Abstract
The aim of this systematic review was to assess a potential benefit of laser use in the treatment of recurrent aphthous stomatitis (RAS). The primary outcome variables were pain relief, duration of wound healing and reduction in episode frequency. A PICO approach was used as a search strategy in Medline, Embase and Cochrane databases. After scanning and excluding titles, abstracts and full texts, 11 studies (ten RCTs and one non-randomised controlled trial) were included. Study selection and data extraction was done by two observers. Study participants varied between 7–90 for the laser and 5–90 for the control groups. Laser treatment included Nd:YAG laser ablation, CO2 laser applied through a transparent gel (non-ablative) and diode laser in a low-level laser treatment (LLLT) mode. Control groups had placebo, no therapy or topical corticosteroid treatment. Significant pain relief immediately after treatment was found in five out of six studies. Pain relief in the days following treatment was recorded in seven studies. The duration of RAS wound healing was also reduced in five studies. However, criteria of evaluation differed between the studies. The episode frequency was not evaluated as only one study addressed this outcome parameter, but did not discriminate between the study (LLLT) and control (corticosteroid) groups. Jadad scores (ranging from 0 to 5) for quality assessment of the included studies range between 0 and 2 (mean = 1.0) for studies analysing pain relief and between 0 and 3 (mean = 1.1) for studies evaluating wound healing. The use of lasers (CO2 laser, Nd:YAG laser and diode laser) to relieve symptoms and promote healing of RAS is a therapeutic option. More studies for laser applications are necessary to demonstrate superiority over topical pharmaceutical treatment and to recommend a specific laser type, wavelength, power output and applied energy (ablative versus photobiomodulation).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent aphthous stomatitis (RAS), also known as recurrent aphthous ulceration and canker sores, is a frequent oral mucosal disease that exhibits different geographical incidence rates of 0.5% (Malaysia) [1], 10–15% (USA) [2], 17.7% (Sweden) [3] and up to 25% (Iran) [4] in the respective populations analysed. In a Caucasian population, young adults, females and people with a higher socioeconomic status are prone to suffer from RAS [5, 6]. RAS is known to be very painful, and it may even have a negative effect on the quality of life of the affected individual, impairing eating, swallowing and speaking [7].
RAS is classified into three different types: minor RAS (MiRAS), major RAS (MaRAS) and herpetiform aphthous ulceration (HAU). The difference between MiRAS and MaRAS was described for the first time in the literature by Truelove and Morris-Owen in 1958 [8], and soon after Cook [9] first mentioned HAU. In 80% of patients with RAS, MiRAS is diagnosed, and thus, it is by far the most common type [10]. Typical initial symptoms are a localised tingling, redness and discreet swelling of the affected non-keratinised mucosa. After a few hours, characteristic ulcers appear with a grey-white centre, a fibrin coverage and a red halo [11]. The diameter of ulcers in MiRAS is usually smaller than 5 mm and does not exceed 1 cm. The ulcers heal after a period of 10–14 days without scarring. MaRAS, also known as Sutton’s disease, is diagnosed in 10% of all patients with RAS and is much more painful than MiRAS. The diameter is usually greater than 1 cm. The healing time is around 6 weeks and often leaves a scar [6, 11]. HAU is clinically characterised by multiple small ulcers distributed in the oral cavity and affects less than 10% of patients with RAS [11, 12].
Currently, the etiopathogenesis of RAS is not fully understood, but there are some suggested predisposing factors such as a family history for aphthous lesions or systemic disorders like deficiencies (e.g. vitamin B12) with or without underlying gastrointestinal disorders, endocrine imbalance (e.g. menarche, menstruation and menopause) or allergies [6, 11, 13]. Mouth ulcers very similar to RAS can appear in some systemic disorders (e.g. Behçet’s disease, MAGIC syndrome, Sweet’s syndrome, cyclic neutropenia and HIV disease), but these conditions should be distinguished from RAS [11, 13]. Known local predisposing factors for RAS are trauma, food hypersensitivity, dental procedures, stress and non-smoking [14, 15]. The aim of RAS therapy is immediate pain alleviation, and ideally, therapy would decrease the frequency or even stop the onset of acute phases. Treatment modalities are topical or systemic. Topical interventions include mouth rinses, pastes, gels, sprays, injections, locally dissolving tablets and laser treatment [16]. The pharmacological agents proposed for topical treatment include anti-inflammatory drugs, antiseptics, antibiotics, anaesthetics and corticosteroids [17]. Systemic medication may be used following ineffective topical treatment, but a Cochrane review showed that the effect of systematic medication does not outweigh the negative side effects of the drugs used [18]. Thus, a commonly accepted treatment modality for RAS is still missing [10, 17, 19].
