Abstract
Background
Periodontal treatment in diabetic patients reduces systemic inflammatory burden and hence should be closely coordinated with the patient’s overall clinical diabetic management.
Objective
To evaluate the effectiveness of diode laser (DL) (Biolase EpicTM, 940 nm, Irvine, CA, USA) as an adjunct to scaling root and planing (SRP) on periodontal health and glycated hemoglobin (HbA1c) level of type 2 diabetes mellitus (T2DM) patients suffering from generalized chronic periodontitis (CP), currently, stage II or above/grade B or C periodontitis.
Materials and methodology
After initial screening of 55 T2DM patients, a total of 44 T2DM-CP patients (between the age group of 30 and 65 years) were selected and randomly assigned into two groups. The groups were divided into control group (n=22), treated with scaling and root planing alone (SRP alone), and experimental group (n=22), treated with scaling and root planing along with laser therapy (SRP + DL). Laser irradiation was accomplished at perio pocket setting of 0.8 W (average) in a pulse interval of 1.0 ms and pulse length of 1.0 ms delivering 24 J of energy using a 300-μm fiber optic delivery system.
Results
Thirty-seven out of 44 enrolled T2DM-CP patients completed the study. Both treatment modalities, i.e., SRP alone and SRP+DL resulted in mean significant (p < 0.001) improvement in periodontal health parameters (plaque index (PI), gingival index (GI), probing pocket depth (PPD) and clinical attachment loss (CAL)) and glycemic level (RBS, FBS, and HbA1c) in T2DM-CP patients after 6 months, and was higher in SRP+DL group in comparison to SRP alone. Among the periodontal health parameters, the mean PPD reduction and CAL gain were 51.78% and 48.26% in control as compared to 61.56% and 62.54% in experimental group respectively, whereas the mean significant reduction in HbA1c was 13.8% in SRP alone and 22.52% in SRP+DL group after 6 months (p < 0.05).
Conclusion
Periodontal treatment involving SRP+DL contributes to improved periodontal health parameters and HbA1c level in T2DM-CP patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a complex, chronic endocrinal ailment of metabolic derangement in carbohydrate, protein, and fat metabolism caused by resistance to insulin action or increased compensatory insulin secretory response, or a combination of both [1, 2]. It is predicted that there are already 415 million adults aged between 20 and 79 years suffering from diabetes worldwide, including 193 million who are undiagnosed. Additional 318 million adults are projected to have impaired glucose tolerance, and are at high risk of emerging the disease. If this rise is not ceased, then by 2040, 642 million people may be having active disease [3]. T2DM has a deep effect on mankind health and needs continuous multidisciplinary management strategies [4].
Periodontal disease has been recognized as a possible modifiable risk factor for poorly controlled DM patients [5]. There are substantial evidence based on observational and few randomized clinical trials that establish a bidirectional relationship between diabetes and periodontitis events [6]. The presence of one condition tends to promote the other, and the meticulous management of either may assist treatment of the other [7]. Current literature of various meta-analysis advocated that periodontal treatment might be effective in improving metabolic control in terms of reduction of HbA1c concentration in diabetic patients [8].
Although scaling and root planing (SRP) is considered the gold standard for the treatment of periodontitis, mechanical therapy alone, however, may not always be predictable and often does not completely eliminate the periodontal infection and associated inflammation. It may fail to eradicate the pathogenic bacterial species because of their location within the periodontal tissues or in other areas inaccessible by periodontal instruments during close debridement, especially in systemically compromised patients and individuals prone to more severe cases of periodontitis, such as diabetic patients [9]. Adjunctive periodontal therapies including the use of anti-microbial agents, use of antimicrobial photodynamic therapy, and use of lasers of various wavelengths are often suggested to enhance the mechanical treatment outcomes [10], and thus resulted in positive clinical impact in periodontitis management [11].
