Abstract
Recently, investigations suggest the benefits of low-level laser (light) therapy (LLLT) in noninvasive treatment of cellulite, improvement of body countering, and control of lipid profile. However, the underlying key mechanism for such potential effects associated to aerobic plus resistance training to reduce body fat and inflammatory process, related to obesity in women still unclear. The purpose of the present investigation was to evaluate the effects of combined therapy of LLLT and aerobic plus resistance training in inflammatory profile and body composition of obese women. For this study, it involved 40 obese women with age of 20–40 years. Inclusion criteria were primary obesity and body mass index (BMI) greater than 30 kg/m2 and less than 40 kg/m2. The voluntaries were allocated in two different groups: phototherapy group and SHAM group. The interventions consisted on physical exercise training and application of phototherapy (808 nm), immediately after the physical exercise, with special designed device. Proinflammatory/anti-inflammatory adipokines were measured. It was showed that LLLT associated to physical exercise is more effective than physical exercise alone to increase adiponectin concentration, an anti-inflammatory adipokine. Also, it showed reduced values of neck circumference (cm), insulin concentration (μU/ml), and interleukin-6 (pg/ml) in LLLT group. In conclusion, phototherapy can be an important tool in the obesity, mostly considering its potential effects associated to exercise training in attenuating inflammation in women, being these results applicable in the clinical practices to control related risk associated to obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, strong evidence has been found that LLLT is an important tool in widely clinical applications, including esthetic treatments, chronic kidney disease, cancer, and different branches of regenerative medicine and dentistry [1]. Supporting this, it was recently demonstrated in experimental conditions that LLLT promoted improvement in some metabolic syndrome parameters in rats [2], although its effects in human obesity are unknown in the literature.
Metabolic syndrome is a constellation of metabolic alterations leading to cardiovascular diseases. In this way, obesity, mostly considering central and visceral fat and insulin resistance, has been strongly associated with the development of metabolic syndrome and cardiovascular diseases [3, 4]. In addition, the prevalence of metabolic syndrome in obese people ranges between 30 and 70 % [5, 6] in adolescents and adults, respectively, showing the necessity for new techniques to optimize the treatment of metabolic comorbidities. Moreover, the related inflammatory process involved in obesity promotes an increase in the proinflammatory adipokines and a reduction in the anti-inflammatory adipokines which are associated with several metabolic disorders such as type 2 diabetes, obesity, and cardiovascular disease [7, 8].
The key inflammatory mediators in obesity are adiponectin, interleukin-6 (IL-6), and TNF-alpha. Adiponectin is a 244-amino acid-long protein that is secreted from adipocytes and has anti-inflammatory and insulin-sensitizing properties [9]. However, reduced levels of this adipokine are linked to increased carotid intima-media thickness (cIMT), visceral adiposity, insulin resistance, diabetes, dyslipidemia, hypertension, hyperleptinemia, cardiovascular disease, metabolic syndrome, and systemic inflammation [10].
On the other hand, the members of proinflammatory cytokines, IL-6 and TNF-alpha, are known to be elevated in obesity and its comorbidities [11, 12] and have been accepted as clinical markers. Additionally, in metabolic syndrome patients, it is known that both are strong inhibitors of adiponectin. Nonetheless, weight loss therapies including physical exercise promote an improvement in the inflammatory biomarkers, showing that an increase in the adiponectin concentration and a reduction in the interleukin-6 and TNF-alpha occur [13, 14].
Interestingly, an initial proof-of-concept was previously shown suggesting that LLLT can be used in some inflammatory diseases to provide a noninvasive and clinical therapeutic strategy due to photochemical effects that change cellular functions in irradiated cells; thus, the therapeutic effect is not thermal [15, 16].
It is well settled in the literature that physical exercise favors the control of obesity and related inflammatory processes, since this kind of strategy leads to a reduction in body weight, visceral fat, and insulin resistance through an increase in the lipolysis [17, 18]. However, the association of LLLT with aerobic plus resistance training in obese women is scarce in the literature. Therefore, in the present study, it was hypothesized that in obese women, changes in proinflammatory markers should be compensated by altered anti-inflammatory markers after treatment with LLLT combined with aerobic plus resistance training.