The use of different types of lasers has been demonstrated to relieve pain and result in a favourable wound healing in RAS patients. Lasers used in these studies were CO2 laser [20,21,22], Nd:YAG laser [23] and diode laser, the last one mainly for photobiomodulation in a low-level laser (LLL) mode [24, 25]. Nevertheless, what type of laser treatment and to which extent it is effective in the treatment of RAS are not known. Furthermore, the potential advantages and disadvantages of laser therapy compared to other therapy modalities have not been established in the literature to date. Therefore, the present systematic review addresses the following focused PICO (patient–intervention–comparison–outcomes) question: What is the effect of laser therapy (LLL/hard laser) for RAS compared with placebo, no or other treatments in terms of (1) pain relief (immediate and during healing), (2) duration of wound healing and (3) reduction in episode frequency?
Materials and methods
Search strategy
A PICO approach was used in the search strategy with MeSH/EMTRÉE and text words (Table 1). The electronic data resources used were Medline and Embase on the OVID platform and the Cochrane database, including all clinical studies until 19 November 2014. The search strategy also included a manual search of selected core journals in the field of interest (mainly oral medicine and oral surgery; Table 1).
Inclusion and exclusion criteria
Publications written in English, German, French, Italian, Swedish, Norwegian and Danish were included. Only studies including patients with RAS were considered. Each study group had to include at least five participants. Studies reporting on the treatment of RAS with any type of laser (soft or hard lasers) were included. Control groups could be a placebo group, a group with the use of a different treatment modality or without any treatment. The duration of observation had to be at least 3 days. The threshold of 3 days was chosen because ulcerative lesions form after 2 to 48 h following initial clinical symptoms and enlarge between 48 and 72 h [12]. The outcomes of the studies included had to report on pain relief and/or the effect on wound healing and/or the reduction of episode frequency.
Study selection
Two reviewers (V.S. and S.S.) independently evaluated the studies in three steps. First, duplicates were identified and eliminated. After the titles, abstracts and full texts of publications agreed by both reviewers were scanned, they were screened using a standardised pre-quality assessment. The studies were eliminated if two of the following issues were present: (1) no randomisation; (2) only one study group (no control or no head-to-head group); (3) group size not clear; (4) post-interventional observation protocol not clear or different in the group; or (5) no statistical analysis performed. In the case of a disagreement between the two reviewers, a third reviewer (M.B.) was consulted. Finally, the full-text evaluation of the included publications was done using a standardised data extraction sheet.
Data extraction
Both reviewers used a standardised data extraction sheet with the following parameters: study type, type of laser, laser parameters, type of treatment, placebo or no treatment in the control group, total number of patients, number of patients in the study laser group, number of patients in the control group, postsurgical treatment protocol regarding observation time points and the total duration of observation. Also, the effects of the laser intervention were recorded: these included reduction of duration of wound healing, pain reduction and reduction in episode frequency. Quality assessment of the included studies was performed using the Jadad score [26]. This score assesses the quality of randomised controlled trials (RCTs) in pain research and the effect of rater blinding. The score has a minimum of 0 and a maximum of 5 points. The first three points are allocated if the study was described as randomised, as double-blinded and if withdrawals and dropouts were accounted for. One additional point is given if the randomisation sequence was appropriate and one point if the method of double-blinding was appropriate. If the sequence of randomisation or the methodology of double-blinding was inappropriate, one point for each issue is deducted.
Results
Study selection
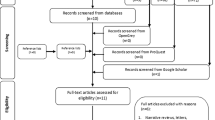
After removal of all duplicates, the electronic database search resulted in a total of 245 studies identified. Additionally, eight studies were identified by manual search, and three of them were included in the final analysis [27,28,29]. After screening the abstracts, 26 studies remained. Finally, 11 studies were included for the systematic review after full-text analysis and screening using a standardised pre-quality assessment (Fig. 1).
