Abstract
Infrared neural stimulation (INS) is a new and developing approach for neural repair, with the advantages of being non-contact, spatially precise, and artifact-free. However, the disadvantage of infrared light is that it is difficult to stimulate deep tissue because of its weak penetrating power. Therefore, this paper introduces an improved method using near-infrared laser to stimulate bullfrog sciatic nerves because of its strong penetrating power. Meanwhile, gold nanorods (Au NRs) are injected into the nerve to increase the absorption of light. The mechanism is the instantaneous temperature rise caused by the absorption of infrared light by Au NRs. The compound muscle action potential (CMAP) associated with the irradiated sciatic nerve is recorded by a multi-channel physiological signal instrument. The peak to peak amplitude (Vpp) of CMAP for sciatic nerves injected with Au NRs increases significantly compared to the CMAP for control nerves without Au NRs. These results demonstrate INS by labeling nerves with nanoparticle exhibiting latent capacity to increase the efficiency, spatial resolution, and the neural responsivity, and especially, can increase the penetration depth and reduce the requisite radiant exposure level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neural functional disease can be repaired by modulating the nerves with external stimuli, such as electrical, optical, chemical, and mechanical methods [1, 2]. Among these methods, traditional electrical stimulation, which has already entered the clinical stage, is the most popular method [3]. However, electrical techniques are challenged by poor spatial resolution, short working life, and low power and data transmission efficiency. In addition, people often suffer from muscle fatigue, pain, and other negative effects from electrical stimulation because thick fibers with lower excitation thresholds are usually excited earlier than fine fibers in an electrical stimulus, which is opposite to normal physiology. In this case, high spatial resolution to realize selective stimulation is needed for treating neurological disorders. Therefore, optical stimulation becomes an alternative approach to electrical technique, with the advantages of being non-contact, spatially precise, and artifact-free [2, 4, 5].
Two kinds of methods can achieve optical stimulation, optogenetics, and direct optical stimulation. Optogenetics is a new technology to combine genetic engineering and light operation to control the target cells. The underlying mechanism is that photosensitive genes are firstly transferred into target cells via gene transfection techniques then light-sensitive ion channels are expressed on the cell membrane which can be opened by light of special wavelength. Thus, the membrane potential of both sides of the cell membrane changes, so as to stimulate or inhibit the cell selectively [6,7,8]. Although optogenetics is highly selective in control of the target neurons, it relies on gene transfection technique which is still highly experimental. Therefore, it is not adapted to human beings [8,9,10]. Also, inhibition of certain photosensitive ion channels requires strong photocurrent, and optogenetics for neurons at deep layer have not been achieved. In contrast, direct optical stimulation can regulate the electrical activity of the unmodified neurons directly by the light without genetic transfection technique. Lots of studies have shown that infrared light with wavelengths between 1450 and 2200 nm were the best choice for nerve stimulation because the tissue absorption coefficient of lasers with those wavelengths is higher [11]. So, direct optical stimulation is usually called infrared neural stimulation (INS). Compared to optogenetic, INS requires no modification of the target tissue as it only relies upon the absorption of infrared light by water in the tissue [12, 13]. So photothermal effect is considered as the most likely mechanism during INS [14], which is different from the mechanism of low-level laser/light therapy (LLLT) that uses near-infrared laser (INR) to modulate neurons based on cytochrome C oxidase absorbance [15, 16]. Wang et al. made a conclusion through the experiment of transcranial infrared laser stimulation (TILS) using LLLT that the observed effects of TILS on cerebral hemodynamics and metabolism are not induced by heating the skin [16]. Unlike LLLT, which continued to irradiate the biological tissue for a few minutes with a low energy laser, INS uses a much higher energy laser to irradiate the nerve for less than one millisecond. So it is believed that the transient tissue heating or the thermal gradient with respect to time excite the action potential, as simply heating the tissue does not generate a response [14]. Infrared light is difficult to penetrate deep tissue and also its selectivity needs to be further improved [17]. Therefore, NIR (650–900 nm) is beginning to be used for direct optical stimulation since this wavelength light has the ability to reach deeper layers.
Recently, scientists found that nanometer particles have very high absorptivity for light shining at their resonance frequency, so they are tried to be used in INS to enhance the absorption of light [2, 4, 5, 8, 10, 18,19,20]. For example, in vivo optical stimulation, gold nanorod (Au NRs) heating has been used to excite unmodified rat sciatic nerves with irradiation from a pulsed 980-nm laser [5]. In vitro study, Au NRs coated with silicon dioxide were used successfully in exciting rat auditory neurons without gene transfection [4]. And Paviolo et al. [20] also investigated the effects of Au NRs on optical stimulation of NG108-15 neurons in vitro. In addition, Au NRs is also used to restrain hippocampal neuronal cells that illuminated by continuous NIR light [10].
