Abstract
This study evaluated the caries resistant effects of sub-ablative Er,Cr:YSGG laser irradiation alone and combined with fluoride in comparison with fluoride application alone on enamel prepared for veneer restorations. And also, evaluated these treatments’ effects on the shear bond strength of all-ceramic veneer restorations. One hundred and thirty-five human maxillary central teeth were assigned to groups of 1a–control, 1b–laser treated, 1c–fluoride treated, 1d–laser + fluoride treated for shear bond testing and to groups of 2a–positive control(non-demineralised), 2b–laser treated, 2c–fluoride treated, 2d–laser + fluoride treated, 2e–negative control (demineralised) for microhardness testing (n = 15, N = 135). Demineralisation solutions of microhardness measurements were used for the ICP-OES elemental analysis. The parameters for laser irradiation were as follows: power output, 0.25 W; total energy density, 62.5 J/cm2 and energy density per pulse, 4.48 J/cm2 with an irradiation time of 20 s and with no water cooling. Five percent NaF varnish was used as fluoride preparate. ANOVA and Tukey HSD tests were performed (α = 5%). Surface treatments showed no significant effects on shear bond strength values (p = 0.579). However, significant differences were found in microhardness measurements and in elemental analysis of Ca and P amounts (p < 0.01). Surface-treated groups showed significantly high VNH values and significantly low ICP-OES values when compared with non-treated (−control) group while there were no significance among surface-treated groups regarding VHN and ICP-OES values. Sub-ablative Er,Cr:YSGG treatment alone or combined with fluoride is as an effective method as at least fluoride alone for preventing the prepared enamel to demineralization with no negative effect on shear bond strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the preventive measures, such as water fluoridation and the use of fluoride containing cariostatic agents have led to distinct decline in the prevalence of dental caries, this disease is still have a high manifestation [1,2,3]. By topical fluoride applications, deposition of surface CaF2 crystals occur which are highly soluble [4,5,6]. Thus, it is stated that several fluoride applications are necessary to maintain the anti-caries effect by topical treatments [7]. In some cases, perhaps faster acting applications may be more useful by alone or with fluoride. And to identify patients with an elevated risk of caries and give them appropriate and individual preventive support may be the right move.
The use of lasers in this field of dentistry has been a subject of discussion ever since lasers were introduced into dental medicine [8]. The possibility of increasing the acid resistance of enamel after laser irradiation was first demonstrated by Sognnaes and Stern in the 1970s using a CO2 laser [9]. When laser energy absorbed by the specific components of dental enamel, it is directly converted into heat which is the main factor leading to the structural and chemical changes in enamel [10]. The theories suggested to explain the reduced acid solubility of heated dental enamel are the decrease of the water permeability of dental enamel after heating [11] and when compared with unheated enamel more hydroxide and pyrophosphate but less carbonate content of lased enamel [12, 13]. Nowadays it is well known that, since they are highly absorbed by water and hydroxyapatite, the wavelengths emitted by the erbium (Er:YAG and Er,Cr:YSGG) and CO2 lasers have higher interaction with dental hard tissues allowing either to cut these tissues or make them acid-resistant depending on the laser energy transmitted [8,9,10,11,12,13,14,15].
The Er,Cr:YSGG (erbium,chromium: yttrium-scandium-gallium-garnet) laser works at a wavelength of 2.78 μm which is suitable for cavity preparation also investigated for caries-prevention purposes. Nevertheless, it is so important that to ensure the caries-prevention effect, lased surfaces must not be ablated only morphological or chemical changes must be provided. For this purpose, several studies have been performed with low energy densities so-called sub-ablative laser irradiation [8,9,10,11,12,13,14,15,16,17].
For a few dacades, since porcelain laminate veneers (PLVs) require less invasive tooth preparation with high esthetic appeal and patient satisfaction there is an increasing demand for treating unaesthetic anterior teeth by using PLVs. These restorations are also biocompatible, resistant to staining and wear [19,20,21]. Improvements in adhesive systems and in new-generation porcelain technology have supported the use of these materials. Currently, different ceramic systems are available in the market, and also there are different methods to fabricate all-ceramic restorations [22, 23]. In recent years, there has been a significant increase in chair side dental computer-aided design/computer-aided manufacturing (CAD/CAM) materials like lithium disilicate glass ceramics, leucite-reinforced glass ceramics, feldspathic glass ceramics, some kind of zirconia polycrystals, polymer-infiltrated ceramics and nanohybrid-composites with inorganic ceramic fillers [24]. Among them, glass ceramics have highly natural looking outcomes, excellent optical properties, mechanical and chemical stability and biocompatibilty.
