Abstract
The aim of this study was to investigate the efficiency of low-level laser therapy (LLLT) to recovery testicular degeneration in rams. In the first study, rams were induced to testicular degeneration by scrotal insulation, and then, they were treated using LLLT at 28 J/cm2 (INS28) or 56 J/cm2 (INS56) energy densities. Sperm kinetics, morphology, and membranes integrity as well as proportion of lumen area in seminiferous tubule were assessed. In the second study, rams were submitted or not to scrotal insulation and treated or not by the best protocol of LLLT defined by experiment 1 (INS28). In this study were evaluated sperm kinetics, morphology, membranes integrity, ROS production, and DNA integrity. Testosterone serum concentration and proportion of lumen area in seminiferous tubule were also analyzed. Insulation was effective in promoting sperm injuries in both experiments. Biostimulatory effect was observed in experiment 1: INS28 presented smaller proportion of lumen area (P = 0.0001) and less degeneration degree (P = 0.0002). However, in experiment 2, there was no difference between the groups (P = 0.17). In addition, LLLT did not improve sperm quality, and there was a decreasing for total and progressive motility (P = 0.02) and integrity of sperm membranes (P = 0.01) in LLLT-treated groups. Moreover, testosterone concentration was not improved by LLLT (P = 0.37). Stimulation of aerobic phosphorylation by LLLT may have led to a deregulated increase in ROS leading to sperm damages. Thus, LLLT at energy of 28 J/cm2 (808 nm of wavelength and 30 mW of power output) can induce sperm damages and increase the quantity of cells in seminiferous tubule in rams.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular degeneration is the most important cause of male infertility and arises in domestic animals and men [1]. This alteration can be occasioned by many factors; the most frequent of them is heat stress (HS) which affects severely the spermatogenesis. HS can be caused by disruption of testicular thermoregulation by inflammations, traumas, and high environmental temperature [1, 2].
Increase of testes temperature results in a raise of cellular metabolism, which increases reactive oxygen species (ROS) production and consequently oxidative stress, DNA fragmentation, and cellular apoptosis [2]. Experimentally, testicular degeneration can be induced by cryptorchidism, immerging testes in hot water or using scrotal bags to promote scrotal insulation. The animals can also be submitted to high environmental temperature, although, unlike the other methods, this practice alters all organism systems [2–5].
Even though testicular degeneration is an important cause of male infertility, there is not yet an efficient treatment established. Some treatments performed nowadays consist in the removal of the cause [6] or administration of nutraceuticals substances [7]. However, responses to nutraceuticals treatment vary widely according to individual characteristics, and its results present low repeatability [7]. Moreover, the removal of the causes can demand many weeks to testicular recovery, being extremely variable according to individual characteristics, duration of the stress, and spermatogenesis [6].
Low-level laser therapy (LLLT) is knew by its biostimulatory effects [8], proliferation of mesenchymal stem cells [9, 10], and acceleration of differentiation of bone marrow-derived mesenchymal stem cells to osteoblasts and neurons [11]. In rats, LLLT doses of 28.05 J/cm2 increased the number of spermatocytes and spermatids, while doses of 46.8 J/cm2 presented a negative affect [12]. In oligospermic men treated by LLLT, sperm count and libido increased and abnormal cells decreased [13]. LLLT promotes ATP synthesis by activating respiratory chain enzymes and aerobic phosphorylation. Moreover, LLLT accelerates metabolism and promotes amplification of antioxidants [8, 14, 15].
According to our knowledge, LLLT was not evaluated as a testicular degeneration treatment. Therefore, the aim of this study was to propose a new method to testicular degeneration treatment based in the LLLT properties. Due to biostimulatory effect, it was expected that LLLT improves seminal characteristics and increases the seminiferous epithelium cells and the plasma testosterone concentration.
Material and methods
This study was conducted at the Animal Reproduction Biotechnology Center from the School of Medicine Veterinary and Animal Science of the University of São Paulo, in Pirassununga. Unless otherwise noted, solutions and chemical reagents used were from Sigma-Aldrich (St. Louis, MO, USA) and Life Technologies (Thermo Fisher Scientific).
All procedures were in agreement with Ethical Principles in Animal Research adopted by “Ethic Committee in the Use of Animals” of the School of Veterinary Medicine and Animal Science of University of São Paulo, protocol number 2467/2012.