Of the included studies, ten were RCTs (five of them with a split-mouth design) [20,21,22,23,24,29,30,25, 28–31] and one was a non-randomised controlled trial [27]. The type of laser, exposition parameters and method of application used in each study are shown in Table 2.
Study results
Four studies used the CO2 laser, one the Nd:YAG laser and six the diode laser (Table 2). The CO2 laser was used in a non-contact and non-ablative mode after application of a non-anaesthetic gel on the aphthous ulcer [20,21,22, 30]. The Nd:YAG laser was applied with a pulsed mode in direct contact to the lesion until the surface epithelium was ablated [23]. The diode laser was used in pulsed [28, 31] or continuous mode [24, 27, 29], in direct contact [25, 27, 31] or non-contact [24, 28, 29] to the lesion, with wavelengths varying between 670 and 940 nm depending on the type of diode laser and an output power ranging from 0.005 to 2 W.
The effect of laser on (1) pain relief (immediate and during healing) and on (2) duration of wound healing was analysed separately (Tables 3 and 4). Study participants varied between n = 7 and n = 90 for the laser groups and from n = 5 to n = 90 for the control groups. The episodes of recurrences were described for all participants only in one paper [25]. However, there was no discrimination between the study (low-level laser treatment, LLLT) and control (topical corticosteroid) groups. Thus, this outcome had to be excluded from further evaluation.
Effect on pain
Ten out of the 11 selected studies addressed the effect on pain as an outcome (Table 3) [20,23,24,28,29,30,, 22–25, 27–31]. Five studies analysed the effect on immediate and late pain relief [20, 22, 24, 25, 30], whilst one investigation looked only at the effect on immediate [28] and four publications only at the effect on late pain relief [23, 27, 29, 31].
Significant immediate pain relief was found after the treatment in five out of six studies [20, 22, 24, 25, 28] (Table 3). Four of these six studies were placebo-controlled [20, 22, 24, 30]. Amongst the three placebo-controlled RCTs using the CO2 laser through a transparent gel, the two studies with a split-mouth design found a significant immediate pain relief [20, 22].
The late effect on pain relief was analysed in nine [20,23,24,30,, 22–25, 27, 29–31] out of ten studies (Table 3). Controls varied between the use of topical corticosteroids [23, 25, 27], topical solcoseryl/granofurin [29], placebo [20, 22, 24, 30, 31] or no therapy [27, 28]. A significant late pain reduction was found in seven studies [20,23,30,, 22–24, 29–31].
Effect on wound healing
Nine of the selected 11 studies evaluated wound healing (Table 4) [21,22,23,24,28,29,25, 27–30]. Wound healing was faster in five studies after laser application [21, 22, 24, 28, 29]. Wound healing was usually graded as completed when the ulcer was absent or the mucosa was epithelialised [21, 22, 28, 29]. In some studies, the ulcer size was compared between the groups [24, 25, 27, 30]. One study did not evaluate the duration of wound healing but analysed the degree of erythema (no difference between both groups) and of exudation (significantly less in the laser group) by using a four-point scale [23].
Quality of the studies
Jadad scores were not higher than 3 for all included studies, ranging between 0 and 2 (mean = 1.0, median = 1) for studies analysing pain relief and between 0 and 3 (mean = 1.1, median = 1) for studies looking at wound healing. Two points were given for appropriate description of randomisation for Lalabonova and Dasklov [29] and Albrektson et al. [31]. Most studies got 1 point for randomisation [20,21,22, 24, 25]. Single blinding with placebo application of an inactivated laser was achieved in four studies [20, 22, 24, 31]. Two studies (Jadad score = 3 [21] and Jadad score = 2 [30]) were double-blinded due to the same placebo method and the involvement of a further researcher not involved in the initial therapy and blinded to the type of treatment. He performed the postsurgical clinical assessment. It was not possible to achieve blinding in the studies with different treatment arms [23, 25, 27, 29]. Two studies left control group subjects without treatment and thus did not achieve blinding [27, 28]. None of the studies included made any statement regarding withdrawals. Thus, according to the Jadad scoring, no points could be attributed for this criterion.