Although studies have reported the long-term clinical success rates, but also, there are still failures even if in low rates. Researchers stated that the most frequent failure was fracture/chipping, but in these studies, caries formation was also detected in changing rates between 1 to 3.5% [19,20,21, 25,26,27,28,29,30,31,32].
So the aim of this in vitro study was to evaluate the acid-resistant effect of sub-ablative laser treatment (alone and combined with fluoride) on dental enamel by using inductively coupled plasma-optical emission spectrometry (ICP-OES) and Vicker’s microhardness tests to analyze mineral solubility and to evaluate the effects of this laser treatment on the shear bond strength of all glass ceramic restorations to enamel. There were two null hypothesis:
1. Sub-ablative laser treatment has the potential decreasing effect on demineralisation of dental enamel caused by acidic conditions.
2. This laser treatment has the negative effect on shear bond strength of glass ceramic laminate restorations to enamel.
Materials and methods
Sample preparation
The protocol of this study plan was approved by the Ethics Committee of Kirikkale University (Approval number 18/13, 29.06.2015). One hundred and thirty-five noncarious human maxillary central teeth extracted within the last 6 months were used in this study. After removal of dental plaque, calculus, and periodontal fibers, the teeth were stored in 0.1% thymol-containing distilled water under refrigeration at 4 °C until they were required for the study. Before the experiment the teeth were observed in a stereomicroscope for cracks, caries, defects and the presence of calculus. After cleansing, the selected teeth crowns were separated from the roots at the cement–enamel junction using a section machine (Minitom, Struers Inc., Westlake, OH, USA) with a diamond disk (Isomet; 10.2 cm × 0.3 mm, arbour size 1/2 in., series 15HC diamond; Buehler Ltd., Lake Bluff, IL, USA) at low speed. Then the teeth were rinsed with distilled water for 15 s and dried with oil-free compressed air. Samples were then assigned randomly to one of the four groups for shear bond testing and to one of the five groups for microhardness testing (n = 15, N = 135). Demineralisation solutions of specimens of microhardness measurements were used for the ICP-OES analysis.
Shear bond groups | Microhardness and ICP-OES groups |
Group 1A—control group | Group 2A—positive (+) control group (non-demineralised) |
Group 1B—laser-treated group | Group 2B—laser-treated group |
Group 1C—fluoride-treated group | Group 2C—fluoride-treated group |
Group 1D—laser + fluoride-treated group | Group 2D—laser + fluoride-treated group |
Group 2E—negative (−) Control group (demineralised) |
Enamel preparation
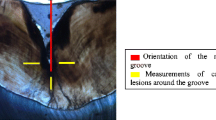
At first, by using a depth preparation bur (Diatech, Coltene/Whaledent, AG, Switzerland) depth-orientation grooves (0.5 mm in depth) were placed on the facial surfaces of the teeth. The preparation surfaces were covered with a water insoluble pen. Then, the surfaces were grinded with silicon carbide abrasive papers of grit sizes of 100, 400, and 600 (LecoR VP 100, Leco Instrumente GmbH, Germany) without exceeding the depth-orientation grooves. The prepared enamel surface areas were approximately 5 mm in diameter. Until the colour was removed from the middle third of the covered facial surfaces preparations were continued. In total, 135 teeth were prepared, among them 60 specimen used for shear bond testing and 75 for microhardness measurements. A 4 × 4 mm area in each of the 135 samples of tooth enamel was identified, with the bottom edge located 2.0 mm above of the cemento-enamel junction. The parts outside this defined area were coated with two layers of acid-resistant varnish sealer.
