Abstract
To characterize oral sites affected by radiation-induced oral mucositis (OM) and related clinical outcomes in oral cancer patients subjected to prophylactic photobiomodulation therapy (PBMT). This study included advanced oral squamous cell carcinoma (OSCC) patients treated with prophylactic PBMT for OM. The site distribution of OM, OM grading (CTCAE NCI, Version 4.0, 2010), OM-related pain (VAS), analgesic protocol (WHO Analgesic Ladder), and use of enteral nutrition were evaluated weekly during treatment. Data analysis was performed using descriptive statistics expressed as median values and percentages. A total of 145 OSCC patients were included. OM most frequently affected the lateral border of the tongue (44.1%), buccal mucosa (37.2%), and labial mucosa (33.8%). Keratinized oral mucosa sites, including the tongue dorsum (6.21%), retromolar trigone (8.3%), and hard palate (2.76%), were less frequently affected. Peak OM scores were observed at weeks 5, 6, and 7, with severe OM (NCI grades 3 and 4) rates of 11%, 20%, and 25%, respectively. The cumulative occurrence of severe OM was 23%, which developed as early as week 3 and as late as week 7. The highest mean value of OM-related pain (2.7) was observed at the sixth week, and 13.8% of the patients required feeding support. This study showed, compared with studies that did not provide PBMT, reduced severity of mucositis, reduced pain and analgesic use, and reduced tube feeding in patients treated with PBMT. OM involving keratinized and non-keratinized surfaces should be included in the prophylactic PBMT to reduce severe OM in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cavity cancer is one of the most common cancers worldwide with annual estimates of approximately 270,000 new cases and 130,000 deaths [1]. An overwhelming majority of cases are oral squamous cell carcinoma (OSCC) with over 70% of patients diagnosed in advanced stages and with 5-year survival rates of approximately 50% [1, 2]. OSCC treatment may include surgery, radiation therapy (RT), and chemotherapy (CT) [2]. RT and chemoradiotherapy (CRT) protocols have been associated with acute toxicities that affect non-targeted tissues, including oral mucositis (OM) [3,4,5,6,7,8].
Recently, The Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) Mucositis Study Group [3] following a systematic review of the literature suggested that OM pathogenesis is strongly linked to inflammatory signaling [3]. Thus, targeting inflammatory mediators and modulating reactive oxygen species continues to be a key consideration in OM management [3,4,5,6]. In this context, photobiomodulation therapy (PBMT) utilizes low-energy red and near-infrared light to reduce inflammation, relieve pain, and ultimately promote tissue regeneration, potentially a non-medication strategy to prevent and reduce the severity of CRT-induced OM [7, 9,10,11,12]. MASCC/ISOO recently recommends the use of PBMT (wavelength 632.8 nm) for the prevention of OM in head and neck cancer patients undergoing RT with or without concomitant CT [7].
During PBMT, the red/near-infrared light photon energy is absorbed by cytochrome c oxidase in the mitochondria, which is the last enzyme in the electron transportation chain, playing a pivotal role in metabolism and production of ATP [12,13,14,15]. The more photons are absorbed by cytochrome c oxidase, the more oxidized (activated) state the cytochrome c oxidase will be; therefore, the accelerated oxygenation process and extra production of ATP may protect the oral mucosa and promote tissue healing [11, 13,14,15,16].
In PBMT, the following treatment parameters have been recommended: wavelength (633–685 nm or 780–830 nm), energy density (laser or light-emitting diode output 10–150 mW), dose (2–10 J/cm2), treatment schedule (two times a week up to daily), emission type pulsed (< 100 Hz), and route of delivery (intraoral or extraoral/transcutaneous) [10, 11, 17,18,19]. It is unclear, however, to what extent the delivered PBMT therapy effectively provides an adequate uniform dose to the at-risk tissues. The objective of this retrospective study was to describe the clinical features and outcomes of OM in a large and homogeneous sample of patients with advanced OSCC treated with prophylactic PBMT therapy while receiving CRT.