Discussion
Pain relief and duration of wound healing were regarded as the two main parameters of interest to clinicians and patients suffering from RAS. This systematic review analysed the effect of lasers for the treatment of RAS regarding these two parameters separately. The third parameter of interest, a potential reduction in episode frequency due to laser treatment, could not be evaluated because the only study assessing this variable did not discriminate between laser and corticosteroid treatment.
Laser types and settings used
On the oral mucosa, hard lasers (such as CO2, Nd:YAG or Er:YAG) are mainly used for soft tissue incisional and excisional biopsies or vaporisation [32, 33], and soft lasers (LLL) are applied for the treatment of inflammatory diseases, to promote wound healing and release pain [34, 35]. According to a consensus of an expert group at the joint congress of the North American Association for Photobiomodulation Therapy (NAALT) and the World Association of Laser Therapy in 2014, the term photobiomodulation therapy has been suggested to replace the term LLLT and all other terms used to describe a similar low-level light treatment [36]. Four studies used the CO2 laser (λ = 10,600 nm), but in contrast to the normal use of a CO2 laser by cutting or ablating oral tissue, laser application was performed through a high water content gel, devoid of anaesthetic, but absorbing part of the irradiation. This technique was described as non-ablative CO2 laser therapy (NACLT) and has been described for the first time in the literature specifically for the therapy of RAS by Zand and co-workers [20]. After irradiation through the gel, the power output drops by 200–500 times, and the result on the tissue is a photobiomodulation effect. Laser mode (continuous wave, defocused), type (spiral motions) and time of application (5–10 s [20, 21], 5–8 s [22] and 5 s [30]) were similar in all four studies using NACLT, whilst the power settings differed, with 0.7 W [22], 1 W [20, 21] and 2 W [30].
No study using the CO2 laser in an ablative mode for the treatment of RAS could be included in this systematic review. Ablation as treatment method for aphthous lesions was only found in one study included in this systematic review [23]. The handpiece of the Nd:YAG laser (λ = 1064 nm) was brought in direct contact with the entire lesion and exposed to the laser energy (2 W, 200 mJ, 20-Hz pulsed emission mode) until the surface epithelium was ablated. Typically, there was bleeding and then coagulation within a few seconds. A significant release in late pain was found in this study compared to local corticosteroid application. However, the power of the study was quite low with ten participants per group. Thus, the results have to be interpreted with some caution.
The other six included studies were all performed with a diode laser (different wavelengths ranging from 658 to 940 nm). The applied output power, exposure time and mode (pulsed versus continuous) varied considerably between these studies (Table 2). Therefore, the range of applied total energy with the diode laser on RAS lesions in the six studies was wide (0.3 J [25], 2 J [29], 3 J [27], 14.4 J [31], 90 J [24] and 150–200 J [28]). All authors described the mode of laser treatment in their respective studies as low-level laser application or photobiomodulation. One exception was Hazeem et al. who used a high energy output with a 940-nm diode laser and described the treatment as a combined effect of sub-ablasive (thermal) and LLLT [28].
Inclusion criteria and quality assessment tool for the studies included
Ten of the 11 included studies were RCTs. Because of the heterogeneous study designs with different laser devices and settings, it was not possible to perform a meta-analysis. The one included non-randomised controlled trial satisfied the pre-quality assessment, and no other case series was adequate to satisfy the minimal criteria to be included in this systematic review. There are certainly clinicians that treat RAS with other laser types or settings (ablative and photobiomodulation) than those reported and discussed here, resulting in good outcomes from the surgeon’s and the patient’s perspectives. However, these experiences are either not published or on a lower evidence level (e.g. case report and expert opinion) and, thus, were not eligible for inclusion in this systematic review. The Jadad score has been chosen for this systematic review because it has been developed to measure the risk of bias in pain investigations, is simple to use with only three criteria and has been demonstrated to result in good inter-rater consistency [26]. In this context, “double-blinding” was achieved, when neither study participant nor the person doing the assessment could identify the type of intervention. In interventional studies using devices such as a laser, the surgeon himself can usually not be blinded. However, when the operator is not involved in any further evaluation (assessment of outcomes), the risk of bias due to knowledge of the type of intervention can be eliminated. This type of “double-blinding” was achieved by two studies included in the present review [21, 30].