Enamel surface treatments
Laser treatment
In groups 1B and 2B, samples were irradiated immediately after removal from the distilled water in order to prevent drying out of the dental hard substance and any associated corruption of the results. Defined 4 × 4 mm areas were irradiated with an Er,Cr:YSGG laser device (Waterlase Millennium™, Biolase Technologies Inc., San Clemente, CA, USA). This equipment emits photons at a wavelength of 2.78 μm. The power output was 0.25 W. The repetition rate was fixed at 20 Hz and pulse duration was fixed on 140 μs. The energy density per pulse was 4.46 J/cm2 which calculated as follows: power, 0.25 W; frequency, 20 Hz; pulse energy, 12.5 mJ; focal spot area, 0.28 × 10−2 cm2 and the energy density, 12.5 mJ × 10−3 / 0.28 × 10−2 = 4.46 J/cm2.
MGG6 sapphire tip was used. The beam diameter at the focal area for the handpiece was 600 μm. These parameters (Table 1) were selected after a careful review of the literature [8,9,10,11,12,13,14,15,16,17,18]. The tip was positioned 1.0 mm from the enamel surface [focused mode]. To ensure consistent spot size with the hand irradiation, an endodontic file was fixed to top of handpiece to keep the distance of 1 mm during the irradiation. The handpiece was positioned 3–5° angle to the enamel surface to protect the tip from reflected rays, and the samples were irradiated by hand in focused mode, screening the test surface with a uniform sweeping motion, moving the handpiece horizontally and vertically for 40 s (20 s vertically and 20 s horizontally) [14, 17].
Fluoride treatment
In groups 1C and 2C, 5% sodium fluoride containing Flor-Opal Varnish (Ultradent Products Inc., South Jordan, UT, USA) was applied immediately after removal from the distilled water and left untouched for 30 min.
Fluoride + laser treatment
In groups 1D and 2D, the specimens were firstly treated with fluoride with the same way and then laser irradiated according to the procedure used in groups 1B and 2B. In groups 1A, 2A and 2E, no treatment was done. All specimens kept in distilled water after surface treatments.
Shear bond strength test
Tooth specimen preparation
Teeth were placed in a flat plane with their whole facial surfaces completely horizontally touched to modelling wax. Then a custom-made Teflon mold put on the wax and self-curing acrylic resin was placed into the mold from the top. After curing, resin blocks were removed from mold and the wax on the facial surfaces of the specimens washed with hot water by gently brushing until all the wax removed. This process was separately done for all surface-treatment groups.
Ceramic specimen preparation
VITABLOCS Mark II (Vita Zahnfabrik, Bad Säckingen, Germany) feldspathic glass ceramic CAD/CAM blocks were used for this study. Sections (n = 60) with a thickness of 2.5 mm were prepared from the blocks by using a slow-speed water-cooling diamond blade (Ernst Leitz GmbH, Wetzlar, Germany). All of the ceramic sections were then ground with silicon carbide abrasive paper of grits 400, 600 and 1200 (Leco1 VP 100, Leco Instrumente GmbH, Germany) from the both sides. A 4 mm in diameter Teflon molds placed on the sections and ceramic sections marked with a fine-tipped water insoluble pen through the mold. Then ceramic sections were carefully milled by hand, using water cooled micro-motor with diamond-coated fissure bur. The edges of discs were grounded with Sof-Lex polishing discs from coarse to fine (3M ESPE, St Paul, MN, USA) and discs were subsequently inspected with a magnifying glass for any existing damage.
Bonding procedure
The ceramic specimens were randomly divided (n = 15) into four groups according to the surface treatment groups of the teeth. Ten percent HF gel (Angelus) was applied to the ceramics for 60 s and rinsed with deionized water for 2 min and dried with oil-free air. On the other hand, enamel surfaces were etched with 37% orthophosphoric acid (AXJA-ETCH, Scientific Pharmaceutic Inc. USA) for 15 s according to the manufacturer’s instructions and rinsed with deionized water for 2 min and dried with oil-free air. Then Scotchbond Universal adhesive (3M ESPE, St Paul, MN, USA) was applied both to the enamel and ceramic surfaces for 20 s and gently air dried for 5 s. Resin cement RelyX Ultimate (3M ESPE, St Paul, MN, USA) was placed to the ceramic through the mixing tip and ceramic placed to enamel surface with a gently press. The excess cement was removed, and the specimens were then light cured on each side (perpendicular, from right and left) for 20 s, a total exposure of 60 s with a LED light-curing unit (Elipar S10, 3M Espe, St. Paul, MN) with an irradiance of 1200 mW/cm2 according to the manufacturer’s instructions. The same operator was prepared all the specimens in same conditions of room temperature and humidity. All operations (etching, priming and ceramic bonding) were carried out according to the manufacturer’s instructions. After storing in 37 °C distilled water for 24 h, specimens were thermocycled in distilled water for 5000 cycles in a 5–55 °C water bath with a dwell time of 30 s and a transfer time of 5 s.