Diode laser (DL) is a compact, portable laser unit that consists of a semi-conductor as the active medium present and electric current as the pumping system. Wavelengths of DLs have great affinity for hemoglobin and melanin pigments and in vitro studies shown them to have bactericidal and detoxification effects [12]. Sgolastra et al. [13] in a meta-analysis described that application of DL, with a wavelength between 655 and 980 nm, can accelerate wound healing through the facilitation of collagen synthesis, promotion of angiogenesis, and augmentation of growth factor release. Many studies also evaluated the adjunctive effect of a diode laser along with nonsurgical periodontal therapy in type 2 diabetic patients and found improvement in their clinical and biochemical parameters and glycemic control after 3 months in different population groups [2, 14,15,16,17,18]. When used as an adjunct to periodontal therapy, DL may have been reported to potentiate the effects of SRP by reducing the bacterial load, altering the inflammatory response, and accelerating healing [2] that further results in improvement in periodontal and diabetic health of the patient. Therefore, the primary objective of this study was to evaluate the effectiveness of SRP with a diode laser (940 nm) on periodontal health parameters and glycemic control in type 2 diabetes mellitus patients suffering from generalized chronic periodontitis (T2DM-CP, currently, stage II or above/grade B or C periodontitis).
Materials and methods
This prospective randomized clinical study was conducted in Department of Periodontology between December 2016 and December 2018. The study protocol was conducted in accordance with the ethical principles described in the declaration of Helsinki 1998 revised 2008 after approval from the Institutional Research and Development Committee (SDC/IRDC/2016/MDS/27) and Institutional Human Ethics Committee (SDC/IHEC/2016/MDS/P-27). The study protocol was explained to the patients, and written informed consent was obtained from the patients who agreed to participate voluntarily.
For the present study, a total of 55 patients (both males and females) non-alcoholic, non-smoking (self-reported) who had a confirmed diagnosis of T2DM with no other contributing medical history, and visited outpatient Department of Periodontology for management of generalized chronic periodontitis (stage II or stage III/grade B or C periodontitis) were recruited, based on the following inclusion criteria.
Inclusion criteria were as follows: Otherwise, systemically healthy male and female patients between the ages of 30 and 65 years, who were diagnosed as T2DM based on the criteria given by WHO (fasting plasma glucose (FPG) ≥ 126 mg/dl, random blood sugar (RBS) level ≥ 200 mg/dl, 2-h post-prandial glucose (PP) ≥ 200 mg/dl) and glycated hemoglobin (HbA1c) levels > 6%) [4]; patients with ≥ 20 teeth, and have ≥ 8 sites with clinical attachment loss (CAL) ≥ 3 mm and pocket probing depth (PPD) ≥ 3 mm (stage II or stage III/grade B or C periodontitis); non-smokers or who had quit smoking in the past 6 months (self-reported); patients who had not received any medication except oral hypoglycemics within the preceding 6 months.
Patients having any other systemic diseases (besides DM) that could affect the progression of chronic periodontitis; or received periodontal therapy over the past 6 months; pregnant and lactating women and alcohol consumers; or with any localized infection other than periodontal disease were excluded from the study.
The sample size was calculated considering a mean PPD reduction of 1 mm between the highest and lowest means. Based on this, eighteen T2DM-CP patients in each group would be enough to provide 80% power with a 5% significance level. The required sample size was also estimated based on the previous study by Kocak et al. [2] that considered HbA1c levels as the primary outcome measurement among the two groups (experimental and control), indicating that 18 T2DM patients in each group would be sufficient to detect a 0.7% difference max (σ1, σ2), with variations of 0.6 (SD of experimental group) and 0.7 (SD of control group) in mean HbA1c with a 5% significance level and 80% power. Thus, considering an attrition rate of 15%, a total of 22 T2DM patients were eventually recruited in each group.