Experiment 1: effect of different energy density (28 J/cm2 × 56 J/cm2) of low-level laser therapy in rams induced testicular to degeneration
Animals
Six healthy rams with an average age of 10 ± 0.8 months and body weight of 30.8 ± 7.5 kg were used to perform this first experiment. The rams were housed in paddock being provided corn silage and concentrate to attend the NRC (1998). Environmental temperature and humidity were evaluated all time (each 10 min) by Climatologic Station located at University of São Paulo in Pirassununga City, SP, Brazil. Collections and evaluations of data were performed between February and April 2013.
Study design
All rams were submitted to scrotal insulation during 72 h by insulation bags, to induce testicular degeneration. Scrotal thermography and seminal evaluations were performed before and after scrotal insulation in specific moments as showed below. LLLT treatment initiated 3 days after the remove of insulation bags. Rams were divided in three groups: INS, without treatment (control group; n = 2); INS28, treated by LLLT using 28 J/cm2 (n = 2); and INS56, treated by LLLT using 56 J/cm2 (n = 2).
Low-level laser therapy
The device used was a GaAlAs (Thera Laser®, DMC Equipment, Sao Carlos, Brazil). The spectrum of near-infrared laser at a continuous wavelength of 808 nm and a 30 mW of power output was used in this study. Treatment protocol was adapted from Taha and Velojerdi [12], and it was performed during 15 days each 48 h. The protocol used daily in INS28 was 5 J/cm2 in the first and second days, 4 J/cm2 in the third and fourth days, 3 J/cm2 in the fifth and sixth days, and 2/cm2 in the seventh and eighth days totalizing 28 J/cm2, while the protocol used daily in INS56 was twice: 10 J/cm2 in the first and second days, 8 J/cm2 in the third and fourth days, 6 J/cm2 in the fifth and sixth days, and 4/cm2 in the seventh and eighth days totalizing 56 J/cm2.
Scrotal thermography and evaluation of images
Scrotal superficies mean temperature (SSMT) mensuration was performed 8, 5, and 1 day before bags were put-on (B-8, B-5, and B-1), in the day that the bags were put-on (B0), in the day that bags were removed (A0), and 1, 2, 10, 17, 24, and 31 days afterward (A1, A2, A10, A17, A24, and A31).
T640® thermography camera (FLIR Systems, USA) was used to take the thermography images, which were analyzed on FLIR Quick Report® software (FLIR Systems, USA). Animals were protected from sun exposure, and the scrotal superficies was not touched at least 30 min before exam. The distance between the animal and the camera was of 0.90 m, and camera emissivity was adjusted to 0.98. It was analyzed the caudal face of the scrota. Environmental temperature and humidity were measured during exam, and the values were used in a mathematic formula, proposed by Basile [16], to allow comparison between the different times of evaluation.
Semen evaluation
Semen evaluation consisted in sperm concentration, sperm kinetic, sperm morphology, and sperm membranes integrity. Semen collection was performed by artificial vagina 8, 5, and 1 day before insulation period (TI-8, TI-5, and TI-1) and 10, 17, 24, and 31 days after the day that bags were removed (TI10, TI17, TI24, and TI31).
Sperm concentration was evaluated by a Neubauer chamber. To assess sperm kinetic, semen was diluted (12.5 × 106 sperm/mL) in Tyrodes Albumin Lactate Pyruvate (TALP) medium [17]. Sperm kinetic was evaluated by Sperm Class Analyzer software (SCA, Microptics, Barcelona, Spain) with setup adjusted to ram’s spermatozoa. A Makler® chamber was used in all evaluations. The parameters evaluated were total motility (%) and progressive motility (%).
To assess sperm morphology, an aliquot of fresh semen was fixed in a 37 °C formaldehyde 4 % solution. Differential interference contrast (DIC, model 80i, Nikon, Tokyo, Japan) was used to evaluate sperm morphology. A total of 200 cells were counted at magnification of ×1000. Cells were classified according to Blom [18] in major, minor, and total defects.