Patients and methods
Study design
This was a single-center retrospective study designed to evaluate the clinicopathological features of OM in patients undergoing RT with or without concomitant CT, for OSCC and who received prophylactic PBMT therapy at Sao Paulo State Cancer Institute (ICESP, Brazil) from January 2009 to December 2014. This study was approved by the Ethics Committee of the School of Medicine of the University of Sao Paulo, Sao Paulo, Brazil (Protocol# 1.897.352), and conducted in accordance with the Declaration of Helsinki. The data collection followed the guideline for reporting observational studies as per Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [20].
Inclusion criteria
This study included previously untreated advanced OSCC patients subjected to postsurgical RT (with or without concomitant cisplatin) using a 6-MV linear accelerator (Synergy Platform, Elekta AB, Stockholm, Sweden) and included patients undergoing curative protocols of head and neck RT, for 5 days/week for 6–7 weeks, with or without concomitant CT.
The RT protocols with radiation volumes encompassed the primary site and areas of lymph nodes at risk and received cumulative doses that ranged from 60 to 70 Gy. All included subjects received stabilization of oral disease before starting RT and all included subjects completed the institutional standard-of-care PBMT protocol for prevention of OM [19].
Exclusion criteria
Subjects who missed one or more RT or PBMT sessions were considered to have received incomplete treatment and were excluded from the study. Subjects that did not have oral disease stabilized and patients that presented tumor site other than the oral cavity and oropharynx were also excluded.
Photobiomodulation protocol
The PBMT institutional protocol used was established by the Sao Paulo State Cancer Institute (ICESP), Brazil [16]. Trained dentists administered the PBMT on outpatient basis. PBMT was provided daily for 5 consecutive days (Monday to Friday), immediately before each RT session. All patients were treated using a Twin Flex (MM Optics, São Carlos, Brazil) PBMT device. The PBMT parameters are described in Table 1. The laser hand piece was activated when positioned flatly against several oral mucosa sites. For prophylaxis, 10 s was used per point applied to the normal or erythematous oral mucosa including the upper and lower lip mucosa, bilateral buccal mucosa, bilateral ventrolateral tongue, bilateral lip commissure, floor of the mouth, and soft palate, whereas 60 s was used in ulcerated lesions for OM treatment [19].
PBMT therapy was not delivered over an active tumor site but was performed in cases of clinically sound mucosa after tumor surgical resection. PBMT was not prophylactically delivered to oral keratinized tissues including the tongue dorsum, hard palate, or gingiva [19]. Thermal effects reported by the patients included in this study were not quantified because there is no evidence for tissue heating for PBMT protocols operating with a wavelength of 660 nm.
During RT treatment the oral complications were recorded daily. All electronic medical records were assessed and subjects with missing or unclear information reported on the records were excluded from the study.
Oral mucositis assessment
A trained dental surgeon conducted OM grading using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, Version 4.0, 2010) on the last day of each week of treatment (D5, D10, D15, D20, D25, D30, and D35) as part of standard of care. The following sites were evaluated: buccal mucosa, floor of the mouth, gingiva, hard palate, labial mucosa, oropharynx, retromolar trigone, soft palate, tongue dorsum, and ventrolateral tongue.
Patient self-reported OM pain was recorded using a visual analog scale (VAS) (where 0 is painless and 10 is the highest level of pain) at the end of each week of RT. Pain levels were not specifically recorded by each oral cavity anatomical sites included in the OM assessment. Medication used for OM analgesia was recorded weekly and classified by levels following the WHO Analgesic Ladder [21]: no analgesics, patients without pain related to OM; level 1, low level of pain (VAS 1–3; paracetamol or dipyrone and/or ketoprofen or celecoxib); level 2, moderate level of pain (VAS 4–6; codeine or tramadol or dipyrone and/or ketoprofen or celecoxib); and level 3, severe level of pain (VAS 7–10; morphine or oxycodone + paracetamol or dipyrone and/or ketoprofen or celecoxib).