Study design
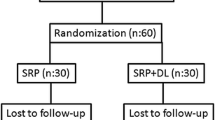
After initial screening of 55 T2DM patients, a total of 44 patients suffering from T2DM-CP (between the age group of 30 and 65 years) were randomly assigned into two groups to receive either the control or experimental treatment modality, as shown below (Fig. 1). To control the effect of confounding variables such sex, metabolic syndrome, cardiovascular diseases, and oral hygiene nearly matched patients were included in both the groups. For the present clinical study, randomization was achieved by selecting patients with the help of identically sealed envelopes consisting of one of the two treatment modalities, ensuring an equal chance of selection. The groups were divided into group A (control), n = 22, i.e., patients receiving scaling and root planing treatment only (SRP alone) and group B (Experimental), n = 22, i.e., patients receiving SRP followed by diode laser application (SRP + DL).
Periodontal therapy
T2DM-CP patients fulfilling the requisite inclusion and exclusion criteria went through a complete anamnesis recording, and were scrutinized to rule out any further systemic involvement other than T2DM. Palliative care consisting of temporary/permanent restoration as per requirements to enhance patient compliance and to reduce bacterial load was performed before enrolling patients for study.
At baseline, after recording gingival index (GI), plaque index (PI) [19, 20], PPD, and CAL, all the patients in both the groups initially underwent a preliminary phase I periodontal therapy that included oral hygiene instructions (patient motivation and education), full-mouth supragingival and subgingival scaling and root planing (SRP) with hand instruments (nos. 1 to 18 Gracey Curettes, Hu-Friedy, Chicago, IL, USA), and ultrasonic scalers under local anesthesia (infiltration or block technique) using 2% lignocaine hydrochloride with adrenaline (1:2,00,000) in a single appointment (approximately 80–90 min) [21]. Randomly divided group A (control) patients received SRP as the only treatment, whereas group B (Experimental) patients underwent SRP followed by laser therapy through a 940-nm InGaAlP (indium–gallium–aluminum–phosphate) diode laser (DL) (Epic, Biolase™, Irvine, CA, USA) as explained below. SRP and DL therapy was performed in the same visit. Patients having PPD > 6 mm at 3-week follow-up received another phase of laser therapy. The clinical follow-up in both groups was executed at 1, 3, and 6 months after the treatment.
Laser therapy
The basic characteristics and laser parameters used for the treatment of periodontal pockets by a 940-nm diode laser are explained in Table 1. Laser-specific glasses (OD 4+ @ 800–820, 920–<980) were worn by the patients and clinician during the procedure. After connecting all the components (pedal and optical fiber), all perio pocket settings were maintained at 0.8 W (average) in a pulse interval of 1.0 ms and pulse length of 1.0 ms delivering 24 J of energy [22]. Laser irradiation was accomplished with a 300-μm fiber optic delivery system (Biolase EpicTM, Irvine, CA, USA). After calibration, the optic fiber tip, curved at an angle of 60°, was introduced into the periodontal pocket base by 1 mm less than the value obtained through the probing procedure, for which an endodontic stop was placed on the fiber to control this irradiation depth. The tip was inserted in parallel alignment with the root surface of the tooth to be treated toward the bottom of the pocket maintaining contact with the soft tissue wall, the device was activated, and the fiber tip was slowly moved within the pocket in apico-cervical scanning movements, as to draw a Z-pattern, leading the tip toward the inner wall of the pocket, in order to remove junctional epithelium migrated and induce bleeding necessary for the formation of a clot [16]. This was done mesially to distally at the buccal aspect and distally to mesially at the lingual aspect for each tooth. Treatment duration was of maximum 15 s per buccal or lingual surface. The length of time of application varies with the extent of disease and surface area, and larger teeth or deeper pockets were treated longer than smaller teeth or shallow pockets [23]. During the treatment, the aspirator was set aside near the site to absorb plumes. The tip was regularly inspected and cleaned with damp sterile gauze soaked in disinfectant before proceeding to the next pocket to remove residues of sulcular epithelium and infected granulation tissue and also to avoid the hot-tip effect. All sites were irrigated with saline solution after each session of irradiation to flush out the debris and to avoid any thermal damage. The periodontal therapy and laser applications were performed by the same investigator. The laser treatment was carried out in a closed room with an appropriate warning sign at the door to inform those outside.
