oxide synthase, and proliferation markers in gingival samples of patients with aggressive and chronic periodontitis. J Periodontol 81:718–726
Ng Y-S, Krilleke D, Shima DT (2006) VEGF function in vascular pathogenesis. Exp Cell Res 312:527–537
Prapulla DV, Sujatha PB, Pradeep A (2007) Gingival crevicular fluid VEGF levels in periodontal health and disease. J Periodontol 78:1783–1787
Sakallıoğlu EE, Aliyev E, Lütfioğlu M, Yavuz Ü, Açıkgöz G (2007) Vascular endothelial growth factor (VEGF) levels of gingiva and gingival crevicular fluid in diabetic and systemically healthy periodontitis patients. Clin Oral Investig 11:115–120
Naruishi K, Nagata T (2018) Biological effects of interleukin-6 on gingival fibroblasts: cytokine regulation in periodontitis. J Cell Physiol 233:6393–6400
Sawada S, Chosa N, Ishisaki A, Naruishi K (2013) Enhancement of gingival inflammation induced by synergism of IL-1β and IL-6. Biomed Res 34:31–40
Vieira Ribeiro F, de Mendonça AC, Santos VR, Bastos MF, Figueiredo LC, Duarte PM (2011) Cytokines and bone-related factors in systemically healthy patients with chronic periodontitis and patients with type 2 diabetes and chronic periodontitis. J Periodontol 82:1187–1196
Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S (2013) Reactive oxygen species in periodontitis. J Indian Soc Periodontol 17:411–416
Liu C, Mo L, Niu Y, Li X, Zhou X, Xu X (2017) The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Front Physiol 8:439
Theodoro LH, Marcantonio RAC, Wainwright M, Garcia VG (2021) LASER in periodontal treatment: is it an effective treatment or science fiction? Braz Oral Res 35:e099. https://doi.org/10.1590/1807-3107bor-2021.vol35.0099
Hakimiha N, Dehghan MM, Manaheji H, Zaringhalam J, Farzad-Mohajeri S, Fekrazad R, Moslemi N (2020) Recovery of inferior alveolar nerve by photobiomodulation therapy using two laser wavelengths: a behavioral and immunological study in rat. J Photochem Photobiol B Biol 204:111785
Firoozi P, Amiri MA, Soghli N, Farshidfar N, Hakimiha N, Fekrazad R (2022) The role of photobiomodulation on dental-derived mesenchymal stem cells in regenerative dentistry: a comprehensive systematic review. Curr Stem Cell Res Ther. https://doi.org/10.2174/1574888X17666220810141411. Online ahead of print
Pawelczyk-Madalińska M, Benedicenti S, Sălăgean T, Bordea IR, Hanna R (2021) Impact of adjunctive diode laser application to non-surgical periodontal therapy on clinical, microbiological and immunological outcomes in management of chronic periodontitis: a systematic review of human randomized controlled clinical trials. J Inflamm Res 14:2515
Dalvi S, Benedicenti S, Hanna R (2021) Effectiveness of photobiomodulation as an adjunct to nonsurgical periodontal therapy in the management of periodontitis-a systematic review of in vivo human studies. Photochem Photobiol 97:223–242
Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, Bruska M, Dominiak M, Mozdziak P, Skiba THI (2020) Photobiomodulation—underlying mechanism and clinical applications. J Clin Med 9:1724
Lee KD, Chiang MH, Chen PH, Ho ML, Lee HZ, Lee HE, Wang YH (2019) The effect of low-level laser irradiation on hyperglycemia-induced inflammation in human gingival fibroblasts. Lasers Med Sci 34:913–920. https://doi.org/10.1007/s10103-018-2675-6
Chen H, Sun S, Pan Z, Tu M, Shi J, Bai H, Han Z, Yao J (2021) Effects of photobiomodulation on high glucose induced oxidative stress in human embryonic skin fibroblasts. IEEE J Sel Top Quantum Electron 27:1–9
Mostafavinia A, Ahmadi H, Amini A, Roudafshani Z, Hamblin MR, Chien S, Bayat M (2021) The effect of photobiomodulation therapy on antioxidants and oxidative stress profiles of adipose derived mesenchymal stem cells in diabetic rats. Spectrochim Acta A Mol Biomol Spectrosc 262:120157. https://doi.org/10.1016/j.saa.2021.120157
Esmaeelinejad M, Bayat M (2013) Effect of low-level laser therapy on the release of interleukin-6 and basic fibroblast growth factor from cultured human skin fibroblasts in normal and high glucose mediums. J Cosmet Laser Ther 15:310–317
Houreld NN, Sekhejane PR, Abrahamse H (2010) Irradiation at 830 nm stimulates nitric oxide production and inhibits pro-inflammatory cytokines in diabetic wounded fibroblast cells. Lasers Surg Med 42:494–502
Nobakht-Haghighi N, Rahimifard M, Baeeri M, Rezvanfar MA, Moini Nodeh S, Haghi-Aminjan H, Hamurtekin E, Abdollahi M (2018) Regulation of aging and oxidative stress pathways in aged pancreatic islets using alpha-lipoic acid. Mol Cell Biochem 449:267–276
