Abstract
Laser etching has an effect on the mineral content of dentin. The aim of this study was to evaluate the mineral content of dentin prepared at three different power settings with an erbium, chromium:yttrium scandium gallium garnet (Er,Cr:YSGG) laser. The enamel of five, lower, wisdom, molar teeth was removed to expose the dentin surface. Four dentin slabs were obtained, then each tooth was randomly divided into four portions (groups 1 W, 2 W, 3 W and control) so that we could evaluate the effect of laser treatment. The Er,Cr:YSGG laser used for the study had a pulse duration of 140 μs, a pulse repetition rate of 20 Hz and a power output range of 0 W to 6 W. Laser energy was delivered through a fiberoptic system to a sapphire tip terminal 6 mm long and 600 μm in diameter, using a non-contact mode. The levels of five elements: magnesium (Mg), phosphorus (P), calcium (Ca), potassium (K), and sodium (Na), in each slab were measured by inductively coupled plasma–atomic emission spectrometry (ICP-AES). There were significant differences between the groups (1 W, 2 W, 3 W and control) for Ca, Mg, Na, P and Ca/P ratio (P < 0.05); however, there were no significant differences for K (P = 0.43). Laser treatment at 1 W significantly affected the mean percentage weight of all element groups except K. Scanning electron microscopy (SEM) photographs indicated that the surface irregularities increased with increasing power setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, lasers are being considered as a potential replacement for conventional mechanical cutting and drilling systems to remove dental hard tissues. Ruby, argon, CO2 and neodymium:yttrium aluminum garnet (Nd:YAG) lasers have been shown by many researchers to produce major thermal effects on dentin, including melting, carbonization, the creation of fissures and cracks in the surrounding tissue, as well as an increase in pulp temperature. With the discovery of two new types of lasers, erbium:yttrium aluminum garnet (Er:YAG) and erbium chromium:yttrium scandium gallium garnet (Er,Cr:YSGG), which were approved by the Food and Drug Administration (FDA), dental hard tissues can now be removed without causing damage [1]. The Er,Cr:YSGG laser allows nearly pain-free cutting without the need for a local anesthetic [2]. This instrument, when used with an air–water spray, has been shown to cut enamel, dentin, cementum, and bone efficiently and cleanly [3, 4]. This laser produces micro-explosions during tissue ablation, resulting in macroscopic and microscopic irregularities [5, 6]. The Er,Cr:YSGG laser initially causes vaporization of water and other hydrated organic components of the tissue [5]. On vaporization, the internal pressure builds within the tissue until the explosive destruction of inorganic substance occurs before the melting point is reached [5]. Laser-induced changes include melting and recrystallization, with the formation of numerous pores and small, bubble-like inclusions. No melting or recrystallization was observed with the Er,Cr:YSGG laser [4, 7].
It has been suggested that lasers might also be used to produce an etched surface [6–9]. Laser etching is painless and does not involve vibration or heat, making it highly attractive for routine use. Furthermore, laser etching of enamel or dentin has been reported to yield a fractured and uneven surface and open dentin tubules, both apparently ideal for adhesion [10]. The surface produced by laser etching is acid resistant, because laser radiation of dental hard tissues modifies the calcium-to-phosphorus ratio (Ca/P ratio), reduces the carbonate-to-phosphate ratio, and leads to the formation of more stable and less acid-soluble compounds, thus reducing susceptibility to acid attack and caries [7, 11, 12]. The alterations in chemical composition may affect the permeability and solubility characteristics of dentin and the adhesion of dental materials to dental hard tissues.
The aim of our study was to evaluate the compositional changes [(magnesium (Mg), phosphorus (P), calcium (Ca), potassium (K) and sodium (Na)] of the dentin surfaces prepared by Er,Cr:YSGG laser irradiation and to compare the Ca/P ratios of the groups using inductively coupled plasma–atomic emission spectrometry (ICP-AES) technique.