Statistical analysis
Data were analyzed using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA) using descriptive statistics. Results were expressed as median values and percentages.
Results
Patient characteristics
There were 145 patients with advanced oral cavity SCC who met the inclusion criteria and were included in this study (Table 2). The mean age was 58.8 years ranging from 35 to 86 years old (standard deviation (SD) = 10.19) and 73.8% of included patients were males. SCC of the lateral border of the tongue was the most frequent primary site (46.9%), followed by the floor of the mouth (16.5%) and retromolar trigone (10.4%). All patients received complete and uninterrupted postoperative RT (n = 60) or CRT (n = 85).
Oral mucositis assessment
All patients developed OM during the treatment period (Table 3). During the first 2 weeks of treatment (D5–D10), OM grades varied from 0 to 2 with a mean pain score of 0 and 0.52, respectively. By the end of the third week (D15), grade 3 lesions developed in 3.7% of patients with a mean pain score of 1.72. Grade 3 OM increased to 5.5% and 11% during weeks 4 and 5 (D20–D25) with mean pain scores of 2.19 and 2.57, respectively. Grade 4 OM were first observed at the end of the sixth week (D30) and remained stable at 1.3% until the end of the treatment (D35) with mean pain scores of 2.69 and 2.22, respectively. In the last week of RT, the most affected OM sites were the ventrolateral tongue (92 (63.4%) patients), buccal mucosa (58 (39.09%) patients), labial mucosa (52 (35.8%) patients), and soft palate (41 (28.2%) patients). Oral sites with severe OM (grade 3) were the labial mucosa, ventrolateral tongue, buccal mucosa, soft palate, floor of the mouth, and oropharyngeal mucosa. Overall higher oral pain levels were observed in those patients affected by OM in the labial mucosa, ventrolateral tongue, buccal mucosa, soft palate, floor of the mouth, and oropharynx. The most frequently oral anatomic sites affected by OM were also those associated with increased pain as seen on Tables 4 and 5. The hard palate, tongue dorsum, and retromolar trigone were rarely affected by OM and its associated pain. Gingival tissue was not affected.
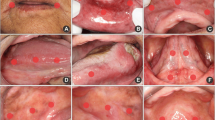
The most common oral sites affected by OM were the lateral border of the tongue (44.1%), buccal mucosa (37.2%), and labial mucosa (33.8%). Keratinized mucosal sites were also affected by ulcerative OM lesions, including the dorsal surface of the tongue (6.21%), the retromolar trigone (8.3%), and hard palate (2.76%) (Fig. 1). On the last day of RT, 113 (77.9%) patients had more than one site affected by OM.
Images of oral mucositis in areas that are not part of the laser application protocols. a Oral mucositis affecting the lip commissure in both sides. b Severe oral mucositis affecting the hard palate. c Severe oral mucositis in the anterior tongue dorsum. d Confluent oral mucositis in the dorsal surface of the tongue. e Ulceration areas in the retromolar trigone. f Severe oral mucositis affecting dorsal surface and lateral border of the tongue
OM-related pain management is summarized in Table 5. At the end of the first week of treatment (D5), no patients required analgesics. At the end of the third week (D15), 95 (65.5%) patients did not report OM-related pain, 23 (16%) patients were using level 1 analgesics, 21 (14.5%) used level 2 analgesics, and 6 (4%) used level 3 analgesics. By the end of RT (D35), 54 (37.2%) patients did not report OM-related pain, 21 (14.5%) patients used level 1, 50 (34.5%) patients level 2, and 20 (13.8%) patients required level 3 analgesics.
On the first day of RT, 51 (35.2%) patients had unrestricted diet, 76 (52.4%) had restricted diet (soft or liquid intake only), and 18 (12.4%) by enteral diet (nasogastric tube or gastrostomy). At completion of treatment (D35), 24 (16.5%) of the patients had an unrestricted diet, 83 (57.3%) had restricted diet (soft or liquid intake only), and 38 (26.2%) were fed by enteral diet (nasogastric tube or gastrostomy) (Table 6). There were no significant differences regarding OM prevalence or any of the investigated outcomes between patients who undergo RT alone or combined with CT.