Sadatmansouri S, Agahikesheh B, Karimi M, Etemadi A, Saberi S (2022) Effect of different energy densities of 915 nm low power laser on the biological behavior of human gingival fibroblast cells in vitro. Photochem Photobiol 98:969–973
Tschon M, Incerti-Parenti S, Cepollaro S, Checchi L, Fini M (2015) Photobiomodulation with low-level diode laser promotes osteoblast migration in an in vitro micro wound model. J Biomed Opt 20:078002–078002
Boström EA, Kindstedt E, Sulniute R, Palmqvist P, Majster M, Holm CK, Zwicker S, Clark R, Önell S, Johansson I (2015) Increased eotaxin and MCP-1 levels in serum from individuals with periodontitis and in human gingival fibroblasts exposed to pro-inflammatory cytokines. PLoS ONE 10:e0134608
Esmaeelinejad M, Bayat M, Darbandi H, Bayat M, Mosaffa N (2014) The effects of low-level laser irradiation on cellular viability and proliferation of human skin fibroblasts cultured in high glucose mediums. Lasers Med Sci 29:121–129. https://doi.org/10.1007/s10103-013-1289-2
Silva DFT, Mesquita-Ferrari RA, Fernandes KPS, Raele MP, Wetter NU, Deana AM (2012) Effective transmission of light for media culture, plates and tubes. Photochem Photobiol 88:1211–1216
Koobotse MO, Schmidt D, Holly JM, Perks CM (2020) Glucose concentration in cell culture medium influences the BRCA1-mediated regulation of the lipogenic action of IGF-I in breast cancer cells. Int J Mol Sci 21:8674
Lewinska A, Wnuk M, Slota E, Bartosz G (2007) Total anti-oxidant capacity of cell culture media. Clin Exp Pharmacol Physiol 34:781–786
Chen H, Tu M, Shi J, Wang Y, Hou Z, Wang J (2021) Effect of photobiomodulation on CCC-ESF reactive oxygen species steady-state in high glucose mediums. Lasers Med Sci 36:555–562. https://doi.org/10.1007/s10103-020-03057-4
Xuan YH, Huang BB, Tian HS, Chi LS, Duan YM, Wang X, Zhu ZX, Cai WH, Zhu YT, Wei TM, Ye HB, Cong WT, Jin LT (2014) High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS ONE 9:e108182. https://doi.org/10.1371/journal.pone.0108182
Wirostko B, Wong TY, Simó R (2008) Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res 27:608–621. https://doi.org/10.1016/j.preteyeres.2008.09.002
Tsai C-H, Chiang Y-C, Chen H-T, Huang P-H, Hsu H-C, Tang C-H (2013) High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochim Biophys Acta 1830:2649–2658. https://doi.org/10.1016/j.bbagen.2012.12.017
Omori K, Naruishi K, Nishimura F, Yamada-Naruishi H, Takashiba S (2004) High glucose enhances interleukin-6-induced vascular endothelial growth factor 165 expression via activation of gp130-mediated p44/42 MAPK-CCAAT/enhancer binding protein signaling in gingival fibroblasts. J Biol Chem 279:6643–6649
Chiu HC, Fu MM, Yang TS, Fu E, Chiang CY, Tu HP, Chin YT, Lin FG, Shih KC (2017) Effect of high glucose, porphyromonas gingivalis lipopolysaccharide and advanced glycation end-products on production of interleukin-6/-8 by gingival fibroblasts. J Periodont Res 52:268–276. https://doi.org/10.1111/jre.12391
Lew JH, Naruishi K, Kajiura Y, Nishikawa Y, Ikuta T, Kido J, Nagata T (2018) High glucose-mediated cytokine regulation in gingival fibroblasts and THP-1 macrophage: a possible mechanism of severe periodontitis with diabetes. Cell Physiol Biochem 50:973–986. https://doi.org/10.1159/000494481
Góralczyk K, Szymańska J, Szot K, Fisz J, Rość D (2016) Low-level laser irradiation effect on endothelial cells under conditions of hyperglycemia. Lasers Med Sci 31:825–831. https://doi.org/10.1007/s10103-016-1880-4
Nibali L, Donos N (2013) Periodontitis and redox status: a review. Curr Pharm Des 19:2687–2697. https://doi.org/10.2174/1381612811319150003
George S, Hamblin MR, Abrahamse H (2018) Effect of red light and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts. J Photochem Photobiol B 188:60–68. https://doi.org/10.1016/j.jphotobiol.2018.09.004
Amaroli A, Ravera S, Baldini F, Benedicenti S, Panfoli I, Vergani L (2019) Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med Sci 34:495–504. https://doi.org/10.1007/s10103-018-2623-5
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
An ethical approval was obtained from the Ethical Committee of Islamic Azad University (NO: IR.IAU.DENTAL.REC.1400.104).
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iranpour, B., Mohammadi, K., Hodjat, M. et al. An evaluation of photobiomodulation effects on human gingival fibroblast cells under hyperglycemic condition: an in vitro study. Lasers Med Sci 39, 9 (2024). https://doi.org/10.1007/s10103-023-03954-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-023-03954-4