Materials and methods
The preparation of the dentin slabs
Five, lower, wisdom, molar teeth that were free of dental caries or restoration were cleaned with gauze and a fine brush and stored in distilled water at room temperature immediately after extraction. The teeth were then mounted in quadrangular molds with an autopolymerizing acrylic resin (Meliodent, Bayer Dental Ltd., Newbury, UK). The enamel of the teeth was removed with a conventional bur under water cooling to expose the dentin surface. The teeth were mounted in a slow-speed, diamond-saw, sectioning machine (Isomet, Buehler Ltd., Lake Bluff, IL, Ill, USA). Occlusal thirds of the crowns were cut with a slow-speed, diamond-saw, sectioning machine under water cooling. To prepare the dentin slabs into 0.60-mm-thick pieces, we made saw cuts perpendicular to the long axis of the tooth. Finally, four dentin slabs were obtained, and then each tooth was randomly divided into four portions so that we could evaluate the effect of laser treatment.
Laser treatment
An Er,Cr:YSGG dental laser (Millennium; Biolase Technologies, San Clemente, CA, USA) with a pulse duration of 140 μs, a pulse repetition rate of 20 Hz and a power output range of 0 W to 6 W was used for laser etching. Laser energy was delivered through a fiberoptic system to a sapphire tip terminal, 6 mm long and 600 μm in diameter, using a non-contact mode with settings of 1 W, 2 W, 3 W (energy densities 2.7 J/cm2, 5.6 J/cm2, 8.2 J/cm2), 55% for water and 65% for air. The area of the beam was 1.8 × 10−2 cm2. The beam was aligned perpendicularly to the dentin at 1 cm distance and was moved in a sweeping fashion by hand to obtain a homogeneous surface appearance over the entire area of each dentin slab.
ICP-AES technique
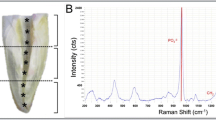
The dentin slabs were stored in plates at 70°C in a cabinet desiccator (Ventisell, Italy) until they reached a fixed weight. That is to say, the specimens were dehydrated. Their weights were recorded with an electronic balance (Electronic Balance AX200, Shimadzu Corporation, Japan). Ten milliliter nitric acid (HNO3) and 3 ml hydrochloric acid (HCl) were added on the specimens and the specimens were burned at 180 PSI and 180 °C in a microwave (CEM, Mars 5, USA) until they were dissolved. After calibration of the ICP-AES instrument (Vista AX, Varian, Australia), 2 ml of solution was taken. In this technique, the solutions are carried in a nebulizer with the help of a peristaltic pump. The specimens turn into aerosols and are carried by an argon spray. The aerosols are heated by conduction and radiation and reach approximately 10,000°C, at which temperature they are completely atomized. Therefore, energy is released. The light is transferred to the detector, and every element is described according to its different wavelength (Fig. 1). In this study, three measurements were performed on each element for each solution, and the means of the measurements were calculated in milligrams per liter (parts per million) by computer.
The levels of five elements: Mg, P, Ca, K and Na, in each specimen were measured by ICP-AES. The mineral contents were calculated as percentage weights.
Scanning electron microscopy examinations
One specimen from each group was prepared for scanning electron microscopy (SEM) (Jeol JSM-5600; Jeol Ltd., Tokyo, Japan). After surface treatment, the specimens were sputter-coated (Polaron SC500 Sputter Coater, VG Microtech, E. Sussex, England) with gold–palladium alloy under high vacuum and photomicrographs were taken (Fig. 2).
Statistical analysis
Differences between the groups (1 W, 2 W, 3 W and control) were statistically analyzed by one way analysis of variance (ANOVA) and Tukey HSD tests. We compared the groups to verify the differences at a significance level set at P < 0.05 using the SPSS 11 for Windows statistical program.