Shear bond strength test
Specimens were mounted on the jig of a universal testing machine (MCE 2000ST, Quicktest Prüfpartner GmbH, Langenfeld, Germany). Bond strength was determined at a crosshead speed of 0.5 mm/min until fracture occurred. Shear bond strength was calculated by dividing the maximum load at failure (N) with the bonding area (mm2) and recorded in megapascals (MPa).
Vickers microhardness measurements
Seventy-five teeth were used for this part of the study. At first, for groups 2B to 2E, demineralisation treatment was carried out. The other 15 teeth of group 2A left untouched to use as positive control (non-demineralised and non-surface treated).
Demineralisation procedure
The applied demineralisation protocol was based on the method that by White has been used in various studies [35,36,37]. Artificial lesions were formed by immersing the specimens for 48 h individually in plastic tubes (Falcon TubesTM, BD, Franklin Lakes, USA) which were containing 0.1 M lactic acid buffer solution[0.75 mM CaC12·2H2O2, 0.45 mM K(H2P04)] with a pH value of 4.75. Demineralisation was performed at 37 °C at a ratio of 10 ml of solution per specimen. At the end of the 48 h, specimens were removed from the solutions and ultrasonically cleaned with distilled water and gently dried. Then, all specimens including group 2A were prepared for microhardness measurements by embedding in self curing acrylic resin as with the same way described before in shear bond strength test section. On the other hand, immersed solutions were used for ICP-OES elemental analysis of Ca and P amounts.
Microhardness test
Microhardness measurements were made with a Vickers diamond microhardness tester (Buehler Micromet 5101, obj. lens, 50×) in Vickers hardness units/numbers (VHN). Three indentations were made using a 300 g load perpendicularly orientated to the indentation surface for 15 s, and the average values were recorded.
ICP-OES analysis
The amounts of calcium and phosphorous released into the demineralization solutions were measured with an inductively coupled plasma-optical emission spectrometer (ICP-OES; Spectro Flame Blue EOP TI, Spectro Analytical Instruments GmbH and Co. KG, Kleve, Germany) in the conditions of the following: plasma power, 1430 W; pump speed, 30 rpm; coolant flow, 13 L/min; auxilory flow, 0.80 and nebulizer flow, 0.75. Before the determination of the elements, calibration was performed with calcium and phosphorous standard solutions (Merck KGaA, Darmstadt, Germany). From each solution, 3 ml samples were taken in triplicate, and three measurements of calcium and three of phosphorous were performed to ensure the precision of the measurements. Both calcium and phosphorous contents were measured in parts per million.
Statistical analysis
Assumptions of normal distribution were checked with Shapiro-Wilk tests for all the variables tested. Descriptive and inferential statistical analyses were performed using the SPSS 16.0 for Windows statistical program at a signicance level of p < 0.05. Differences between the groups for all tests (shear bond, microhardness, ICP-OES) were statistically analyzed by one-way analysis of variance (ANOVA) and Tukey HSD tests.
Results
Shapiro-Wilk tests showed that all data obtained in this study had normal distribution. Mean values and standard deviations of all the tests and the tested groups are shown in Table 2. There were slightly differences in shear bond strength values among groups, but ANOVA revealed that the surface treatments had no significant effects on shear bond strength values (p = 0.579 at Table 3). However, significant differences were found in microhardness measurements (p < 0.01, VHN at Table 4) and in elemental analysis (p < 0.01, ICP-OES at Table 5). In VHN and ICP-OES tests, parallel results were obtained. Such that surface-treated groups showed significantly different high VNH values and significantly different low ICP-OES values when compared with non-treated “- control” group. Although there was a slight difference on behalf of the laser-treated group, there was no significance among surface-treated groups regarding VHN and ICP-OES values.