Effect on pain
Pain relief was differentiated between an immediate effect after treatment and relief in the days following laser use. Immediate pain relief was studied by the three research groups treating RAS with NACLT [20, 22, 30]. In the study of Sattayut and co-workers [30], no initial pain relief was found, which was in contrast to the two other studies having used lower laser powers [20, 22]. However, it has to be said that in the study of Sattayut and co-workers, a smaller number of participants (n = 7) were included than in the studies of Zand and co-workers (n = 15) [20] and Prasad and Pai (n = 25) [22], both using a split-mouth design. Significant pain relief in the days following treatment was found for all studies using NACLT, but in two studies, this was found only on specific days: at 24 h [22] or on day 3 [30].
Animal studies showed that LLLT could achieve a reduction of inflammatory processes similar to the use of NSAID [37]. Pain has also been shown to be lower after photobiomodulation in inflammatory processes and after surgical interventions [38, 39]. Furthermore, it has also been shown in animal studies that LLLT may promote neural regeneration, which could also explain the pain reduction observed in clinical trials [40]. A meta-analysis evaluating the efficacy of low-power lasers concluded an overall positive treatment effect on pain [41]. The authors found that a wavelength of 830 nm had the most significant treatment effect on pain, but only four studies could be considered [41]. Vale et al. [42] suggested that a wavelength of 809 or 810 nm was favourable to reduce pain in RAS treatment by using a laser. In contrast, in our systematic review, we found a broader spectrum of LLLT wavelengths to be effective in the reduction of pain: reduction of immediate pain at 670 nm [25], 810 nm [24], and 940 nm [28] and reduction of late pain at 658 nm [29], 809 nm [31] and 810 nm [24]. However, there was no improvement in late pain with LLLT at 670 nm [25].
Effect on wound healing
The healing of an ulcer is a dynamic process usually involving the classic three phases of wound healing known as inflammation, proliferation and remodelling. Evaluating and comparing wound healing based on clinical criteria only has limitations. Wound healing after RAS laser treatment was evaluated using various criteria (i.e. ulcer size, ulcer healing, erythema and reepithelialisation) and with different follow-up intervals. Criteria like the evaluation of complete reepithelialisation [21] or absence of ulcer [22] seem quite reliable (yes/no answer). On the other hand, the four-point scale evaluation of erythema and exudation is more difficult to interpret and may be sensitive to intra- and inter-rater variations, which were not evaluated [23]. A similar tool evaluating erythema has, however, been described earlier to analyse the effect of aphthous ulcer treatment [43]. Sattayut et al. [30] evaluated wound healing by comparing the ulcer size to a plastic sheath grid. Specifically, for wound healing, the method used for evaluation needs to be critically assessed as it may influence the outcome due to bias resulting from intra- or inter-observer variability. Furthermore, the methodology in the control groups was heterogeneous in the included studies as every study had another protocol for the control.
LLLT, which is also known as biostimulation or photobiomodulation, causes photochemical reactions in the tissue cells [44]. One of the first clinical applications of LLLT was to influence wound healing [45, 46]. The effect of LLLT on wound healing has been examined experimentally in vitro and in vivo, and it is suggested that wound healing is enhanced by promoting cell proliferation and procollagen synthesis, accelerating the formation of granulation tissue and increasing ATP synthesis within the mitochondria [41, 44]. The effects are dependent on irradiation parameters like wavelength, output power and energy density, but also on the different cell types affected [44, 47]. The energy density seems to play an important role as, at low doses (<2 J/cm2), photobiostimulation is favourable, whilst at higher doses (>16 J/cm2) it has mostly been described as suppressive. The total energy applied on the ulcers varied between 0.3 and 90 J in the studies using a diode laser included in this review. One study on wound healing after LLLT was placebo-blinded [24] and had a split-mouth design. The others had no therapy [27, 28] or used topical corticosteroid as controls [23, 27, 29]. Thus, comparison between the studies is difficult, and results have to be interpreted with some caution.
The effect of NACLT on wound healing has, to the best of our knowledge, not been studied elsewhere than in the included studies. Two of three studies found an improvement of wound healing after NACLT on aphthous lesions. All three studies were placebo-controlled RCTs, but evaluation of healing differed.