Discussion
Combined CRT protocols represent the standard of care for advanced stage OSCC, although treatment increases acute toxicities, including OM [22], which reinforces the need to develop protocols to prevent and treat oral toxicities [23].
This study described the frequency and distribution of OM lesions in patients with OSCC undergoing head and neck RT with or without concomitant CT and receiving prophylactic PBMT. The results of our study suggest that although the main affected sites were non-keratinized tissues [24], such as the lateral border of the tongue, buccal mucosa, and lip mucosa; and the highly keratinized areas of the oral mucosa, such as the dorsal surface of the tongue, the retromolar trigone, and hard palate, were affected in less than 10% of patients, suggesting that PBMT protocols may be further optimized for best results to include the entire field of high-dose RT. As the keratinized sites are not typically considered to be at high risk for OM, these sites were not included in the PBMT protocol.
The current study did not include a concurrent control group as at our institution all OSCC patients are treated with PBMT for prevention of OM as a routine standard of care [19]. However, we attempted to address this issue by comparing the results of this study with those of previously published, randomized controlled trials that included treatment outcomes of head and neck cancer patients treated with multimodal therapy.
The epidemiology and severity of OM reported in the present study are similar to those found in previously published phase III studies in patients undergoing RT for head and neck cancer who underwent prophylactic PBMT. Less than 30% of the study patients developed severe OM (grades 3 and 4), although almost all patients developed some grade of OM during the course of treatment, similar to the existing literature [25]. Our finding of 23% of patients with severe OM (grades 3 and 4) at the end of RT is very similar to those found in the literature by Gouvêa de Lima et al. [10], who observed that 22% of the patients showed grades 3 and 4 of OM after receiving prophylactic PBMT, and by Gautam et al. [26] who observed that oral cavity and head and neck cancer patients presented 29% and 23.4% grade 3 and 4 OM, respectively.
The literature describes similar PMBT treatment schedules (5 days per week during weekdays prior to RT) and exclusion of active tumor areas from the PBMT application sites [19, 25,26,27,28]. Differences in PBMT dose with values of 2, 3, 4, and 10 J/cm2 are reported [19, 25,26,27,28]. A high heterogeneity in oral site distribution treated with PBMT is reported, whereas the institutional protocol in this study included sites with a total of 7 different oral sites [19] while other studies reported 6, 5, or 3 oral sites included for the PBMT prophylactic application [25,26,27,28]. Although PBMT is well established as a prophylactic approach for OM, these considerable differences in protocols adopted by different institutions may influence the response to the treatment. According to Wang et al. [15], the PBMT parameters used in the current study would not be able to generate tissue heating, discomfort, or thermal changes with potential to impact the OM management outcomes. Hence, the treatment effects observed in this manuscript were most probably induced by increased cytochrome c oxidase and related with higher ATP synthesis as described by Karu et al. [12].
The rates of severe OM observed in the present study were considerably lower when compared with those of the placebo groups of phase III OM studies reported in the literature, where 1% of the patients developed grade 4 OM while studies reported values ranging from 4.2 to 20.8% for HNC and OSCC patients, respectively [26, 29].
Low grades of OM similar to the ones found in the present study are expected to occur in treatment of other primary tumor sites such as the larynx and hypopharynx, due to the lower RT dose delivered to the oral mucosa [10, 28]. The OM assessment in the present study may be considered remarkable when considering the large number of patients with stage IV oral cancer receiving highly cytotoxic therapy, supporting the use of PBMT. All patients included in this study received the same PBMT protocol delivery encompassing the upper and lower lip mucosa, bilateral buccal mucosa, bilateral ventrolateral tongue, bilateral lip commissure, floor of the mouth, and soft palate except active tumor site. This result may drive the development of new PBMT protocol strategies optimized for different oral anatomic sites during the course of CRT-induced OM.