Au NRs, compared to other nanoparticle shapes, have the optical absorption spectrum coincides with the therapeutic Window (650–900 nm) of biological tissues [20]. Moreover, Au NRs are biocompatible, can be selectively gathered in target cells with simple surface modification, and can be injected into cells with small dimensions [2]. Therefore, in the present paper, Au NRs is used as an extrinsic absorber of a near-infrared laser at 808 nm to stimulate the bullfrog sciatic nerve. The compound muscle action potential (CMAP) associated with the irradiated sciatic nerve is recorded by a multi-channel physiological signal instrument. Since the light absorption coefficient of water for 808 nm is very small (absorption coefficient ≈ 0.05 cm−1) [21], the increase of the tissue temperature in this experiment is mainly caused by the absorption of light by the Au NRs. In order to test the enhancement of Au NRs, the peak to peak amplitude (Vpp) of CMAP for sciatic nerves injected with Au NRs is compared to the CMAP for control nerves without Au NRs. In addition, the effects of different laser energies on the sciatic nerves injected with Au NRs and the safety test of the tissue after laser irradiation were also studied.
Materials and methods
Optical stimulation
A fiber-coupled laser diode (MDL-808, Changchun New Industries Optoelectronics Technology Co., Ltd., Changchun, Jilin, China) was selected to illuminate the bullfrog sciatic nerve. The laser operates at a wavelength of 808 nm and the beam is coupled into a fiber which is controlled by a 3D micromanipulator (STW-3, Chengdu Instrument Factory, Chengdu, Sichuan, China) to locate above the sciatic nerve at a held constant distance of 1 mm. The diameter of the fiber is 400 μm and the numerical aperture is 0.22. So the spot size is 0.57 mm2 according to the knife-edge method [22]. The maximum laser output is 10 W. The laser has two luminescence modes: continuous mode and pulse mode. In this paper, the laser works in pulse mode, and the pulse peak power, pulse width, and repetition frequency can be adjusted.
Electrical recording system
Optically evoked compound muscle action potentials were recorded with three needle electrodes (one electrode was inserted into the gastrocnemius muscle as the recording electrode, one was inserted into the tendon as reference electrode, and the other one was inserted into the toe as the ground electrode.) Then, the CMAP associated with the irradiated sciatic nerve was recorded by a multi-channel physiological signal instrument (MD3000, Anhui Zheng Hua Biological Instrument and Equipment Co., Ltd. Anhui, China). CMAP signals were amplified (× 1000), band-pass filtered (0.2–3000 Hz).
Animal preparation
Bullfrog (n = 12, 5 for histological analysis, 1 for laser irradiation without Au NRs, and 6 for laser irradiation with Au NRs), weighing 300–400 g (Central Biomedical Services of Jinan University, Guangzhou, China), was performed following protocols approved by the Animals (Scientific Procedures) Act of 1986. The frogs were euthanized by decapitation. Following decapitation, the animals’ brain was pithed to ensure no feeling of pain prior to central tissue death. Map pins held positioned through the toad’s forearms stabilized the upper body during the procedure. The skin along the bullfrog’s legs was torn off and the thigh muscle covering the sciatic nerve was carefully cut away to expose the nerve surface and the nerve was dissociated by glass needle, then expose the sciatic from the upper abdominal incision to the proximal end of the knee, and tore off the muscular fascia above the nerve but kept the nerve’s epineurial layer intact. During the experiment, Ringer’s solution was periodically dripped onto the nerve to keep the nerve moist.
Gold nanorod characterization and injection
Au NRs (DKAU-4.1, Beijing DK Nano S&T Ltd., Beijing, China) with a concentration of 0.3 g/L were used in this paper. The characterization of Au NRs was observed by a transmission electron microscope (TEM) (FEI, TECNAI10, Holland). And the absorption spectrogram was tested with an ultraviolet-visible spectrophotometer (UV-2550, Shimadzu, Japan). Au NRs were injected into the prepared sciatic nerve with a microprocessor-controlled injection system (Nanoject III, Drummond Scientific Company, Broomall, PA, USA) at the rate of 46 nL/s.