Discussion
It is well established in literature that sub-ablative laser treatment have the potential of increasing the caries resistance of tooth enamel. And there are various studies stated the caries-preventive effects of different laser types like Nd:YAG, Argon, CO2 and Erbium family lasers [14,15,16,17,18, 32,33,34,35]. Among them, Er,Cr: YSGG laser can be widely used for many dental purposes, ranging from hard tissue operations to soft tissue operations. In this study, this laser type was investigated to contribute to its usage for another field, because of the high surface absorption due to hydroxyapatite. Although many researchers have investigated the caries resistant effect of this laser, it was the first that in this study, this effect evaluated on the enamel which prepared for all-ceramic laminate restorations and additionally, the ICP-OES analysis was used to support the microhardness measurement results in determining the mineral loss. According to the results obtained from this study, the first hypothesis “sub-ablative laser treatment have the decreasing effect on demineralization of dental enamel” is accepted, but the second “this laser treatment have the negative effect on shear-bond strength of all glass ceramic laminate restorations to enamel” is rejected.
In high-risk areas such as around orthodontic brackets or around composite restorations, it has been demonstrated through several studies that laser use alone or combined with fluoride treatment can control caries [32, 36, 37]. However, it is a mystery that laser treatment has such an effect under or around all ceramic restorations. Numerous clinical studies with evaluation periods varying from 3 to 25 years have reported the high success rates of ceramic laminate restorations but these studies still refer to the formation of caries between rates of 1 to 3.5% [19,20,21, 25,26,27,28,29]. No doubt, possible treatment methods that can be made to raise this rate to 100% cannot be ignored. But at the same time, these methods should not adversely affect the bonding performance of the restorations to tooth hard substances. Fortunately, according to the results of this study ANOVA (Table 3) showed that there were no significant differences of shear bond strength values among the groups. Moreover, considering the demineralisation prevention there were significant positive effects of treatment methods compared to control group.
It is well known that to prevent ablation is has the primary importance in laser-assisted caries prevention [13,14,15,16,17,18, 32,33,34,35,36,37,38]. To increase the enamel’s acid resistance by using laser irradiation at sub-ablative parameters, photothermal effect is more dominant rather than photomechanical effect [21]. In several studies, it is stated that to achieve this effect, temperature raise ranging from 100 °C to 600 °C is necessary [12, 35, 38]. And regarding the use of Er,Cr:YSGG laser, the required energy density to ensure enamel acid resistance effect changes between 4 to 13 J/cm2 [8, 14, 20, 32, 35]. So following a detailed review of the literature sub-ablative parameters that given in Table 1 were selected to use in the present study.
For preventing enamel demineralisation, the combined use of fluoride and laser irradiation evaluated in several studies and it is stated that due to increased fluoride retention capacity, lased enamel may show more acid resistance [39]. It is also stated that the heating effect of laser irradiation can cause melting in enamel structure and in the presence of fluoride the transformation of hydroxyapatite to fluorapatite may induced in this melted layers [40]. In this study, considering this synergistic effect, laser treatment done both alone and in combination with fluoride application and corresponded with each other, with fluoride application only and with control groups. When the results were evaluated in terms of caries preventing effects, although there was a slight difference in favor of the lased and fluoride-assisted lased groups there were no statistically significance in treatment groups. But when compared to the non-treated-control group there were significantly different preventing effects of treatment groups of lased, lased with fluoride combination and only fluoride applied groups. That is to say that laser treatment is found to be as effective as fluoride treatment alone or with laser combination. These results are consistent with some other previous studies [8, 28, 32, 41]. But at the contrary in some other studies, a synergistic effect between laser and fluoride that increases the acid resistance of sound enamel have been shown [18, 33, 35, 42]. This differences probably occured due to the differences among the studies. Namely, 1—in the present study only demineralisation process was applied but in Liu et al.’s [42] and de Freitas et al.’s [18] studies demineralisation-remineralisation cycles applied with different time intervals. 2—in Zezell et al.’s [33] and Anaraki et al.’s [35] studies different types of CO2 and Nd:YAG lasers were used. This laser types differently interacts with tooth structures that may exhibit different melting effects. 3—the other point was the differences in the fluoride preparate types and the concentrations used in the studies.