The mean pain rating related to OM in our study was considerably lower than those reported in the literature. Gautan et al. [26] observed the highest mean pain rating related to OM to be 4.67 in the laser group on the fifth week of treatment, whereas in the present study, the highest mean value of pain was 2.69, in the sixth week of treatment. When compared with the pain rating of the patients in placebo groups of phase III studies, the low pain rating found in our study suggests that the PBMT is capable of reducing the severity of pain reported by the patients. Considering this result, further studies are needed to determine if the use of prophylactic PBMT may be correlated with reducing the need of opioid use and the need of tube feeding, resulting in reduced cost of care and improving the quality of life of these patients [28,29,30].
Although a number of interventions are available to relieve pain associated with OM, there is weak evidence to support one intervention over another. According to a recent Cochrane review [31], randomized clinical trials designed to access the efficacy of OM treatments are scarce and offer little clinical guidance. In this context, our results, in terms of pain scores, reinforce the potential of PBMT to prevent OM-related pain, especially after the third week of treatment which is a time point when increased oral pain is reported by patients [32].
Additional evidence of the benefits of PBMT to prevent oral pain related to OM is evident in the smaller number of subjects that required enteral feeding observed in the present study (20 (13.8%)), when compared with existing literature that shows rates of up to 35% of the patients requiring the placement of enteral feeding tubes [23]. The institutional protocol for placement of a nasogastric tube is usually according to the patients’ needs regarding poor nutritional intake due to odynophagia or dysphagia. This lower percentage of patients requiring enteral feeding is expected to be associated with lower cost and improved quality of life during therapy, and furthermore that return to improved function following treatment would be facilitated [23].
Our findings suggest that the PBMT may offer the potential to reduce the occurrence and severity of OM and associated pain and reducing the use of enteral feeding and opioid analgesic use. Although not usually reported by the literature, the dorsal surface of the tongue, the retromolar trigone, and the hard palate were often affected by OM, which suggests that PBMT treatment should include these regions when included in the high-dose radiation volume and provides an adequate uniform dose to the at-risk oral mucosa tissues of advanced OSCC patients. Nonetheless, future prospective randomized controlled trials including keratinized mucosa sites in areas of prophylactic PBMT application would be ideal to further validate these results and also improve the development of new PBMT protocols for CRT-induced OM.
Limitations
The limitations of the present study include its retrospective nature in that it was a single institutional trial and, most importantly, it does not include a concurrent control group, as all OSCC patients are treated at our institution with PBMT for prevention of OM as routine standard of care. Because of the retrospective nature of this study, we could not collect pain outcomes specifically for each oral mucosa subsite. These limitations may guide the design of future clinical prospective studies.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Marta GN, William N Jr, Feher O, Carvalho AL, Kowalski LP (2015) Induction chemotherapy for oral cavity cancer patients: current status and future perspective. Oral Oncol 51:1069–1075. https://doi.org/10.1016/j.oraloncology.2015.10.009
Bowen J, Gibson R, Coller J, Blijlevens N, Bossi P, Al-Dasooqi N, Bateman E, Chiang K, De Mooji C, Mayo B, Stringer A, Tissing W, Wardill H, Van Sebille Y, Ranna V, Vaddi A, Lalla R, Cheng K, Elad S (2019) Systematic review of agents for the management of cancer gastrointestinal mucositis and clinical practice guidelines. Support Care Cancer 10:4011–4022. https://doi.org/10.1007/s00520-019-04892-0