Material and methods
Population
For this study, it involved 40 obese women with age of 20–40 years. Inclusion criteria were primary obesity and body mass index (BMI) greater than 30 kg/m2 and less than 40 kg/m2. Noninclusion criteria were the use of cortisone, antiepileptic drugs, history of renal disease, alcohol intake, smoking, and secondary obesity due to endocrine disorders.
The main reasons for dropout (n = 4) in our study were financial and family problems, followed by job opportunities. The study was conducted with the principles of the Declaration of Helsinki and was approved by the ethics committee on research at the Universidade Federal de São Carlos-UFSCar with the number (237.050), Clinical Trial: 231.286. All procedures were clear to the volunteers, and it obtained consent for research. All evaluations were performed at two different times (baseline and at the end of therapy: after 4 months of interdisciplinary intervention).
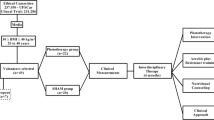
The voluntaries were allocated in two different groups: phototherapy group and SHAM group. The interventions consisted in to participate of physical exercise intervention, and immediately after physical exercises, the voluntaries received, individually, the application of phototherapy. The SHAM group participated the same interventions of phototherapy group, although, during the application of phototherapy, this group did not receive the incidence of laser light. It is important to note that the voluntaries do not know which group they belonged (Fig. 1).
Anthropometric measurements
Weight and height were measured for all patients who wore minimum clothing. After obtaining, the data was calculated using the BMI by dividing the weight by height squared (kg/m2). Fat mass (% and kg) and lean mass (% and kg) were obtained through the Bioelectrical Impedance InBody®.
Serum analysis
Blood samples were collected at the outpatient clinic at approximately 8:00 A.M. after an overnight fast (12 h). Insulin resistance was assessed using the homeostasis model assessment-insulin resistance (HOMA-IR) calculated by the fasting blood glucose (FBG) and the immunoreactive insulin (I): [FBG (in milligrams per deciliter) × I (in milliunits per liter)]/405. The cutoff value determined for Brazilian population is HOMA-IR > 2.71 for classifying the subjects with insulin resistance [19]. The normal range for insulin is 2.60–24.90 μU/ml [20]. The adipokines adiponectin (ng/l) and interleukin-6 (pg/ml) concentrations were measured using a commercially available multiplex assay (EMD Millipore; HMHMAG-34 K). Manufacture-supplied controls were included to measure assay variation, and all samples were analyzed on the same day to minimize day-to-day variation. A minimum of 100 beads were collected for each analyzed using a Luminex MagPix System (Austin, Texas), which was calibrated and verified prior to sample analysis. Unknown sample values were calculated offline using Milliplex Analyst Software (EMD Millipore) [21].
Descriptive methodology of weight loss therapy
All voluntaries visited the team of health professionals (endocrinologist, nutritionist, and physical educator) three times during the intervention period: (1) baseline (before the participation in the physical exercise and LLLT interventions); (2) after 2 months of participation in the interventions; and (3) after therapy (in the end of 4 months of interventions). They monitored and evaluated all clinical exams of voluntaries and treated health problems during intervention. The medical follow-up included the initial medical history, and a physical examination of blood pressure, cardiac frequency, and body composition were checked.