Results
The mean percentage weights of the five elements (Mg, P, Ca, K and Na) in the dentin after treatment with the laser are shown in Table 1. One-way ANOVA showed that there were significant differences between the groups (1 W, 2 W, 3 W and control) for Ca, Mg, Na, P and Ca/P ratio (P < 0.05); however, there were no significant differences for K (P = 0.43). There were significant increases in all element group levels except for K after laser treatment at 1 W when compared with the other groups (2 W, 3 W and control) (P < 0.01). Significant differences in the mean percentage weights of Na were found between 1 W-3 W groups (P < 0.05), and there were no significant differences among other groups (P = 0.39).
Scanning electron microscopy
SEM views of Er,Cr:YSGG laser-treated dentin surfaces are shown in Fig. 2. The surface of the specimen treated with 1 W laser irradiation was the smoothest. The surface treated with 3 W laser irradiation was rougher than those of the other groups. There was no recrystallization or melting of surfaces.
Discussion
In the study, the compositional changes of the dentin surfaces prepared by Er,Cr:YSGG laser irradiation were evaluated and the Ca/P ratios of the groups were compared using an ICP-AES technique. Mean percentage weights of Ca, Mg, Na and P and the Ca/P ratio of the groups were affected by the laser irradiation.
The laser technique might have an advantage because it produces an etching behavior and does not damage the underlying tissues and dental pulp. The use of laser therapy also shows promise from the current research; laser therapy induced surface roughness comparable with that of acid etching [13–15] and facilitated, or even improved, bond strength [10, 16]. Therefore, the acid etch step can be easily avoided with laser treatment [17]. Surface alterations of the enamel and dentin after Er,Cr:YSGG laser irradiation showed that these surfaces are associated with micro-irregularities, and there was also the absence of a smear layer [18]. Visuri et al. [10] suggested that the greater presence of peritubular dentin, which has a greater mineral content than intertubular dentin has, might result in better bonding to the dentin. In their study they obtained a higher shear bond strength of composite when it was bonded to Er:YAG laser-prepared dentin than with acid-etched dentin. Another difference between acid etchant and laser actions related to dentin is their effect on the structure of dentin tubules. When an acid etchant is applied, the peritubular dentin is preferentially etched, resulting in funnel-shaped openings to the tubules. This structure may contribute, with polymerization shrinkage, to pull the tags away from the walls. Laser irradiation produces no demineralization of peritubular dentin, and the dentinal tubules remain open, with no widening [5]. In our study, SEM views of the irradiated surfaces achieved with the Er,Cr:YSGG laser system showed that the surface irregularities increased with increasing power setting.
In previous studies, SEM and energy dispersive spectrometry was used to determinate the levels of the mineral content of dentin [19–23]. The measurements using SEM and energy dispersive spectrometry were not repeated exactly at the same point [23, 24]. The average content of an area was recorded in each sample. In addition, dentin porosity may produce secondary diffraction. Therefore, dentin surfaces must be polished perfectly. However, the smear layer produced by polishing may be responsible for different elements found in dentin surfaces [23]. Kaufman et al. [20] showed that it was possible to eliminate this smear layer by immersing the samples in an ultrasonic bath of distilled water. However, in clinical situations, this layer is always on the radicular surfaces. The mineral contents of dentin vary, depending on the types and anatomical locations of the dentin tissue samples, so such an experiment is generally difficult [23, 25, 26]. The inductively coupled plasma technique uses a sample that is passed through argon in a ray fluorescence (RF) field. When the sample is introduced into the plasma, the atoms are excited and emit very stable light of varying wavelengths that permit identification of the elements. This technique has become highly popular for element analysis [27]. A previous study showed that ICP-AES was one of the most attractive detection systems for the determination of trace elements in dentin [23, 24]. The study showed that polishing was not necessary and that dentin chips obtained with Gates-Glidden drills were sufficient to make the test with the ICP-AES technique. The mineral content of dentin can be measured by SEM and energy dispersive spectrometry, with amounts detectable at the parts per million (milligram per liter) level. However, with ICP-AES it can be detected at the parts per billion (micrograms per liter) level. In addition, multiple elements can be measured at the same time by ICP-AES. The measurements should be repeated for a second element [23]. The ICP-AES technique was preferred for this study due to these advantages.