To evaluate the treated enamel specimens’ responses to the acidic condition, mineral loss was evaluated. For this purpose, Vickers microhardness test and ICP-OES elemental analysis were used. Vickers microhardness test is one of the widely preferred methods in the field of dental research. In the present study, ANOVA showed significant differences in VHN among groups (p = 0.00). When the results are evaluated considering Tukey HSD test, treatment groups of 2B, 2C and 2D showed significant higher VHN values corresponded to (−)control group of 2E; meanwhile, they showed significant lower VHN values corresponded to (+)control group of 2A. But there were no significance among the treatment groups of 2B, 2C and 2D. Since ICP-OES allows to determine the mineral content, it was used to support the VHN measurement values. For this purpose, methods such as SEM and energy dispersive spectrometry, ICP-AES (inductively coupled plasma–atomic emission spectrometry) or atomic absorption spectrometry can be used, but in SEM and energy dispersive spectrometry, measurement were not repeated exactly at the same point and also, it is necessary to coat the specimens for this method which may cause the specimens not to be used for further studies. In atomic absorption spectrometry and ICP-AES, it is necessary to dehydrate, burned and dissolved that again cause the specimens not to be used for further studies. ICP-OES does not require this situations which permits to use the specimens in further studies and in addition, this method can detect elements in parts per billion (micrograms per liter). Because of these advantages, ICP-OES was preferred. Similar to the results of VHN, ANOVA showed significant differences in ICP-OES among groups regarding Ca and P levels (p = 0.00). In both elements, when the results are evaluated considering Tukey HSD test, treatment groups of 2B, 2C and 2D were showed significant lower Ca and P amounts in demineralisation solutions corresponded to the untreated (−)control group of 2E; meanwhile, they were showed significant higher Ca and P amounts in demineralisation solutions corresponded to the control solution. But again there were no significance among the treatment groups of 2B, 2C and 2D in terms of Ca and P amounts in solutions.
So according to this result, it can be argued that these treatment methods have the capability of preventing enamel demineralisation, and there were no difference in efficiency between them.
Adhesion is one of the most important parameters affecting the success of restorations. And shear bond testing is the one of the most accepted and used methods for assessing the adhesion performance of dental materials to dental structures [43]. It has been stated in the literature that the required shear bond strength of adhesives to enamel to compensate the polymerization shrinkage stresses should be at least 20 MPa [43, 44]. In the present study, all preparation margins were in enamel and all groups showed the adequate shear bond strength values contrary to estimates. Moreover, although there was a slight difference in favor of the laser group, this difference was not significant. According to these results, the application of enamel preventive methods to demineralisation did not negatively affect the adhesive bonding to prepared enamel. Further studies are needed to support these results and also to evaluate these effects of laser treatment in prepared dentin structure.
Conclusions
Within the limitations of this study, the following conclusions can be addressed:
-
1.
The sub-ablative Er,Cr:YSGG treatment alone or combined with fluoride is as an effective method as at least fluoride alone for preventing the prepared enamel to demineralisation. Thus, it can be an alternative choice of enamel prevention.
-
2.
While sub-ablative Er,Cr:YSGG treatment shows preventive effects, it also does not negatively affect the shear bond strength to enamel.