Identification of laser-mediated tissue damage
To test the tissue damage after laser irradiation, the nerve was cut off immediately and fixed in the 4% paraformaldehyde solution, then embedded in paraffin. The nerve was cut into 5-μm-thick slices and stained using hematoxylin and eosin (H&E), and then observed with an optical microscope (Axiophot, Carl Zeiss, Germany).
Results and discussion
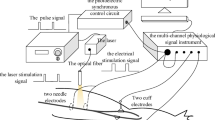
Figure 1a shows the sketch map of our experimental setup. The optical fiber of the 808-nm laser is irradiated above the target nerve, and the distance between the nerve and the fiber end face is kept 1 mm. Since the numerical aperture of the fiber with 400-μm diameter is 0.22, so the spot size is about 0.57 mm2 [22]. Real-time monitoring of CMAP in response to the infrared stimulation is recorded with multi-channel physiological signal instrument. Because the gold nanorods are purple, they can be clearly seen where gold nanoparticles are injected into the nerve (Fig. 1b).
Sketch map of near infrared stimulation enhanced by Au NRs. a Au NRs that distributed near the plasma membrane of the nerve tissue have strong absorption to the 808-nm wavelength laser, and then convert the light energy into heat energy, so that the local temperature of the surrounding nerve tissue rises. b The photograph of Au NRs distributed in the nerve, the powder purple represents Au NRs
Figure 2a shows that the size ratio of the nanorod is 4.1 with 79 nm length and 19 nm width which were measured by averaging more than 40 gold nanorods. The absorption spectra for the nanorods of a concentration of 3e−5 g/L are shown in Fig. 2b. As we can see from the diagram, the strongest absorption is at 810 nm, which is very close to the 808-nm laser that we used. Since the surface plasmon resonance of nanoparticles can make the nerve tissue an increase absorption of the resonance light [5], we considered that the laser energy needed to activate nerve tissue is greatly reduced with the enhancement of nanoparticles. So it should reduce the thermal damage of the laser to the tissue under the enhancement of Au NRs.
The sciatic nerve is exposed and injected with Au NRs; the bullfrog is placed on the frog board. As shown in Fig. 3a, fiber-coupled 808-nm laser diode illuminated the proximal part of the nerve bundle while extracellular electrical recording was performed at the gastrocnemius muscle. The fiber is controlled by a 3D micromanipulator to locate above the sciatic nerve at a held constant distance of 1 mm. By adjusting the three dimensional micromanipulator, the position of the spot on the nerve and the size of the spot can be adjusted. The optical evoked CMAPs were amplified, filtered, and superposed average in the experiment. Figure 3b exhibits the typical example of recorded CMAP with or without Au NRs. When sciatic nerve is irradiated under identical laser exposure of 0.351 J/cm2, the recorded CMAP with Au NRs is more obvious than that without Au NRs.
Unlike the result of Eom [5], it seems the nerve without Au NRs is not excited by the 808-nm laser since the CMAP is not obvious in our experiment (Fig. 3b), and there was no obvious change in the recorded CMAPs when we continued to increase the laser energy until the neural tissue burned, which was also found in Well’s experiments using 780 nm [11]. This may because NIR with the wavelength around 800 nm exhibited lower absorption by the water in tissue than that with wavelength around 980 nm which was used by Eom [21]. However, consistent with Eom’s results, we also found the nerve with Au NRs was activated under NIR irradiation. The findings indicate that Au NRs can increase the absorption of laser because of their surface plasmon resonance effect. The light energy is absorbed by Au NRs and then converted into heat energy, which causes the local temperature to rise rapidly; thus, the nerve can be excited due to heat conduction.
Many experiments have already shown that with the enhancement of the nanoparticle, NIR can be used to activate the nerve. Yong et al. found that action potentials could be evoked in vitro with in primary auditory neurons cultured with Au NRs under exposure to 780 nm light [4]. Farah et al. introduced micron scale photo-absorbing particles (iron oxide) to cultured rat cortical cells [23]. They found significant reductions in the energy required to achieve stimulation using the combination of photo-absorbers and patterned light. And Paviolo et al. has also investigated that near-infrared light can be used in vitro neural stimulation with Au NRs in cells as a selective absorber [18]. We can see that the INS can activate the action potential not dependent on a certain wavelength. The optothermal transduction of gold nanomaterials due to localized surface plasmon resonance makes them particularly suitable as optical absorbers for neural stimulation [14]. So with the enhance absorption of Au NRs, NIR can also activate the action potential.