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284. https://doi.org/10.1038/nrc1318
Treister N, Sonis S (2007) Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg 15:123–129. https://doi.org/10.1097/MOO.0b013e3280523ad6
Sonis ST (2009) Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 45:1015–1020. https://doi.org/10.1016/j.oraloncology.2009.08.006
Zadik Y, Arany P, Fregnani E, Bossi P, Antunes H, Bensadoun R-J, Gueiros L, Majorana A, Nair R, Ranna V, Tissing W, Vaddi A, Lubart R, Migliorati C, Lalla R, Cheng K, Elad S (2019) Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 10:3969–3983. https://doi.org/10.1007/s00520-019-04890-2
Iqbal MS, Chaw C, Kovaric J, Aslam S, Jackson A, Kelly J, Dobrowsly W, Kelly C (2017) Primary concurrent chemoradiation in head and neck cancers with weekly cisplatin chemotherapy: analysis of compliance, toxicity and survival. Int Arch Otorhinolaryngol 21:171–177. https://doi.org/10.1055/s-0036-1594020
Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, Viot M, Dejou J, Tardieu C, Benezery K, Nguyen TD, Laudoyer Y, Dassonville O, Poissonnet G, Vallicioni J, Thyss A, Hamdi M, Chauvel P, Demard F (1999) Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer 7:244–252. https://doi.org/10.1007/s005200050256
Gouvêa de Lima A, Villar RC, de Castro G Jr, Antequera R, Gil E, Rosalmeida MC, Federico MH, Snitcovsky IM (2012) Oral mucositis prevention by low-level laser therapy in head-and-neck cancer patients undergoing concurrent chemoradiotherapy: a phase III randomized study. Int J Radiat Oncol Biol Phys 82:270–275. https://doi.org/10.1016/j.ijrobp.2010.10.012
Oberoi S, Zamperlini-Netto G, Beyene J, Treister NS, Sung L (2014) Effect of prophylactic low level laser therapy on oral mucositis: a systematic review and meta-analysis. PLoS One 9:e107418. https://doi.org/10.1371/journal.pone.0107418
Karu TI (2008) Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol 84:1091–1099. https://doi.org/10.1111/j.1751-1097.2008.00394.x
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. https://doi.org/10.1109/JSTQE.2016.2561201
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40:516–553. https://doi.org/10.1007/s10439-011-0454-7
Wang X, Tian F, Reddy DD, Nalawade SS, Barett DW, González-Lima F, Liu H (2017) Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: a broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab 37:3789–3802. https://doi.org/10.1177/0271678X17691783
Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H (2016) Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 6:30540. https://doi.org/10.1038/srep30540
Sonis TS, Hashemi S, Epstein JB, Nair RG, Raber-Durlacher JE (2016) Could the biological robustness of low level laser therapy (photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol 54:7–14. https://doi.org/10.1016/j.oraloncology.2016.01.005
Zecha JA, Raber-Durlacher JE, Nair RG, Epstein JB, Sonis ST, Elad S, Hamblin MR, Barasch A, Migliorati CA, Milstein DM, Genot MT, Lansaat L, van der Brink R, Arnabat-Dominguez J, van der Molen L, Jacobi I, van Diessen J, de Lange J, Smeele LE, Schubert MM, Bensadoun RJ (2016) Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer 24:2781–2792. https://doi.org/10.1007/s00520-016-3152-z
Brandão TB, Morais-Faria K, Ribeiro ACP, Rivera C, Salvajoli JV, Lopes MA, Epstein JB, Arany PR, de Castro G Jr, Migliorati CA, Santos-Silva AR (2018) Locally advanced oral squamous cell carcinoma patients treated with photobiomodulation for prevention of oral mucositis: retrospective outcomes and safety analyses. Support Care Cancer 26:2417–2423. https://doi.org/10.1007/s00520-018-4046-z
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) STROBE Initiativem. The Strengthening the Reporting of Observational Studies inEpidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Ferreira KASL, Kimura M, Teixeira MJ (2006) The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer 14:1086–1093. https://doi.org/10.1007/s00520-006-0086-x