References
Kidd EA, Fejerskov OE (2004) What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res 83:35–38
Petersen PE, Lennon MA (2004) Effective use of fluorides for the prevention of dental caries in the 21st century: the WHO approach. Community Dent Oral Epidemiol 32:319–321
Bratthall D, Hansel-Petersson G, Sundberg G (1996) Reasons for the caries decline: what do the experts believe? Eur J Oral Sci 104:433–435
Featherstone JDB (2000) The science and practice of caries prevention. J Am Dent Assoc 131:887–899
Feathestone JDB (2004) The continuum of dental caries—evidence for a dynamic process. J Dent Res 83:C39–C42
McCann HG (1968) The solubility of fluorapatite and its relationship to that of calcium fluoride. Arch Oral Biol 13:987–1001
Dijkman AG, Boer P, Arends J (1983) In vivo investigation on the fluoride content in and on human enamel after topical applications. Caries Res 17:392–402
Apel C, Meister J, Schmitt N, Gräber HG, Gutknecht N (2002) Calcium solubility of dental enamel following sub-ablative Er:YAG and Er:YSGG laser irradiation in vitro. Lasers Surg Med 30:337–341
Stern RH, Vahl J, Sognnaes RF (1972) Lased enamel: ultrastructural observations of pulsed carbon dioxide laser effects. J Dent Res 51:455–460
Niemz M (1996) Laser tissue interaction: fundamentals and applications. Springer-Verlag, Berlin Heidelberg
Sato K (1983) Relation between acid dissolution and histological alteration of heated tooth enamel. Caries Res 17:490–495
Fowler BO, Kuroda S (1986) Changes in heated and in laser irradiated human tooth enamel and their probable effects on solubility. Calcif Tissue Int 38:197–208
Secilmis A, Altintas S, Usumez A, Berk G (2008) Evaluation of mineral content of dentin prepared by erbium, chromium: yttrium scandium gallium garnet laser. Lasers Med Sci 23:421–425
Geraldo-Martins VR, Lepri CP, Palma-Dibb RG (2013) Influence of Er,Cr:YSGG laser irradiation on enamel caries prevention. Lasers Med Sci 28:33–39
Bader C, Krejci I (2006) Indications and limitations of Er:YAG laser applications in dentistry. Am J Dent 19:178–186
Apel C, Birker L, Meister J, Weiss C, Gutknecht N (2004) The caries-preventive potential of sub-ablative Er:YAG and Er:YSGG laser radiation in an intraoral model: a pilot study. Photomed Laser Surg 22:312–317
Apel C, Meister J, Götz H, Duschner H, Gutknecht N (2005) Structural changes in human dental enamel after sub-ablative erbium laser irradiation and its potential use for caries prevention. Caries Res 39:65–70
de Freitas PM, Rapozo-Hilo M, Eduardo CP, Featherstone JD (2010) In vitro evaluation of erbium, chromium:yttrium-scandiumgallium-garnet laser-treated enamel demineralization. LasersMed Sci 25:165–170
Strassler HE (2007) Minimally invasive porcelain veneer: indications for a conservative esthetic dentistry treatment modality. Gen Dent 55:686–712
Calamia JR (1989) Clinical evaluation of etched porcelain veneers. Am J Dent 2:9–15
Calamia JR, Calamia CS (2007) Porcelain laminate veneers: reasons for 25 years of success. Dent Clin N Am 51:399–417
Guess PC, Schultheis S, Bonfante EA, Coelho PG, Ferencz JL, Silva NR (2011) All-ceramic systems: laboratory and clinical performance. Dent Clin N Am 55:333–352
Conrad HJ, Seong WJ, Pesun IJ (2007) Current ceramic materials and systems with clinical recommendations: a systematic review. J Prosthet Dent 98:389–404
Elsaka SE (2014) Bond strength of novel CAD/CAM restorative materials to self-adhesive resin cement:the effect of surface treatments. J Adhes Dent 16:531–540
Granell-Ruiz M, Fons-Font A, Labaig-Rueda C, Martínez-González A, Román-Rodríguez JL, Solá-Ruiz MF (2010) A clinical longitudinal study 323 porcelain laminate veneers. Period of study from 3 to 11 years. Med Oral Patol Oral Cir Bucal 15:531–537
Shaini FJ, Shortall AC, Marquis PM (1997) Clinical performance of porcelain laminate veneers. A retrospective evaluation over a period of 6.5 years. J Oral Rehabil 24:553–559
Gurel G, Sesma N, Calamita MA, Coachman C, Morimoto S (2013) Influence of enamel preservation on failure rates of porcelain laminate veneers. Int J Periodontics Restorative Dent 33:31–39