The effect on wound healing may not be totally separated from the effect on pain. Two studies with faster wound healing [24, 29] also reported a significant reduction in late pain, whilst the two without faster wound healing did not report improvement in pain relief. Thus, a shorter period of wound healing could have a direct effect on the reduction of pain.
Laser compared to other treatments for RAS
Treating RAS with lasers is considered as an alternative option to the common symptomatic treatment approaches. The first line of treatment is usually with topical pharmaceuticals, such as (1) antiseptics, anti-inflammatory agents and analgesics or (2) antibiotics or (3) corticosteroids or (4) hyaluronic acid or (5) topical anaesthetics or (6) other (i.e. herbal medication) [17]. Topical corticosteroids are widely used for immune-mediated oral diseases and in RAS therapy to shorten the healing time and reduce pain [17]. One study using Nd:YAG laser [23] and two studies with LLLT [25, 27] compared head-to-head the laser effect to the topical application of triamcinolone acetonide 0.1%. The patients in these studies were not blinded to their group allocation, which might be considered as a disadvantage. However, comparing different treatment arms is of clinical interest. Regarding wound healing, less exudation was observed during wound healing after Nd:YAG laser than when triamcinolone acetonide was applied, and pain was significantly reduced after laser treatment. After LLLT, no improvement in wound healing was observed compared to the effect of topical corticosteroid. A typical side effect of the topical application of corticosteroids is fungal infection [48]. This risk is absent after laser therapy. More serious side effects are described in connection with systemic pharmacological treatments for RAS [17, 18]. These treatments are usually restricted to patients with severe RAS and/or as a second line of therapy. A proper evaluation of the superiority of the beneficial over the adverse effects should always be performed before and during treatment. None of the studies compared laser effect to systemic medication. Due to the low frequency or absence of side effects when using lasers, potential applications of laser in severe cases of RAS should be considered in future research.
Conclusions
This systematic review demonstrated that the application of lasers (CO2 laser, Nd:YAG laser and diode laser) is an option to relieve symptoms and promote wound healing in patients suffering from RAS with a low risk of side effects. Different laser applications (ablative or photobiomodulation) exhibited favourable results when compared to placebo, other therapies or no therapy. But due to the high variation of laser types and laser settings used, none can be currently considered as a standard laser application for the treatment of RAS. One further common disadvantage of the included studies is the limited power of the publications, with study participants varying between 7–90 for the laser and 5–90 for the control groups. More studies using similar parameters and on larger patient groups are necessary to demonstrate whether there is any superiority using a laser to a topical pharmaceutical treatment. A uniform methodology to evaluate wound healing of RAS in future studies would be advantageous. Any effect of laser on the severity of recurrences and episode frequency is also of interest for future projects.
References
Zain RB (2000) Oral recurrent aphthous ulcers/stomatitis: prevalence in Malaysia and an epidemiological update. J Oral Sci 42:15–19
Chattopadhyay A, Shetty KV (2011) Recurrent aphthous stomatitis. Otolaryngol Clin North Am 44:79–88
Axéll T, Henricsson V (1985) The occurrence of recurrent aphthous ulcers in an adult Swedish population. Acta Odontol Scand 43:121–125
Davatchi F, Tehrani-Banihashemi A, Jamshidi AR, Chams-Davatchi C, Gholami J, Moradi M, Akhlaghi M, Foroozanfar MH, Barghamdi M, Noorolahzadeh E, Samadi F, Hadj-Aliloo M, Ghaznavi K, Ghaznavi K, Soroosh M, Khabazi A, Salari AH, Sharif SK, Karimifar M, Salessi M, Essalat-Manesh K, Nadji A, Shahram F (2008) The prevalence of oral aphthosis in a normal population in Iran: a WHO-ILAR COPCORD study. Arch Iran Med 11:207–209