Regarding sperm membranes integrity evaluation, sperm was diluted in TALP medium [17] to adjust sperm concentration to 25 × 106 sperm/mL. For each 150 μL of semen diluted was added 2 μL of Hoescht 33342 (0.5 mg/mL, Life Technologies), 3 μL of propidium iodide (0.5 mg/mL, Sigma), 50 μL of fluorescein isothiocyanato-labeled Pisum sativum agglutinin (FITC-PSA, 100 μg/mL, Sigma), and 2 μL of 5,5′,6,6′tetracloro 1,1′,3,3′tetraetilbenzimidazolil carbocianin iodide (JC-1, 153 μM, Life Technologies) incubated per 8 min at 37 °C in the dark according to Celeghini [19]. Two hundred cells were analyzed under epifluorescence microscopy (Nikon, model 80i) at ×1000 magnification using a triple filter (D/F/R, C58420) featuring the UV-2E/C (340–380 nm excitation and 435–485 emission), B-2E/C (465–495 excitation and 515–555 emission), and G-2E/C (excitation 540–525 and 605–655 emission). Cells were classified in PIAIHM (sperm with plasma membrane integrity, acrosome membrane integrity and high mitochondrial membrane potential), sperm with plasma membrane integrity (PI), sperm with acrosome membrane integrity (AI), and sperm with high mitochondrial membrane potential (HM).
Histopathology
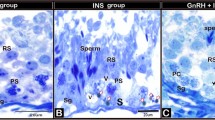
Rams were submitted to bilateral orchiectomy 32 days after insulation period, and testicular fragments were fixed in Bouin® solution during 24 h; then, it was subsequently maintained in alcohol 70 %, being finally stained using hematoxylin-eosin.
Images of fragments were taken by a camera attached in a light microscope (model 80i, Nikon, Tokyo, Japan). Proportion of lumen area in seminiferous tubule was measured in each image by Image Pro Plus® software, using the same program configuration for all analyses. Moreover, fragments were also classified in degeneration degrees to certify that the evaluation by the program was accurate. For this purpose, blind analyses were performed and fragments were classified in 0 (normal parenchyma), 1 (light degeneration degree), 2 (moderate degeneration degree), and 3 (severe degeneration degree).
Statistical analyses
Three treatments and seven different periods of evaluation were considered. The Shapiro-Wilk test was used to verify homogeneity of variances and transformations, and removal of outliers was performed when necessary. Data were evaluated by analysis of variance with PROC MIXED of SAS (SAS Institute 9.2). Respecting the completely randomized design, the command repeated measures was added to verify interaction between treatment and the different times of each period. For histopathology analyses, it was performed the PROC MIXED command and Tukey test. It was considering significant difference when P ≤ 0.05.
Experiment 2: treatment of induced testicular degeneration in rams by low-level laser therapy
Animals
Twenty healthy White Dorper rams with an average age of 17.5 ± 2.8 months and body weight of 65.7 ± 8.8 kg were used to perform this second experiment. The rams were housed in paddock. Hay and concentrate were provided to attend the NRC (1998). Environmental temperature and humidity were evaluated at all experimental times (each 10 min) by a data logger (OPUS 20 THI - 8120.00, Lufft, Germany). Data collection and evaluation were performed between August and December 2013.
Study design
Scrotal thermography and seminal evaluations were performed 5 days before (TL-5) LLLT treatment (corresponding to 21 days after insulation period) and 8 (TL8), 22 (TL22), 36 (TL36), and 50 days after (TL50) LLLT treatment. Study was conducted in 2 × 2 factorial design, considering insulation and LLLT treatment. The experimental groups were CC, not submitted to scrotal insulation and not treated with LLLT (control group; n = 5), CL, not submitted to scrotal insulation and treated with LLLT (n = 6), IC, submitted to scrotal insulation and not treated with LLLT (n = 3), and IL, submitted to scrotal insulation and treated with LLLT (n = 6). Rams were submitted to scrotal insulation during 72 h with insulation bags. LLLT protocol was defined in experiment 1, and it was used the same protocol established for INS28.
Scrotal thermography and evaluation of images
Scrotal superficies mean temperature mensuration was performed before and after LLLT treatment period. The same material and method of experiment 1 was employed. However, it was used a climatic chamber to control the environmental temperature (20 °C) and humidity (60 %) in all evaluations, being the rams maintained there for at least 12 h before thermography exam. Therefore, for this experiment was not necessary to adjust the values by mathematic formula.
Semen evaluation
In experiment 2, semen evaluation was submitted to a deeper approach than evaluation performed in experiment 1. The analyses consisted in sperm kinetic, sperm morphology, sperm membranes integrity, sperm production of reactive oxygen species, and sperm DNA fragmentation. Semen collection was performed by artificial vagina on times TL-5, TL8, TL22, TL36, and TL50. Sperm kinetic, sperm morphology, and sperm membranes integrity were evaluated with the same material and methods of experiment 1 described above.