Zhang AM, Fan Y, Wang XX, Xie QC (2012) Increased treatment-related mortality with additional cisplatin-based chemotherapy in patients with nasopharyngeal carcinoma treated with standard radiotherapy. Radiother Oncol 104:279–285. https://doi.org/10.1016/j.radonc.2012.08.022
He Y, Guo T, Guan H, Wang J, Sun Y, Peng X (2018) Concurrent chemoradiotherapy versus radiotherapy alone for locoregionally advanced nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy: a meta-analysis. Cancer Manag Res 10:1419–1428. https://doi.org/10.2147/CMAR.S160469
Lalla RV, Sonis ST, Peterson DE (2008) Management of oral mucositis in patients with cancer. Dent Clin North Am 52: 61–viii. https://doi.org/10.1016/j.cden.2007.10.002
Bensadoun RJ, Le Page F, Darcourt V, Bensadoun F, Ciais G, Rostom YA, Poissonnet G, Dassonville O, Demard F, MASCC/ISOO mucositis group (2006) Radiation-induced mucositis of the aerodigestive tract: prevention and treatment. MASCC/ISOO mucositis group’s recommendations. Bull Cancer 93:201–211
Gautam AP, Fernandes DJ, Vidyasagar MS, Maiya AG, Vadhiraja BM (2012) Low level laser therapy for concurrent chemoradiotherapy induced oral mucositis in head and neck cancer patients - a triple blinded randomized controlled trial. Radiother Oncol 104:349–354. https://doi.org/10.1007/s00520-012-1684-4
Antunes HS, Herchenhorn D, Small IA, Araújo CM, Viégas CM, Cabral E, Rampini MP, Rodrigues PC, Silva TG, Ferreira EM, Dias FL, Ferreira CG (2013) Phase III trial of low-level laser therapy to prevent oral mucositis in head and neck cancer patients treated with concurrent chemoradiation. Radiother Oncol 109:297–302. https://doi.org/10.1016/j.radonc.2013.08.010
Antunes HS, Schluckebier LF, Herchenhorn D, Small IA, Araújo CM, Viégas CM, Rampini MP, Ferreira EM, Dias FL, Teich V, Teich N, Ferreira CG (2016) Cost-effectiveness of low-level laser therapy (LLLT) in head and neck cancer patients receiving concurrent chemoradiation. Oral Oncol 52:85–90. https://doi.org/10.1016/j.oraloncology.2015.10.022
Elting LS, Cooksley CD, Chambers MS, Garden AS (2007) Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 68:1110–1120. https://doi.org/10.1016/j.ijrobp.2007.01.053
Nonzee NJ, Dandade NA, Patel U, Markossian T, Agulnik M, Argiris A, Patel JD, Kern RC, Munshi HG, Calhoun EA, Bennett CL (2008) Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis. Cancer 113:1446–1452. https://doi.org/10.1002/cncr.23714
Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S (2010) Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 8:CD001973. https://doi.org/10.1002/14651858.CD001973.pub4
Konopka-Filippow M, Zabrocka E, Wojtowicz (2015) Pain management during radiotherapy and radiochemotherapy in oropharyngeal cancer patients: single institution experience. Int Dent J 65:e242–e248. https://doi.org/10.1111/idj.12181
Acknowledgments
Alan Roger Santos-Silva is a research fellow of the Brazilian National Council for Scientific and Technological Development (CNPq).
Funding
The authors received financial support from the São Paulo Research Foundation (FAPESP), Brazil, process numbers 2018/02233-6, 2018/04657-8, 2017/13098-0, 2013/18402-8, and 2012/06138-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of the School of Medicine of the University of Sao Paulo, Sao Paulo, Brazil (Protocol# 1.897.352), and conducted in accordance with the Declaration of Helsinki. The data collection followed the guideline for reporting observational studies as per Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was approved by the Ethics Committee of the School of Medicine of the University of Sao Paulo, Sao Paulo, Brazil (Protocol# 1.897.352).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Pauli Paglioni, M., Faria, K.M., Palmier, N.R. et al. Patterns of oral mucositis in advanced oral squamous cell carcinoma patients managed with prophylactic photobiomodulation therapy—insights for future protocol development. Lasers Med Sci 36, 429–436 (2021). https://doi.org/10.1007/s10103-020-03091-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03091-2