McCullough MJ, Abdel-Hafeth S, Scully C (2007) Recurrent aphthous stomatitis revisited; clinical features, associations, and new association with infant feeding practices? J Oral Pathol Med 36:615–620
Slebioda Z, Szponar E, Kowalska A (2014) Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz) 62:205–215
Al-Omiri MK, Karasneh J, Alhijawi MM, Zwiri AM, Scully C, Lynch E (2015) Recurrent aphthous stomatitis (RAS): a preliminary within-subject study of quality of life, oral health impacts and personality profiles. J Oral Pathol Med 44:278–823
Truelove SC, Morris-Owen RM (1958) Treatment of aphthous ulceration of the mouth. BMJ 15:603–607
Cook BE (1960) The diagnosis of bullous lesions affecting the oral mucosa. I. Br Dent J 109:83–96
Scully C, Porter S (2008) Oral mucosal disease: recurrent aphthous stomatitis. Br J Oral Maxillofac Surg 46:198–206
Jurge S, Kuffer R, Scully C, Porter SR (2006) Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis 12:1–21
Chavan M, Jain H, Diwan N, Khedkar S, Shete A, Durkar S (2012) Recurrent aphthous stomatitis: a review. J Oral Pathol Med 41:577–583
Baccaglini L, Lalla RV, Bruce AJ, Sartori-Valinotti JC, Latortue MC, Carrozzo M, Rogers RS 3rd (2011) Urban legends: recurrent aphthous stomatitis. Oral Dis 17:755–770
Graykowski EA, Barile MF, Lee WB, Stanley HR Jr (1966) Recurrent aphthous stomatitis. Clinical, therapeutic, histopathologic, and hypersensitivity aspects. JAMA 196:637–644
Koybasi S, Parlak AH, Serin E, Yilmaz F, Serin D (2006) Recurrent aphthous stomatitis: investigation of possible etiologic factors. Am J Otolaryngol 27:229–232
Taylor J, Glenny AM, Walsh T, Brocklehurst P, Riley P, Gorodkin R, Pemberton MN (2014) Interventions for the management of oral ulcers in Behçet’s disease. Cochrane Database Syst Rev 9:CD011018
Belenguer-Guallar I, Jiménez-Soriano Y, Claramunt-Lozano A (2014) Treatment of recurrent aphthous stomatitis. A literature review. J Clin Exp Dent 6:e168–174
Brocklehurst P, Tickle M, Glenny AM, Lewis MA, Pemberton MN, Taylor J, Walsh T, Riley P, Yates JM (2012) Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database Syst Rev 9:CD005411
Ship JA (1996) Recurrent aphthous stomatitis. An update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81:141–147
Zand N, Ataie-Fashtami L, Djavid GE, Fateh M, Alinaghizadeh MR, Fatemi SM, Arbabi-Kalati F (2009) Relieving pain in minor aphthous stomatitis by a single session of non-thermal carbon dioxide laser irradiation. Lasers Med Sci 24:515–520
Zand N, Fateh M, Ataie-Fashtami L, Djavid GE, Fatemi SM, Shirkavand A (2012) Promoting wound healing in minor recurrent aphthous stomatitis by non-thermal, non-ablative CO2 laser therapy: a pilot study. Photomed Laser Surg 30:719–723
Prasad RS, Pai A (2013) Assessment of immediate pain relief with laser treatment in recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol 116:189–193
Tezel A, Kara C, Balkaya V, Orbak R (2009) An evaluation of different treatments for recurrent aphthous stomatitis and patient perceptions: Nd:YAG laser versus medication. Photomed Laser Surg 27:101–106
Aggarwal H, Singh MP, Nahar P, Mathur H, Gv S (2014) Efficacy of low-level laser therapy in treatment of recurrent aphthous ulcers—a sham controlled, split mouth follow up study. J Clin Diagn Res 8:218–221
De Souza TO, Martins MA, Bussadori SK, Fernandes KP, Tanji EY, Mesquita-Ferrari RA, Martins MD (2010) Clinical evaluation of low-level laser treatment for recurring aphthous stomatitis. Photomed Laser Surg 28:S85–S88
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Salman H, Kashmoola MA, Al-Waiz MM, Al-Sandooq TA (2008) Differences between low level laser therapy and triamcinolone acetonide kenalog on healing of recurrent aphthous ulceration. Ann Coll Med 34:35–41
Hazeem MI, Rajab MS, Badeia RA (2013) Treatment of recurrent aphthous stomatitis with 940 nm diode laser. Tikrit J Dental Sci 1:77–82
Lalabonova H, Daskalov H (2014) Clinical assessment of the therapeutic effect of low-level laser therapy on chronic recurrent aphthous stomatitis. Biotechnol Biotechnol Equip 28:929–933