Postoperative instructions
Standardized patient-care instructions were followed for laser-assisted periodontal therapy given to the patients from both the group A (control) and group B (experimental) to avoid any bias related to post-operative instructions. Patients from both the groups were asked: To refrain themselves from spicy, sharp, crunchy foods and alcohol-containing products for 24 h; evade seeds or husks for 3 to 5 days; rinse with saltwater (1 tsp in 8 oz of warm water) three times daily until tissues are comfortable; over-the-counter pain reliever may be taken to manage mild discomfort; in case of more severe pain contact the investigator; thorough but gentle cleaning of the treated site to enhance the healing; in treated areas, use an extra-soft toothbrush for 1 or 2 days, and floss gently; regular brushing and flossing may be done in all other areas; oral irrigation may begin after 24 h [23].
Parameters recorded
Clinical parameters
The periodontal health parameters recorded at baseline and 1, 3 and 6 months after therapy were the PI, GI, PPD, and clinical attachment loss (CAL) with the help of an UNC-15 probe. Care was taken to insert the periodontal probe parallel to the long axis of the teeth. PI and GI measurements were performed at four sites (distofacial, facial, mesiofacial, and lingual) around each tooth; PPD and CAL measurements were performed at six sites (distofacial, midfacial, mesiofacial, mesiolingual, midlingual, and distolingual) around each tooth. To reduce the individual variability, all the clinical measurements were recorded by a single investigator throughout the study.
Systemic health parameters
Vitals, body mass index and systemic parameters, fasting plasma glucose (FPG), random blood sugar (RBS), and glycated hemoglobin (HbA1c) from blood samples obtained through venous puncture of the ante-cubital vein. Body mass index (BMI): calculated as weight in kilograms divided by square of height in meters. [BMI = weight (kg) / height (m2)]. Random blood sugar: measured by chair side glucometer. Glycated hemoglobin (HbA1c): the collected sample was assayed for glycated hemoglobin (HbA1c) levels by the Nephelometry method using glycated hemoglobin kit as explained in our previous study [21].
Intra-observer reliability
A single observer who was unaware of the groups recorded all the clinical parameters. To verify intra-observer reliability, probing pocket depth (PPD) and clinical attachment loss (CAL) were evaluated by an observer randomly 2 times in ten patients at the interval of 24 h and analyzed by intra-class correlation (ICC) analysis. The ICC values showed a significant (p < 0.001) and positive correlation for both PPD (ICC = 0.9888) and CAL (ICC = 0.996) measurements between the 2 time intervals, representative of high intra-observer reliability for the periodontal health parameters.
Statistical analysis
The results were analyzed using descriptive statistics and comparisons were made among various groups. Discrete (categorical) data were summarized as proportions and percentages (%) and quantitative data were summarized as mean ± SD. The intra-observer agreement was done by intra-class coefficient (ICC) analysis. Analyses were performed on SPSS software (Windows version 17.0). Level of significance was set at p < 0.05. The following statistics were calculated in the present analysis; chi-square test, arithmetic mean, the standard deviation, Student’s unpaired t test, and paired t test.