There are many interactions between light and organisms, such as photothermal effects, photomechanical pressure waves, photochemical mechanisms, photobiomodulation, and so on. Transient heating of neurons is thought to be the mechanism of INS [11, 14], which is considered differently with respect to the mechanism of LLLT. Wang et al. used a heat generator to demonstrate that the observed effects of LLLT on cerebral hemodynamics and metabolism are not induced by heating the skin [16]. There may be two factors that cause the difference between the principles of INS and LLLT. Firstly, cytochrome C oxidase is proved to be the main absorption substance of NIR laser to modulate neurons in LLLT, while water is proved by Shapiro et al. using heavy water (D2O) to be the primary chromophore-absorbing laser irradiation during INS [13]. Secondly, the laser energy and irradiation time are different for INS and LLLT; low energy density laser is used for a long period (several minutes) irradiation in LLLT while high energy density laser is used for a short time (several nanoseconds) irradiation in INS. Although transient heating of neurons is proved to be the main mechanism of INS, the changes in the molecular level of the cells after the temperature rise still remain to be further studied.
Optically evoked CMAPs of sciatic nerve with Au NRs under different radiant exposures were recorded from six bullfrogs, and the laser operated in pulse mode with 500-μs pulse duration repeated at 2 Hz. The peak power of the laser pulse can be adjusted by adjusting the laser control power supply. The relationships between the laser radiant exposure and the control parameters are listed in Table 1. Figure 4 displays that the Vpp of CMAP of each sciatic nerve increased linearly with the increase of irradiation energy. Figure 4a illustrated the typical examples of CMAPs recorded from one sciatic nerve with Au NRs by varying laser energy during experimental stimulation. The results showed a similar trend as Eom’s experiments results with the increasing radiation energy [5]. When the energy of one laser pulse is 0.175 mJ/cm2, the Vpp of CMAP was only about 0.9683 mV, whereas the Vpp of CMAP reached 2.5797 and 3.0565 mV for the laser pulse of 0.702 and 0.877 mJ/cm2, respectively. Moreover, the change of Vpp of CMAP from 0.175 to 0.877 J/cm2 was calculated for each subject and the averaged result with standard deviation across six animals is also shown in Fig. 4b.
CMAPs recorded with Au NRs while stimulating six sciatic nerves with an increasing laser intensity. a CMAPs evoked with Au NRs using an 808-nm laser. From the top to the bottom, the laser radiant exposure is 0.175, 0.351, 0.702, and 0.877 J/cm2, respectively. The gray block represents the trigger time of the laser pulse. b The Vpp of CMAP increases with the increase of radiant exposure. The data of six nerves are displayed by different marks, and the average data of all the nerves are shown by a black triangle with the standard deviation
In the end, we performed H&E stain of the nerve tissue after laser irradiation to observe the histomorphology and evaluate the safety of the biological tissue under laser irradiation. The laser used in our experiments was divided into two pulse durations, 500 and 1000 μs, and both were repeated at 2 Hz. As shown in Table 1, when the peak power is the same, the radiant exposure of 1000-μs pulse is two times that of 500-μs pulse. We use all the laser pulse parameters in Table 1 on neural irradiation. For each experiment, the pulse was repeated 40 times, that is, the laser was irradiating for 20 s when the pulse repetition rate is 2 Hz. After irradiation, the bullfrog sciatic nerves were embedded using paraffin. The whole nerve was cut into 5-μm-thick slices and then the slices were stained with hematoxylin and eosin (H&E). Figure 5a is the nerve tissue without laser irradiation for control experiment and there is no tissue damage. Figure 5b, c shows the tissue changes with 500 and 1000-μs pulse duration respectively when the peak energy is 10 W. No obvious morphological changes were observed in Fig. 5b when the radiant energy is 0.877 J/cm2 with 500-μs pulse, but obvious cell damage was found in Fig. 5c when the radiant energy is 1.754 J/cm2 with 1000-μs pulse. Therefore, in our experiment, only the laser when the radiant energy is less than 0.877 J/cm2 was chosen to ensure no damage to the nerve tissue.