Sattayut S, Trivibulwanich J, Pipithirunkarn N, Danvirutai N (2013) A clinical efficacy of using CO2 laser irradiating to transparent gel on aphthous stomatitis patients. Laser Ther 22:283–289
Albrektson M, Hedström L, Bergh H (2014) Recurrent aphthous stomatitis and pain management with low-level laser therapy: a randomized controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol 117:590–594
Bornstein MM, Suter VG, Stauffer E, Buser D (2003) The CO2 laser in stomatology. Part 2. Schweiz Monatsschr Zahnmed 113:766–785 (in German, in French)
Suter VG, Altermatt HJ, Dietrich T, Warnakulasuriya S, Bornstein MM (2014) Pulsed versus continuous wave CO2 laser excisions of 100 oral fibrous hyperplasias: a randomized controlled clinical and histopathological study. Lasers Surg Med 46:396–404
Carvalho PA, Jaguar GC, Pellizzon AC, Prado JD, Lopes RN, Alves FA (2011) Evaluation of low-level laser therapy in the prevention and treatment of radiation-induced mucositis: a double-blind randomized study in head and neck cancer patients. Oral Oncol 47:1176–1181
Doeuk C, Hersant B, Bosc R, Lange F, SidAhmed-Mezi M, Bouhassira J, Meningaud JP (2015) Current indications for low level laser treatment in maxillofacial surgery: a review. Br J Oral Maxillofac Surg 53:309–315
NAALT (2017) https://www.naalt.org/index.php/whitepapers/25-nomenclature-whitepaper. Last accessed 8 February 2017
Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA (2006) Low-level laser therapy in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg 24:158–168
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40:516–533
He WL, Yu FY, Li CJ, Pan J, Zhuang R, Duan PJ (2015) A systematic review and meta-analysis on the efficacy of low-level laser therapy in the management of complication after mandibular third molar surgery. Lasers Med Sci 30:1779–1788
Miloro M, Halkias LE, Mallery S, Travers S, Rashid RG (2002) Low-level laser effect on neural regeneration in Gore-Tex tubes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:27–34
Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD (2004) The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg 22:323–329
Vale FA, Moreira MS, de Almeida FC, Ramalho KM (2015) Low-level laser therapy in the treatment of recurrent aphthous ulcers: a systematic review. ScientificWorldJournal 2015:150412. doi:10.1155/2015/150412
Greer RO Jr, Lindenmuth JE, Juarez T, Khandwala A (1993) A double-blind study of topically applied 5% amlexanox in the treatment of aphthous ulcers. J Oral Maxillofac Surg 51:243–248
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22:348–364
Mester E, Spiry T, Szende B, Tota JG (1971) Effect of laser rays on wound healing. Am J Surg 122:532–535
Mester E, Nagylucskay S, Döklen A, Tisza S (1976) Laser stimulation of wound healing. Acta Chir Acad Sci Hung 17:49–55
Wagner VP, Meurer L, Martins MA, Danilevicz CK, Magnusson AS, Marques MM, Filho MS, Squarize CH, Martins MD (2013) Influence of different energy densities of laser phototherapy on oral wound healing. J Biomed Opt 18:128002. doi:10.1117/1.JBO.18.12.128002
Pereira Tdos S, Silva Alves Jde F, Gomes CC, Rocha do Nascimento A, Stoianoff MA, Gomez RS (2014) Kinetics of oral colonization by Candida spp. during topical corticotherapy for oral lichen planus. J Oral Pathol Med 43:570–575
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Funding
The study was funded by departmental funds of the Department of Oral Surgery and Stomatology, University of Bern.
Ethical approval
Ethical approval was not needed for this systematic review.
Informed consent
Informed consent was not needed for this systematic review.
Additional information
Valerie G. A. Suter and Sophia Sjölund contributed equally to this work.
Rights and permissions
About this article
Cite this article
Suter, V.G.A., Sjölund, S. & Bornstein, M.M. Effect of laser on pain relief and wound healing of recurrent aphthous stomatitis: a systematic review. Lasers Med Sci 32, 953–963 (2017). https://doi.org/10.1007/s10103-017-2184-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2184-z