Reactive oxygen species production was evaluated by CellROX Deep Red® probe according to the protocol established by Alves [20]. Therefore, semen was diluted (25 × 106 sperm/mL) in TALP medium [17]. At a volume of 200 μL was added 0.5 μL of CellROX® (1 mM, Invitrogen, Life Technologies) and 2 μL of Hoescht 33342 (0.5 mg/mL, Life Technologies) and incubated per 30 min at 37 °C. The sample was submitted to centrifugation per 5 min at 2000g, and it was analyzed 200 cells. Evaluation was performed in an epifluorescence microscopy (Nikon, model 80i) at ×1000 magnification using a triple filter (D/F/R, C58420) featuring the UV-2E/C (340-380 nm excitation and 435-485 emission), B-2E/C (465-495 excitation and 515-555 emission), and G-2E/C (excitation 540-525 and 605-655 emission) sets.
Ovine Halomax® kit (Halotech, Madrid, Spain) was used to evaluate sperm DNA fragmentation, and it was followed the protocol preconized by it. It was used 0.5 μL of propidium iodide probe (0.5 mg/mL) to stain the cell’s DNA. Sperm was classified in nonfragmented DNA (without presence of halo) and fragmented DNA (presence of halo), and 500 cells were evaluated on epifluorescence microscope (Nikon, model 80i) at ×1000 magnification, using the same triple filter cited before.
Blood collections and testosterone concentration evaluation
Blood was collected in the days TL-5, TL8, TL22, TL36, and TL50 at 6 a.m. and 15 p.m. There was no difference on testosterone serum concentration among the different hour of evaluation, so it was considered the average. Vacutainer® system (BD, USA) was used to collect blood from jugular vein. As soon as collected, blood was centrifuged in 2100g during 15 min to separate the serum and then kept in freezer (−80 °C). Serum testosterone concentration was determined by radioimmunoassay using commercial kit (Testosterone DA kit, MP, USA) in Laboratory of Neuroendocrinology and Reproduction, Department of Physiology, Faculty of Medicine, USP, Ribeirão Preto, SP, Brazil.
Histopathology
Rams were submitted to bilateral orchiectomy 56 days after LLLT treatment period. As described in experiment 1, tissue fragments were fixed in Bouin® solution during 24 h, stored in alcohol 70 %, and were stained using hematoxylin-eosin followed by image capture using a camera attached in a light microscope (model 80i, Nikon, Tokyo, Japan). It was also measured the proportion of lumen area present in seminiferous tubule by Image Pro Plus® software using the same program configuration for all analyses.
Statistical analyses
Two factors were considered (insulation and LLLT) and five different periods. Shapiro-Wilk test was used to verify the homogeneity of variances, transformations, and removal of outliers that were performed when necessary. Data were evaluated by analysis of variance using PROC MIXED of SAS (SAS Institute 9.2). Respecting the completely randomized design was added the command repeated measures to verify interaction between treatment and the different times of each period. For histopathology analyses, it performed the command PROC MIXED and Tukey test. Significant difference was considered when P ≤ 0.05.
When there was no effect of interaction treatment × time, the effects were considered isolated (insulation effect, LLLT effect, or time effect).
Results
Experiment 1: effect of different energy density (28 J/cm2 × 56 J/cm2) of low-level laser therapy in rams induced to testicular degeneration
Insulation was efficient to increase scrotal superficies mean temperature (SSMT), as showed in Fig. 1. After insulation, on the day that bags were removed (A0) and 1 day afterward (A1), all rams independently of the treatment group presented increase of SSMT (time effect: P = 0.0012). In A0, SSMT was 36.25 ± 0.49 °C for all groups and in A1 was 34.75 ± 0.24 °C; in the other days of evaluation, temperature varied from 32.78 ± 0.53 °C in A31 to 33.87 ± 0.41 °C in B-1. Besides to alter SSMT, insulation was efficient to affect negatively semen characteristics. Total and progressive motility, PIAIHM cells, PI cells, and HM cells decreased, and abnormal sperm morphology increased by insulation, characterizing testicular degeneration, as seen in Table 1 by the effect of time.
However, LLLT did not increase SSMT (Fig. 1) and did not affect negatively the seminal characteristics. In addition, LLLT decreased (P = 0.05) the number of cells with minor defects in INS28 treatment and also decreased (P = 0.01) the quantity of AI cells in INS56 treatment (Table 1).