Physical exercise intervention
Aerobic plus resistance training
During the 4-month period, the voluntaries followed a combined exercise training therapy. The protocol was performed three times per week and included 30 min of aerobic training and 30 min of resistance training per session. At each training session, subjects were instructed to invert the order of the physical exercises, that is, in one session, the individual started the training session with resistance training, and in the subsequent session, the same individual started with aerobic training. The aerobic training consisted of running on a motor-driven treadmill (Movement®) at a 50–75 % of cardiac frequency maximum intensity established by Bruce test adapted. The resistance training was made recruiting the muscle groups: pectoralis major, quadriceps, back, hamstrings, calf, deltoid, biceps, triceps, abdomen, and extensor muscles by performing the following exercises: chest press, leg press, lat pulldown, hamstring curls, calf raises, military press, arm curls, bench press, sit ups, and lower back. The first 2 weeks were used for training adaptation and to learn the movements (3 sets of 15–20 maximal repetitions [MRs]). The protocol consisted of weekly changes of the load, divided into weeks of high loads (6–8 MR), weeks of moderate loads (10–12 MR), and weeks of light loads (15–20 MR). The volunteers performed 18 sets per session, divided into 3 sets of each exercise, followed by rest intervals between the series and exercises: 15–20 MR = 45 s, 10–12 MR = 1 min, and 6–8 MR = 1.5 min of rest. The physical exercise intervention was based on the guidelines from the American College of Sports Medicine (ACSM) [22, 23].
Device description
The phototherapy equipment was developed by the Laboratory Technology Support-LAT, Center for Research in Optics and Photonics Institute of Physics in São Carlos city at University of São Paulo-USP. The device is composed of four plates made of rubberized material measuring 20 by 20 cm each. Each two plates are connected to an electronic control box. The emitters of Ga-Al-As diode Lasers are distributed in the plate every 2.5 cm, totaling 16 emitters per plate and 64 emitters in total. The device is illustrated in Fig. 2a, and irradiation parameters are in Table 1 [24].
Phototherapy intervention
The application of phototherapy by continuous wave lasers (808 nm) occurs always at the final of training session. Thus, in each week, the patients received 3 sessions of phototherapy. The emitters were arranged perpendicularly to the skin and were allocated in the anterior region: abdominal and quadriceps simultaneously during 8 min. After this, change the position to irradiate the posterior region: gluteus and biceps femoral during 8 min, totalizing 16 min of its application (Fig. 2b).
According with the disposition of the emitters, the irradiance per emitter was 6.0 W/cm2. The energy delivered per session, per point, was 96 J. The diameters of elliptical spot were 0.3692 cm for horizontal and 0.0582 cm for vertical. The value of spot area was 0.0169 cm2. The emission and device parameters are described in Table 1 to become reproducible conditions.
Statistical analysis
Statistical analysis was performed using the program STATISTICA version 7.0 for Windows. The adopted significant value was α < 5 %. Data normality was verified with the Kolmogorov-Smirnov test. Parametric data were expressed as mean ± SD, and nonparametric data were expressed as median, minimum, and maximum values. To analyze the effects of intervention and difference between the groups, it applied ANOVA for repeated measures (ANOVA two-way) followed by Tukey post hoc test. The delta values (Δ) were used for the statistical analysis obtained from the difference between the after therapy and baseline values for each variable: Δ variable = after therapy value − baseline value. Comparing the delta values between the groups was performed by t test independent by groups to parametric variables and Mann-Whitney test to nonparametric variables.
Results
In the beginning of interdisciplinary intervention, no statistical differences were observed between the groups for the variables: age (years), height (m), weight (kg), BMI (kg/m2), fat mass (% and kg), lean mass (% and kg), neck circumference (cm), glucose (mg/dl), insulin (μU/ml), HOMA-IR, adiponectin (ng/l), IL-6 (pg/ml), adiponectin/IL-6 ratio, adiponectin/fat mass (kg and %) ratio, and lean mass/fat mass (kg and %) ratio. These data are important to show that the groups present in the investigation were paired at the beginning of the study and future alterations could result by the possible influence of the purpose interdisciplinary therapy in the study (Table 2).
Effects of weight loss therapy in the phototherapy group
After 4 months of interdisciplinary intervention associated with the phototherapy sessions, a reduction was observed in the phototherapy group in the body mass (kg), BMI (kg/m2), fat mass (kg and %), neck circumference (cm), insulin (μU/ml), interleukin-6 (pg/ml) [from 1.11 (0.56–6.8) to 0.56 (0.28–3.62) p = 0.01] and an increase in the lean mass (%), adiponectin concentration (ng/l) [from 7.01 (2.44–15.14) to 8.44 (5.42–17.14) p = 0.00], adiponectin/interleukin-6 ratio, lean mass/fat mass (kg and %) ratio, and adiponectin/fat mass (kg and %) ratio. No statistical differences were observed for the variables lean mass (kg), glucose (mg/dl), and HOMA-IR (Table 2 and Fig. 3a, b).