Results
A total of 44 patients (aged between 30 and 65 years) were initially selected and screened for vitals, random blood sugar, and routine blood investigations. Among them, 7 patients (4 in the control group and 3 in the experimental group) were non-compliant and failed to follow-up (1 patient died due to accident, 2 patients shifted to an unknown place, 3 patients refused to continue, and 1 patient lost to follow-up as she got pregnant. Data of 37 patients (21 males and 16 females) was finally collected and analyzed. The number of pockets with PPD > 6 mm in SRP+DL group that needed to be treated twice were too few (a total of 8 pockets in 4 patients) to be computed by statistical analysis. Furthermore, mean of all pockets measured in each patient was computed for final statistical analysis. Out of 18 subjects in the control group, 8 (44.4%) were females and the rest (10, 55.6%) were males, while in the experimental groups, out of 19 subjects 8 (42.1%) were females and the rest (11, 57.9%) were males. No significant difference was found in male versus female proportion between the two groups (p=0.886).
On both intra- and intergroup comparisons, the temperatures, pulse rate (PR), and respiratory rate (RR) showed non-significant differences at all time intervals in both groups revealing no deviation from systemic health during the study period except periodontal health status and diabetes control level. The mean change in anthropometric parameters between the two groups revealed no significant difference in weight (Wt), height, and BMI (p > 0.05) (Table 2). Both treatment modalities, i.e., SRP and SRP+DL, resulted in mean significant improvement (p < 0.001) in periodontal health parameters (PI, GI, PPD, CAL), and glycemic level (HbA1c) in T2DM patients with CP after 6 months (Tables 3, 4). Between the two groups, the improvement in periodontal health parameters (PI, GI, PPD, CAL) and glycemic level (RBS, FBS, and HbA1c) was higher in the experimental group (SRP+DL) as compared to the control group (SRP alone) and the mean difference in the improvement was significant (Tables 5, 6) (p < 0001). Among the periodontal health parameters, the mean PPD reduction and CAL gain were 51.78% and 48.26% in the control group (SRP alone) as compared to 61.56% and 62.54% in the experimental group (SRP+DL) respectively, whereas the mean significant reduction in HbA1c was 13.8% in the control group (SRP alone) and 22.52% in the experimental (SRP+DL) group after 6 months.
Discussion
Primarily, the present study demonstrated that DL (940 nm) when used as an adjunct to SRP and T2DM-CP patients exhibited enhancement in the periodontal health clinical measurements and glycemic level (HbA1c) as compared to SRP alone. Kocak et al. [24] suggested that reduction in the local microbial load (other than red-complex microorganism) in treated T2DM-CP patients resulted in improvement in periodontal health as well as the systemic inflammatory burden, which in turn decrease long-term blood glucose levels, measured as HbA1c. Similar to Crispino et al. [16], the results of the present study showed a mean PPD reduction of 51.78% in the control group and 61.56% in the experimental group, and gain in CAL was 48.26% in the control group and 62.54% in the experimental group after 6 months.
Comparative analysis within the groups revealed significant changes at 3 months and 6 months in both control and experimental groups for both RBS and FBS, and the mean change was more significant in experimental groups at 6 months for both the groups. Le et al. [25] reported that semiconductor laser-assisted minocycline therapy in chronic periodontitis treatment was able to control blood glucose, weaken the effect of hyperglycemia on the periodontal tissue, and enhance the therapeutic effect of chronic periodontitis. Intergroup comparison in the present study revealed no significant difference in mean values of HbA1c at baseline, 1 month, 3 months, and 6 months in the control group and experimental group. However, similarly to Kocak et al. [2], intra-group comparison revealed that HbA1c showed significant improvement at 1 month, 3 months, and 6 months in the control group and at 3 and 6 months in the experimental group, and more mean changes were observed in the experimental group as compared to the control group. Janket et al. [26], in a meta-analysis, suggested that reduction of ≥ 10% is considered clinically relevant from a diabetes point of view.
Advanced glycation end products (AGEs) increased during DM may result in increased production of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, matrix metalloproteinase (MMP), and tumor necrosis factor (TNF)-α [27, 28], and RANTES (regulated upon activation, normal T cell expressed and secreted) that can worsen insulin resistance and thereby impair glycemic control and also involved in jeopardizing periodontal tissues [2]. Clinical studies have shown beneficial effects of DL application along with SRP in significantly reducing the levels of IL-6 in the periodontal tissues [2, 29]. It may be hypothesized that improvement in the levels of HbA1c could be the direct influence of this cytokine level reduction through DL application.