Histopathological evaluation showed that INS and INS56 were more affected by insulation than INS28. Thereby, the quantity of cells in the seminiferous tubule was greater in the INS28 than in the INS or INS56, as seen in Table 2.
Experiment 2: treatment of induced testicular degeneration in rams by low-level laser therapy
As observed in experiment 1, SSMT was also not altered by LLLT. Animals that were not treated by LLLT (CC and IC groups) presented SSMT of 30.26 ± 0.10 °C, while animals that were treated by LLLT (CL and IL groups) presented SSMT of 30.32 ± 0.09 °C (P = 0.77). Besides, SSMT was not altered 21 days (TL-5) after scrotal insulation and neither after LLLT treatment according to Table 3.
Likewise, in experiment 1, insulation was also effective to affect negatively sperm total (insulation × time effect: P = 0.01) and progressive motility (insulation effect: P = 0.007) according to Fig. 2, sperm morphology (insulation × time effect: major defects: P = 0.007, total defects: P = 0.006) according to Fig. 3, PIAIHM cells (insulation × time effect: P = 0.002), PI cells (insulation × time effect: P = 0.0004), AI cells (insulation × time effect: P = 0.05), and HM cells (insulation × time effect: P = 0.001) according to Fig. 4, and production of ROS (insulation × time effect: P = 0.03) and sperm DNA integrity (insulation effect: P = 0.02) according to Fig. 5. Except to ROS production, after insulation and before LLLT treatment (TL-5), groups that were submitted to scrotal insulation (IC and IL) presented semen with worst quality than the groups that were not submitted to scrotal insulation (Table 4).
Mean of total* and progressive motility** on control (CC), control treated (CL) by low-level laser therapy (LLLT), insulated (IC) and insulated treated (IL) by LLLT groups 5 days before LLLT treatment (TL-5) and 8 (TL8), 22 (TL22), 36 (TL36), and 50 days (TL50) afterward. (*Insulation effect: P = 0.001; LLLT effect: P = 0.02; insulation × LLLT interaction: P = 0.42; time effect: P < 0.0001; insulation × time interaction: P = 0.01; LLLT × time interaction: P = 0.62; insulation × LLLT × time interaction: P = 0.54. **Insulation effect: P = 0.007; LLLT effect: P = 0.02; insulation × LLLT interaction: P = 0.25; time effect: P = 0.001; insulation × time interaction: P = 0.09; LLLT × time interaction: P = 0.24; insulation × LLLT × time interaction: P = 0.75)
Mean of sperm with major defects* and sperm with total defects** on control (CC), control treated (CL) by low-level laser therapy (LLLT), insulated (IC) and insulated treated (IL) by LLLT groups 5 days before LLLT treatment (TL-5) and 8 (TL8), 22 (TL22), 36 (TL36), and 50 days (TL50) afterward. (*Insulation effect: P = 0.005; LLLT effect: P = 0.28; insulation × LLLT interaction: P = 0.49; time effect: P = 0.0002; insulation × time interaction: P = 0.007; LLLT × time interaction: P = 0.66; insulation × LLLT × time interaction: P = 0.53. **Insulation effect: P = 0.03; LLLT effect: P = 0.31; insulation × LLLT interaction: P = 0.80; time effect: P < 0.0001; insulation × time interaction: P = 0.006; LLLT × time interaction: P = 0.46; insulation × LLLT × time interaction: P = 0.19)
Mean of sperm with plasma and acrosome membranes integrity and mitochondrial membrane with high potential (PIAIHM)*, sperm with plasma membrane integrity (PI)**, sperm with acrosome membrane integrity (AI)***, and sperm with mitochondrial membrane with high potential (HM)**** on control (CC), control treated (CL) by low-level laser therapy (LLLT), insulated (IC), and insulated treated (IL) LLLT groups 5 days before LLLT treatment (TL-5) and 8 (TL8), 22 (TL22), 36 (TL36), and 50 days (TL50) afterward. (*Insulation effect: P = 0.02; LLLT effect: P = 0.01; insulation × LLLT interaction: P = 0.32; time effect: P = 0.004; insulation × time interaction: P = 0.002; LLLT × time interaction: P = 0.45; insulation × LLLT × time interaction: P = 0.50. **Insulation effect: P = 0.02; LLLT effect: P = 0.15; insulation × LLLT interaction: P = 0.56; time effect: P = 0.0001; insulation × time interaction: P = 0.0004; LLLT × time interaction: P = 0.22; insulation × LLLT × time interaction: P = 0.50. ***Insulation effect: P = 0.15; LLLT effect: P = 0.06; insulation × LLLT interaction: P = 0.21; time effect: P = 0.74; insulation × time interaction: P = 0.05; LLLT × time interaction: P = 0.22; insulation × LLLT × time interaction: P = 0.89. ****Insulation effect: P = 0.01; LLLT effect: P = 0.02; insulation × LLLT interaction: P = 0.48; time effect: P = 0.0006; insulation × time interaction: P = 0.001; LLLT × time interaction: P = 0.19; insulation × LLLT × time interaction: P = 0.33)