Peumans M, De Munck J, Fieuws S, Lambrechts P, Vanherle G, Van Meerbeek B (2004) A prospective ten-year clinical trial of porcelain veneers. J Adhes Dent 6:65–76
Cötert HS, Dündar M, Oztürk B (2009) The effect of various preparation designs on the survival of porcelain laminate veneers. J Adhes Dent 11:405–411
White DJ (1987) Reactivity of fluoride dentifrices with artificial caries. I. Effects on early lesions: F uptake, surface hardening and remineralization. Caries Res 21:126–140
Dehailan LA, Martinez-Mier EA, Lippert F (2016) The effect of fluoride varnishes on caries lesions: an in vitro investigation. Clin Oral Invest 20:1655–1662
Fornaini C, Brulat N, Milia G, Rockl A, Rocca JP (2014) The use of sub-ablative Er:YAG laser irradiation in prevention of dental caries during orthodontic treatment. Laser Ther 23:173–181
Zezell DM, Boari HG, Ana PA, Eduardo Cde P, Powell GL (2009) Nd:YAG laser in caries prevention: a clinical trial. Lasers Surg Med 41:31–35
Raucci-Neto W, de Castro-Raucci LM, Lepri CP, Faraoni-Romano JJ, da Silva JM, Palma-Dibb RG (2015) Nd:YAG laser in occlusal caries prevention of primary teeth: a randomized clinical trial. Lasers Med Sci 30:761–768
Anaraki SN, Serajzadeh M, Fekrazad R (2012) Effects of laser-assisted fluoride therapy with a CO2 laser and Er, Cr:YSGG laser on enamel demineralization. Pediatr Dent 34:92–96
Harazaki M, Hayakawa K, Fukui T, Isshiki Y, Powell L (2001) The Nd-YAG laser is useful in prevention of dental caries during orthodontic treatment. Bull Tokyo Dent Coll 42:79–86
Klein ALL, Rodrigues LKA, Eduardo CP, Nobre dos Santos M, Cury JA (2005) Caries inhibition around composite restorations by pulsed carbon dioxide laser application. Eur J Oral Sci 113:239–244
Moslemi M, Fekrazad R, Tadayon N, Ghorbani M, Torabzadeh H, Shadkar MM (2009) Effects of Er,Cr:YSGG laser irradiation and fluoride treatment on acid resistance of the enamel. Pediatr Dent 31:409–413
Ana PA, Bachmann L, Zezell DM (2006) Lasers effects on enamel for caries prevention. Laser Phys 16:865–875
Phan ND, Fried D, Feathestone JDB (1999) Laser-induced transformation of carbonated apatite to fluorapatite on bovine enamel. Proc SPIE 3593:233–239
Geraldo-Martins VR, Lepri CP, Faraoni-Romano JJ, Palma-Dibb RG (2014) The combined use of Er,Cr:YSGG laser and fluoride to prevent root dentin demineralization. J Appl Oral Sci 22:459–464
Liu Y, Hsu CY, Teo CM, Teoh SH (2013) Potential mechanism for the laser-fluoride effect on enamel demineralization. JDent Res 92:71–75
Oztürk E, Bolay S, Hickel R, Ilie N (2013) Shear bond strength of porcelain laminate veneers to enamel, dentine and enamel-dentine complex bonded with different adhesive luting systems. J Dent 41:97–105
Eick JD, Gwinnett AJ, Pashley DH, Robinson SJ (1997) Current concepts on adhesion to dentin. Critical Reviews of Oral Biology & Medicine 8:306–335
Acknowledgements
The author would like to thank to Prof. Dr. Aslihan USUMEZ DDS; PhD for the supports about the calculation of the laser energy density.
The author would like to thank the Department of Restorative Dentistry, Faculty of Dentistry, Kirikkale University, Turkey, for allowing the use of the Er,Cr:YSGG laser device.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare no potential conflicts of interest with respect to the authorship and/or publication of this article, and the author do not have any financial interests in the companies whose materials and devices are included in this article.
Ethical approval and informed consent
The protocol of this study plan was approved by the Ethics Committee of Faculty of Medicine, Kirikkale University (Approval Number: 18/13, 29.06.2015). Teeth donators signed an informed consent form and the experiments followed the principles of the Helsinki Declaration.
Rights and permissions
About this article
Cite this article
Bağlar, S. Sub-ablative Er,Cr:YSGG laser irradiation under all-ceramic restorations: effects on demineralization and shear bond strength. Lasers Med Sci 33, 41–49 (2018). https://doi.org/10.1007/s10103-017-2326-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2326-3