Histology (H&E stain) results of nerve tissue irradiated by different laser energies. a There was no abnormal pathology in the negative control specimens. b No obvious thermal damage was found in the nerve tissue when the laser energy is 0.877 J/cm2. c Obvious histological changes were found in the tissue when the laser energy is 1.754 J/cm2 (positive control)
Conclusion
Our study presents a novel stimulation method using 808-nm laser light to effectively modulate the bullfrog sciatic nerve with the enhancement of Au NRs. The results from this paper show that 808-nm laser can activate sciatic nerve under the enhancement of Au NRs; however, when there is no Au NRs, the neuronal electrical behavior is not affected by the 808-nm laser. So the principal superiority of this method is that with the enhancement of nanoparticles, near-infrared light with strong penetrability can activate the nerve activity in the deep layer of the tissue. In addition, the new technique not only allows non- or minimally invasive neural stimulation of a high spatial resolution and cell specificity, but also improves the safety by significantly reducing the required power of the primary stimulus [5, 24].
References
Hassler C, Boretius T, Stieglitz T (2010) Polymers for neural implants. J Polym Sci B Polym Phys 49(3):18–33
Wang Y, Guo L (2016) Nanomaterial-enabled neural stimulation. Front Neurosci 10:69
Ackermann DM, Foleds EL, Bhadra N et al (2010) Conduction block of peripheral nerve using high frequency alternating currents delivered through an intrafascicular electrode. Muscle Nerve 41(1):117–119
Yong J, Needham K, Brown WGA et al (2014) Gold-nanorod-assisted near-infrared stimulation of primary auditory neurons. Adv Healthc Mater 3(11):1862–1868
Eom K, Kim J, Choi JM et al (2014) Kang T, Chang JW, Byun KM, et al., Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods, Small 10(19):3853–3857
Packer AM, Roska B, Häusser M et al (2013) Targeting neurons and photons for optogenetics. Nat Neurosci 16(7):805–815
Zhang F, Wang L, Boyden ES et al (2006) Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3(10):785–792
Carvalho-de-Souza JL, Treger JS, Dang B et al (2015) Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86(1):207–217
Ginn SL, Alexander IE, Edelstein ML et al (2013) Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 15(2):65–77
Yoo S, Hong S, Choi Y et al (2014) Photothermal inhibition of neural activity with near infrared sensitive nanotransducers. ACS Nano 8(8):8040–8049
Wells J, Kao C, Konrad P et al (2007) Biophysical mechanisms of transient optical stimulation of peripheral nerve. J Biophys 93(7):2567–2580
Richter CP, Matic AI, Wells JD et al (2011) Neural stimulation with optical radiation. Laser Photonics Rev 5(1):68–80
Shapiro MG, Homma K, Villarreal S et al (2012) Infrared light excites cells by changing their electrical capacitance. Nat Commun 3(2):736
Thompson AC, Stoddart PR, Jansen ED (2014) Optical stimulation of neurons. Curr Mol Imaging 3(2):162–177
Wang X, Tian F, Soni SS et al (2016) Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 6:30540
Wang X, Reddy DD, Nalawade SS et al (2018) Impact of heat on metabolic and hemodynamic changes in transcranial infrared laser stimulation measured by broadband near-infrared spectroscopy. Neurophotonics 5:011004
Peterson EJ, Tyler DJ (2014) Motor neuron activation in peripheral nerves using infrared neural stimulation. J Neural Eng 11(1):016001
Paviolo C, Haycock JW, Yong J et al (2013) Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol Bioeng 110(8):2277–2291
Paviolo C, Haycock JW, Cadusch PJ et al (2014) Laser exposure of gold nanorods can induce intracellular calcium transients. J Biophotonics 7(10):761–765
Paviolo C, McArthur SL, Stoddart PR (2015) Gold nanorod-assisted optical stimulation of neuronal cells. J Vis Exp 98(98):e52566
Hale GM, Querry MR (1973) Optical constants of water in the 200-nm to 200-mm wavelength region. Appl Opt 12(3):555–563
Zhang K, Katz E, Kim DH et al (2010) Common-path optical coherence tomography guided fibre probe for spatially precise optical nerve stimulation. Electron Lett 46(2):118–120
Farah N, Zoubi A, Matar S et al (2013) Holographically patterned activation using photo-absorber induced neural-thermal stimulation. J Neural Eng 10(5):056004
Huang H, Delikanli S, Zeng H et al (2010) Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol 5(8):602−606
Funding
This work was supported by the Nature Science Foundation of China Grant (grant number: 31500796).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the protocols approved by the Animals (Scientific Procedures) Act of 1986.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Mou, Z., You, M. & Xue, W. Gold nanorod-assisted near-infrared stimulation of bullfrog sciatic nerve. Lasers Med Sci 33, 1907–1912 (2018). https://doi.org/10.1007/s10103-018-2554-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2554-1