Although current study showed significant improvement after single adjunct application of DL along with SRP, however, multiple applications of laser therapy could improve these results [16]. Further studies with larger samples must be conducted to elucidate the potential of DL in conjunction with SRP to improve systemic parameters. Other limitations of the study were low sample size, no distinction was made between moderate and severe periodontitis as well as between controlled and uncontrolled diabetes, separately, and the lack of a negative control group.
A recent observational study reported that more than 95% of 427 T2DM patients surveyed had some periodontal destruction that consequently require periodontal management to reduce additional inflammatory burden responsible for increased insulin resistance [30]. Henceforth, the broader insight of the present study may be to provide benefit of multidisciplinary approach for the management of T2DM that may become an epidemic [31]. It further emphasizes on close collaboration between the physician, oral health professionals, and the primary health care providers for management as well as reducing the complications related to T2DM at individual as well as community level.
Conclusion
The results of the present study revealed that both treatment modalities, i.e., SRP alone and SRP with DL, showed improvement in periodontal health and glycemic parameters after 3 and 6 months, and the mean improvement was higher for experimental group as compared. However, no statistically significant differences for systemic parameters were observed between groups. A single application of DL may show additional benefits as an adjunct to SRP in T2DM-CP patients in terms of their periodontal and systemic health parameters.
References
Taylor GW (2001) Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodntol 6(1):99–112
Koçak E, Sağlam M, Kayış SA, Dündar N, Kebapçılar L, Bruno G et al (2016) Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci 31:343–353
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Grazziani F et al (2017) Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol 00:1–12
American Diabetes Association (2018) Introduction: standards of medical care in diabetes. Diabetes Care 41(1):S1–S2
Taylor GW, Borgnakke WS (2008) Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis 14:191–203
Awartini FA (2009) Evaluation of the relationship between type 2 diabetes and periodontal disease. Saudi Med J 30:902–906
Obradovic R, Kesic L, Mihailovic D, Jovanovic G, Antic S, Brkic Z (2012) Low-level laser therapy as an adjunct in periodontal therapy in patients with diabetes mellitus. Diabetes Technol Ther 14:799–803
Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram Z (2017) Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med Sci 32:449–459
Barbosa FI, Araújo PV, Machado LJC, Magalhães CS, Guimarães MMM, Moreira AN (2018) Effect of photodynamic therapy as an adjuvant to non-surgical periodontal therapy: periodontal and metabolic evaluation in patients with type 2 diabetes mellitus. Photodiagn Photodyn Ther 22:245–250
Castro dos Santos NC, Andere NMRB, Araujo CF, de Marco AC, dos Santos LM, Jardini MAN et al (2016) Local adjunct effect of antimicrobial photodynamic therapy for the treatment of chronic periodontitis in type 2 diabetics: split-mouth double-blind randomized controlled clinical trial. Lasers Med Sci 31:1633–1640
Bunjaku V, Popovska M, Grcev A, Mrasori S, Kameri A, Sllamniku Z et al (2017) Non-surgical periodontal treatment and low level laser therapy (LLLT) outcomes for patients suffering from type 2 diabetes mellitus, obesity and chronic periodontitis. J Int Dent Med Res 10:214–221
Aoki A, Miyuki K, Sasaki, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontol 36:59–97
Sgolastra F, Severino M, Gatto R, Monaco A (2013) Effectiveness of diode laser as adjunctive therapy to scaling root planning in the treatment of chronic periodontitis: a meta-analysis. Lasers Med Sci 28:1393–1402
Chandra S, Shashikumar P (2019) Diode laser - a novel therapeutic approach in the treatment of chronic periodontitis in type 2 diabetes mellitus patients: a prospective randomized controlled clinical trial. J Lasers Med Sci 10:56–63