Mean of sperm producing ROS* and sperm DNA integrity** on control (CC), control treated (CL) by low-level laser therapy (LLLT), insulated (IC) and insulated treated (IL) by LLLT groups 5 days before LLLT treatment (TL-5) and 8 (TL8), 22 (TL22), 36 (TL36), and 50 days (TL50) afterward. (*Insulation effect: P = 0.28; LLLT effect: P = 0.43; insulation × LLLT interaction: P = 0.25; time effect: P = 0.0001; insulation × time interaction: P = 0.03; LLLT × time interaction: P = 0.68; insulation × LLLT × time interaction: P = 0.19. **Insulation effect: P = 0.02; LLLT effect: P = 0.34; insulation × LLLT interaction: P = 0.42; time effect: P = 0.22; insulation × time interaction: P = 0.25; LLLT × time interaction: P = 0.45; insulation × LLLT × time interaction: P = 0.60)
LLLT was able to affect some sperm characteristics. Sperm total (LLLT effect: P = 0.02) and progressive motility (LLLT effect: P = 0.02), PIAIHM cells (LLLT effect: P = 0.01), and HM cells (LLLT effect: P = 0.02) were negatively affected by LLLT as seen in Table 5 and Figs. 2 and 4. Acrosome membrane integrity displayed statistical tendency (LLLT effect: P = 0.06) (Table 5).
Although the groups that were treated by LLLT presented a higher testosterone serum concentration, the difference was not significant (LLLT effect: P = 0.37). CL and IL groups presented 11.25 ± 0.87 and 9.58 ± 1.16 ng/mL, respectively, while CC and IC presented 9.61 ± 1.29 and 8.57 ± 1.34 ng/mL. Likewise, the proportion of lumen area was not altered by insulation neither by LLLT (Table 6); thus the quantity of cells was similar in the four groups evaluated despite the proportion of lumen area that was apparently minor in group IL.
Discussion
The experimental goal was to establish a new treatment to testicular degeneration in rams. For this, a method to induce testicular degeneration was used. Scrotal insulation was able to increase testicular temperature and to cause injuries in semen quality. Other authors utilized this technique with success to induce seminal injuries and testicular degeneration in bulls and rams [21–24]. Regarding sperm kinetic, insulation was able to decrease total and progressive motility as observed by Arman [23]. Fernandes [22] and Pérez-Crespo [5] observed an increase in DNA abnormalities of Nellore bulls and mice, respectively, submitted to scrotal heat stress. This DNA fragmentation is the consequence of oxidative stress [3, 4]. Although there were observed injuries in DNA promoted by insulation, our data did not show an increase in ROS production 21 days after insulation, being apparently this production more intensive during heat stress and immediately afterward.
The first experiment aimed to establish an LLLT treatment protocol that would be used in the second experiment. An important concern was whether LLLT affected the testicular temperature. Thus, scrotal superficies mean temperature was monitored before and after LLLT in both experiments and was not observed an increase in scrotal temperature after the therapy. Mester [25] reported that low-level laser therapy is unable to increase temperature being that a nonthermal therapy. However, Gnyawali [26] reported that LLLT is able to increase superficies temperature as soon as it was irradiated. While in our study was not observed an increase of scrotal temperature in both experiments, we did not measure the scrotal temperature as soon as therapy was done.
Another question, to be answered in experiment 1, was on the best energy dose to apply on the testicles of rams. The results of histopathological characteristics of proportion of lumen and degeneration degree suggest that LLLT with 28 J/cm2 was better than LLLT with 56 J/cm2. Thus, this protocol was elected in the second experiment. However, it was observed no improvement in semen quality in both experiments and even with worsening of some characteristics caused by LLLT in the second experiment.