Dentin composition has been described on the basis of its organic and inorganic components. Ca and P present in hydroxyapatite crystals are the major inorganic components of dental hard tissue. The Ca/P ratio of hydroxyapatite in dentin, which implies the basic composition of dental hard tissue surfaces, depends on the crystal type, the availability of Ca, the anatomic location, and the technique of determination [23, 28, 29]. It was reported that some chemical agents caused alterations in the chemical structure of human dentin and changed the Ca/P ratio of the dentin surface [19, 23, 25, 26]. The alterations in the Ca/P ratio may change the original ratio between organic and inorganic components that, in turn, change the permeability and solubility characteristics of dentin and also affect the adhesion of dental materials to dental hard tissues [19, 23]. In our study, there was a significant increase in the Ca/P ratio in the group treated with 1 W laser irradiation.
In addition to Ca and P, a small amount of Mg that is always detectable in the mineralized tissues has been considered to influence the mineralization process, especially crystal growth [23, 30]. There was a significant increase in the Mg level after laser treatment at 1 W when compared with the other groups. However, further investigations using greater sample sizes are required to confirm these results.
Conclusion
This study demonstrated that laser etching with the Er,Cr:YSGG laser system affects compositional structure of the dentin surfaces. Mean percentage weights of Ca, Mg, Na and P and the Ca/P ratios of the groups were affected by 1 W laser irradiation.
References
Lee BS, Lin PY, Chen MH, Hsieh TT, Lin CP, Lai JY, Lan WH (2007) Tensile bond strength of Er,Cr:YSGG laser-irradiated human dentin and analysis of dentin-resin interface. Dent Mater 23:570–578
Lin S, Caputo AA, Eversole LR, Rizoiu I (1999) Topographical characteristics and shear bond strength of tooth surface cut with a laser-powered hydrokinetic system. J Prosthet Dent 82:451–455
Eversole LR, Rizoiu IM (1995) Preliminary investigations on the utility of an erbium, chromium YSGG laser. J Calif Dent Assoc 23:41–47
Eversole LR, Rizoiu I, Kimmel AI (1997) Pulpal response to cavity preparation by an erbium, chromium: YSGG laser-powered hydrokinetic system. J Am Dent Assoc 128:1099–1106
Ceballos L, Osorio P, Toledano M, Marshall GW (2001) Microleakage of composite restorations after acid or Er-YAG laser cavity treatments. Dent Mater 17:340–346
Usumez A, Aykent F (2003) Bond strengths of porcelain laminate veneers to tooth surfaces prepared with acid and Er,Cr:YSGG laser etching. J Prosthet Dent 90:24–30
Usumez S, Orhan M, Usumez A (2002) Laser etching of enamel for direct bonding with an Er,Cr:YSGG hydrokinetic laser system. Am J Orthod Dentofacial Orthop 122:649–656
Von Fraunhofer JA, Allen DJ, Orbell GM (1993) Laser etching of enamel for direct bonding. Angle Orthod 63:73–76
Corpus-Pastor L, Moreno JV, Lopez-Gonzalez J, Muriel VP, Moore K, Elias A (1997) Comparing the tensile strength of brackets adhered to laser etched enamel vs acid etched enamel. J Am Dent Assoc 128:732–737
Visuri SR, Gilbert JL, Wright DD, Wigdor HA, Walsh JT (1996) Shear strength of composite bonded to Er:YAG laser-prepared dentin. J Dent Res 75:599–605
Fowler BO, Kuroda S (1986) Changes in heated and in laser-irradiated human tooth enamel and their probable effects on solubility. Calcif Tissue Int 38:197–208