Saglam M, Kantarci A, Dundar N, Hakki SS (2014) Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29:37–46
Crispino A, Figliuzzi MM, Iovane C, Giudice TD, Lomanno S, Pacifico D et al (2015) Effectiveness of a diode laser in addition to non-surgical periodontal therapy: study of intervention. Ann Stomatol VI:15–20
Paul P, Bilichodmath S, Sameera U (2018) Clinical and glycemic level evaluation of the efficacy of diode laser and antibiotics with scaling and root planing in treating chronic periodontitis patients with diabetes. J Dent Lasers 12:24–30
Cao R, Li Q, Wu Q, Yao M, Chen Y, Zhou H (2019) Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health 19:176
Löe H, Silness J (1963) Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 21:533–551
Silness J, Löe H (1964) Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121–135
Mohan M, Jhingran R, Bains VK, Gupta V, Madan R, Rizvi I et al (2014) Impact of scaling and root planing on C-reactive protein levels in gingival crevicular fluid and serum in chronic periodontitis patients with or without diabetes mellitus. J Periodontal Implant Sci 44:158–168
Qadri T, Javed F, Johannsen G, Gustaffson A (2015) Role of diode lasers (800–980Nm) as adjuncts to scaling and root planing in the treatment of chronic periodontitis: a systematic review. Photomed Laser Surg 33:568–575
Convissar RA (2016) Principles and practice of laser dentistry, 2nd edn. Elsevier, New York
Kocak E, Sağlam M, Arslan U et al (2020) Effect of diode laser application as an adjunct to nonsurgical periodontal therapy on the reduction of red complex microorganisms in type 2 diabetics with chronic periodontitis. Lasers Med Sci 35:1403–1410
Le R, Zhe Z, Daxu L, Chunni D, Hong T (2018) Clinical efficacy of semiconductor laser-assisted minocycline in moderate-to-severe chronic periodontitis patients with type 2 diabetes mellitus. Trop J Pharm Res 17:1165–1170
Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA (2005) Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res 84:1154–1159
Takeda M, Ojima M, Yoshioka H, Inaba H, Kogo M, Shizukuishi S et al (2006) Relationship of serum advanced glycation end products with deterioration of periodontitis in type 2 diabetes patients. J Periodontol 77:15–20
Lalla E, Lamster IB, Stern DM, Schmidt AM (2001) Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetics: mechanisms and insights into therapeutic modalities. Ann Periodontol 6:113–118
Kolbe MF, Ribeiro FV, Luchesi VH, Casarin RC, Sallum EA, Nociti FH Jr et al (2014) Photodynamic therapy during supportive periodontal care: clinical, microbiologic, immune inflammatory, and patient centered performance in a split-mouth randomized clinical trial. J Periodontol 85:e277–e286
Singh M, Bains VK, Jhingran R, Srivastava R, Madan R, Maurya SC et al (2019) Prevalence of periodontal disease in type 2 diabetes mellitus patients: a cross-sectional study. Contemp Clin Dent 10:349–357
ICMR Guidelines for management of type 2 diabetes 2018. https://medulletin.com. Accessed 28 Jan 2021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Study is financed by the authors themselves. Infrastructural support provided by the Institute.
Ethics approval
Research protocol and study design approved by Institutional Human Ethics Committee and Institutional Research & Development Committee. Written informed consent obtained from human participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key messages
Diode laser as adjunct to SRP showed additional benefits in T2DM-CP patients in terms of their periodontal and systemic health parameters.
Rights and permissions
About this article
Cite this article
Soi, S., Bains, V.K., Srivastava, R. et al. Comparative evaluation of improvement in periodontal and glycemic health status of type 2 diabetes mellitus patients after scaling and root planing with or without adjunctive use of diode laser. Lasers Med Sci 36, 1307–1315 (2021). https://doi.org/10.1007/s10103-021-03261-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03261-w