According to Farivar [27], biostimulation of LLLT is caused by mitochondrial stimuli. Infrared light is able to stimulate mitochondria to produce ATP. This production is able to increase levels of ROS. However, it was reported that LLLT increases also the antioxidant agents. Nevertheless, in the present study, it was observed injuries in semen quality, mainly in total and progressive motility, PIAIHM, and HM cells. This effect can be occasioned by a possible increase in testicular temperature caused by LLLT that was not detected by thermography made 8 days after LLLT treatment and/or by a deregulated production of ROS promoted by LLLT. Even though it was not observed an increase in sperm ROS production, it is possible that this increase occurred during the LLLT treatment and seminal characteristics that were not evaluated during this period.
With the stimulating production of ATP, laser promotes proliferation of cells [8, 14, 15, 27, 28]. This proliferation was observed in histopathological characteristics of testes in experiment 1. The proportion of lumen area of seminiferous tubule was smaller in the group treated by LLLT with 28 J/cm2 than in group not treated and treated by LLLT with 56/cm2 as observed by Taha and Valojerdi [12]. Consequently, it denotes that the number of cells were greater in the group that had smaller proportion of lumen area. Although the same effect was not observed in experiment 2, it is possible to note that apparently, the proportion of lumen in the group insulated treated with LLLT was smaller than in the group insulated and not treated. This difference between the patterns of histopathology in experiment 1 and in experiment 2 can be explained by the different times of evaluation. In experiment 1, the testes were collected 32 days after LLLT, while in experiment 2, the testes were collected 56 days after LLLT treatment. Moreover, it was not observed the same effect in animals that were not submitted to testicular degeneration and were treated by LLLT. Likewise, testosterone serum concentration was not affected by LLLT. However, it appeared to be increased in the groups that were treated by LLLT suggesting a biostimulatory effect promoted by LLLT in Leydig cells.
Conclusions
Thus, in concern to the results, it is possible to conclude that LLLT at energy of 28 J/cm2, 808 nm of wavelength, and 30 mW of power output can induce semen injuries and increase the quantities of cells in seminiferous tubule when detected 32 days after LLLT treatment. However, this increase did not occur in a long time after the therapy and in animals that did not present testicular degeneration. LLLT in this condition is not efficient to increase serum testosterone concentration, and consequently, it is not efficient to stimulate Leydig cells. It is an evident need for further studies investigating the effect of LLLT in testicular degeneration with other protocols and other methods to measure its biostimulatory effects.
References
Hansen PJ (2009) Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond B Biol Sci 364:3341–3350. doi:10.1098/rstb.2009.0131
Setchell BP (1998) The parkes lecture. Heat and the testis. J Reprod Fertil 114:179–194
Paul C, Teng S, Saunders PTK (2009) A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod 919:913–919. doi:10.1095/biolreprod.108.071779
Paul C, Murray AA, Spears N, Saunders PTK (2008) A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 136:73–84. doi:10.1530/REP-08-0036
Pérez-Crespo M, Pintado B, Gutiérrez-Adán A (2008) Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev 75:40–47. doi:10.1002/mrd
Bicudo SD, Siqueira JB, Meira C (2007) Patologias do sistema reprodutor de touros. Biológico 69:43–48
Arruda RP, Silva DF, Alonso MA, Andrade AFC, Nascimento J, Gallego AM et al (2010) Nutraceuticals in reproduction of bulls and stallions. Rev Bras Zootec 39:393–400. doi:10.1590/S1516-35982010001300043
Navratil L, Kymplova J (2002) Contraindications in noninvasive laser therapy: truth and fiction. J Clin Laser Med Surg 20:341–343
Barboza CAG, Ginani F, Soares DM, Henriques ÁCG, Freitas RDA (2014) Low-level laser irradiation induces in vitro proliferation of mesenchymal stem cells. Einstein (São Paulo) 12:75–81. doi:10.1590/S1679-45082014AO2824
Ginani F, Soares DM, Barreto MPEV, Barboza CAG (2015) Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med Sci. doi:10.1007/s10103-015-1730-9
Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts—an in vitro study. Lasers Med Sci 27:423–430. doi:10.1007/s10103-011-0930-1
Taha MF, Valojerdi MR (2004) Quantitative and qualitative changes of the seminiferous epithelium induced by Ga. Al. As. (830 nm) laser radiation. Lasers Surg Med 34:352–359. doi:10.1002/lsm.20027
Hasan P, Rijadi SA, Purnomo S, Kainama H (1989) The possible application of low reactive level laser therapy in the treatment of male infertility. Laser Ther 1:49–59
Karu T (1987) Photobiological fundamentals of low-power laser therapy. IEEE J Quantum Electron 23:1703–1717. doi:10.1109/JQE.1987.1073236
Karu T (2003) Cellular mechanisms of low power laser therapy: new questions. Lasers Med Dent 3:79–100
Basile RC, Albernaz RM, Pereira MC, Araújo R, Ferraz GC, Queiroz-Neto A (2010) Guia prático de exames termográficos em equinos. Rev Bras Med Equina 6:24–28
Bavister D, Lorraine M (1983) Development of preimplantation in a defined embryos of the golden culture medium. Biol Trace Elem Res 28:235–247
Blom E (1973) The ultrastructure of some characteristic sperm defects and a proposal for a new classification of the bull spermiogram. Nord Vet Med 25:383–391
Celeghini ECC, Nascimento J, Raphael CF, Andrade AFC, Arruda RP (2010) Simultaneous assessment of plasmatic, acrosomal, and mitochondrial membranes in ram sperm by fluorescent probes. Arq Bras Med Vet e Zootec 62:536–543. doi:10.1590/S0102-09352010000300006
Alves MBR, Andrade AFC, Arruda RP, Batissaco L, Florez-Rodriguez SA, Lançoni R et al (2015) An efficient technique to detect sperm reactive oxygen species: the Cell Rox Deep Red® fluorescent probe. Biochem Physiol Open Access 4:1–5. doi:10.4172/2168-9652.1000157
Brito LFC, Silva AEDF, Barbosa RT, Unanian MM, Kastelic JP (2003) Effects of scrotal insulation on sperm production, semen quality, and testicular echotexture in Bos indicus and Bos indicus × Bos taurus bulls. Anim Reprod Sci 79:1–15. doi:10.1016/S0378-4320(03)00082-4
Fernandes CE, Dode MAN, Pereira D, Silva AEDF (2008) Effects of scrotal insulation in Nellore bulls (Bos taurus indicus) on seminal quality and its relationship with in vitro fertilizing ability. Theriogenology 70:1560–1568. doi:10.1016/j.theriogenology.2008.07.005
Arman C, Quintana Casares PI, Sanchez-Partida LG, Setchell BP (2006) Ram sperm motility after intermittent scrotal insulation evaluated by manual and computer-assisted methods. Asian J Androl 8:411–418. doi:10.1111/j.1745-7262.2006.00145.x
Kastelic JP, Cook RB, Coulter GH, Saacke RG (1996) Insulating the scrotal neck affects semen quality and scrotal/testicular temperatures in the bull. Theriogenology 45:935–942
Mester E, Szende B, Gärtner P (1968) The effect of laser beams on the growth of hair in mice. Radiobiol Radiother 9:621–626
Gnyawali SC, Chen Y, Wu F, Bartels KE, Wicksted JP, Liu H, Sen CK, Chen WR (2008) Temperature measurement on tissue surface during laser irradiation. Eng Comput 46:159–168. doi:10.1007/s11517-007-0251-5
Farivar S, Malekshahabi T, Shiari R (2014) Biological effects of low level laser therapy. J Lasers Med Sci 5:58–62
Karu TI (1988) Molecular mechanism of the therapeutic effect of low-intensity laser irradiation. Lasers Life Sci 2:53–74
Acknowledgments
The authors thank Dr. Fábio Pogliani and Dr. Fábio Sellera for assistance to laser therapy protocols, Dr. Ricardo Strefezi for assistance in histopathology images evaluations, and Dr. José Antunes Rodrigues, technicians Marina Holanda, and Rogério Azevedo for assistance to testosterone assay. They also thank Dr. Eduardo Harry Birgel Junior, Dr. Daniela Becker Birgel, Mr. João Carlos Pinto de Campos, Mr. Márcio Donizete De Carli, and Mr. José Maria Bernardi for assistance to the animals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, processes numbers 2011/16744-3, 2012/00040-0, 2012/15087-1, 2013/15745-1).
Rights and permissions
About this article
Cite this article
Alves, M.B.R., de Arruda, R.P., Batissaco, L. et al. Low-level laser therapy to recovery testicular degeneration in rams: effects on seminal characteristics, scrotal temperature, plasma testosterone concentration, and testes histopathology. Lasers Med Sci 31, 695–704 (2016). https://doi.org/10.1007/s10103-016-1911-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-1911-1