Keller U, Hibst R (1990) Ultrastructural changes of enamel and dentin following Er:YAG laser radiation on teeth. Proc SPIE 1200:408–415
Zakariasen KL, Macdonald R, Boran T (1991) Spotlight on lasers. A look at potential benefits. J Am Dent Assoc 122:58–62
Arcoria CJ, Lippas MG, Vitasek BA (1993) Enamel surface roughness analysis after laser ablation and acid-etching. J Oral Rehabil 20:213–224
Walsh LJ, Abood D, Brockhurst PJ (1994) Bonding of resin composite to carbon dioxide laser-modified human enamel. Dent Mater 10:162–166
Martinez-Insua A, Da Silva Dominguez L, Rivera FG, Santana-Penin UA (2000) Differences in bonding to acid etched or Er:YAG-lased-treated enamel and dentin surfaces. J Prosthet Dent 84:280–288
Hossain M, Nakamura Y, Tamaki Y, Yamada Y, Murakami Y, Matsumoto K (2003) Atomic analysis and knoop hardness measurement of the cavity floor prepared by Er,Cr:YSGG laser irradiation in vitro. J Oral Rehabil 30:515–521
Hossain M, Nakamura Y, Yamada Y, Kimura Y, Matsumoto N, Matsumoto K (1999) Effects of Er,Cr:YSGG laser irradiation in human enamel and dentin: ablation and morphological studies. J Clin Laser Med Surg 17:155–159
Rotstein I, Dankner E, Goldman A, Heling I, Stabholz A, Zalkind M (1996) Histochemical analysis of dental hard tissues following bleaching. J Endod 22:23–26
Kaufman D, Mor C, Stabholz A, Rotstein I (1997) Effect of gutta-percha solvents on calcium and phosphorus levels of cut human dentin. J Endod 23:614–615
Doğan H, Çalt S (2001) Effect of chelating agents and sodium hypochlorite on mineral content of root dentin. J Endod 27:578–580
Doğan H, Tasman F, Çehreli ZC (2001) Effect of gutta-percha solvents at different temperatures on the calcium, phosphorus and magnesium levels of human root dentin. J Oral Rehabil 28:792–796
Ari H, Erdemir A (2005) Effect of endodontic irrigation solutions on mineral content of root canal dentin. J Endod 31:187–189
Erdemir A, Eldeniz AU, Belli S (2004) Effect of gutta-percha solvents on mineral contents of human root dentin using ICP-AES technique. J Endod 30:54–56
Hennequin M, Pajot J, Avignant D (1994) Effects of different pH values of citric acid solutions on the calcium and phosphorus contents of human root dentin. J Endod 20:551–554
Hennequin M, Douillard Y (1995) Effects of citric acid treatment on the Ca, P and Mg contents of human dental roots. J Clin Periodontol 22:550–557
Deutsch AS, Cohen BI, Musikant BL (2003) Inductively coupled plasma-emission spectroscopy and atomic absorption for the use of elemental analysis of a root canal after lasing with a holmium:YAG laser. J Endod 29:404–406
Cohen M, Garnick JJ, Ringle RD, Hanes PJ, Thompson WO (1992) Calcium and phosphorus content of root exposed to the oral environment. J Clin Periodontol 19:268–273
Grayson W, Marsall JR (1993) Dentin: microstructure and characterization. Quintessence Int 24:606–617
Wiesmann HP, Tkotz T, Joos U, Zierold K, Stratmann U, Szuwart T, Plate U, Hohling HJ (1997) Magnesium in newly formed dentin mineral of rat incisor. J Bone Miner Res 12:380–383
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Secilmis, A., Altintas, S., Usumez, A. et al. Evaluation of mineral content of dentin prepared by erbium, chromium:yttrium scandium gallium garnet laser. Lasers Med Sci 23, 421–425 (2008). https://doi.org/10.1007/s10103-007-0498-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-007-0498-y