Effects of weight loss therapy in the SHAM group
After 4 months of interdisciplinary intervention, a reduction was observed in the SHAM group in the body mass (kg), BMI (kg/m2), fat mass (kg and %), neck circumference (cm), increase in the lean mass (% and kg), and lean mass/fat mass (kg and %) ratio. No statistical differences were observed for the variables glucose (mg/dl), insulin (uU/ml), HOMA-IR, adiponectin (ng/l) [from 6.20 (2.38–16.53) to 5.76 (3.14–9.22) p = 0,86], IL-6 (pg/ml) [from 0.91 (0.28–2.79) to 0.56 (0.28–3.34) p = 0.13], and adiponectin/fat mass (kg/%) ratio (Table 2 and Fig. 3a, b).
Effects of weight loss therapy between the groups
Comparing the delta values between the groups, it was observed that the phototherapy group showed a statistical reduction in the values of neck circumference (cm) [−2.08 ± 1.49 to −0.88 ± 1.08; p = 0.01], insulin concentration (μU/ml) [−5.72 ± 3.68 to −1.60 ± 4.05; p = 0.00], interleukin-6 (pg/ml) [−0.97 (−4.29–0.76) to −0.20 (−0.81–1.8); p = 0.00] compared to SHAM group. Also, an increase in the adiponectin concentration (ng/ml) [1.08 (0.04–3.62) to −0.42 (−3.15–2.26); p = 0.03] and adiponectin/fat mass (%) ratio [0.09 ± 0.13 to 0.003 ± 0.04; p = 0.05] was shown compared with SHAM group (Table 3; Figs. 4 and 5a–d).
Discussion
The most important finding in the present investigation is that we were able to show an increase in adiponectin concentration associated with a reduction in IL-6 after 20 weeks of treatment with LLLT associated with aerobic plus resistance training (Fig. 3a, b). There is strong evidence that demonstrates that LLLT was effective as a supporting noninvasive tool in the treatment for the reduction of body measurements and cellulite and improvement of lipid profile [20, 21]. However, the underlying key mechanism of actions for such potential effects to reduce body fat and the inflammatory process related to obesity in women is still unclear.
As we know, adiponectin has a great anti-inflammatory effect, mediated by an increase in the insulin sensitivity and improvement of glucose metabolism, providing an antiatherogenic effect in humans [22, 25]. This is supported by the present investigation where a significant reduction in the insulin concentration was only shown in the phototherapy group, suggesting a possible improvement in insulin sensitivity. However, no changes were observed in the glucose concentration and HOMA-IR, probably because the volunteers showed glucose concentration and insulin levels according to reference values [26]. Nevertheless, this needs to be confirmed in a long-term therapy using LLLT.
Furthermore, there is a consensus that a state of hypoadiponectinemia was present in the metabolic syndrome and obesity population exacerbating the inflammatory process and increasing cardiovascular risk [22]. It has also been shown that hypoadiponectinemia was inversely correlated with cIMT, an important subclinical surrogate of inflammation, confirming its key role in atherogenesis [27]. This is substantiated by our results which may support the evidence that LLLT is an important tool in the inflammatory process related to obesity since we were able to show a significant increase in adiponectin concentration in the LLLT group alone; it is important to note that in the SHAM group, this adipokine concentration was not changed.
In corroboration, Wu and colleagues [28] recently showed that human adipose-derived stem cells (hADSCs) expressed toll-like receptors (TLR) and that lipopolysaccharide (LPS) increased the production of proinflammatory interleukin-6 (IL-6). On the other hand, it was proposed that LLLT markedly inhibited LPS induction, corroborating the reduction in the expression of proinflammatory cytokines. In fact, LLLT promoted a reduction in IL-1β, IL-6, and TNF-α in experimental studies [29, 30]. Some mechanisms are proposed to explain the LLLT action; one is based on the production of transient pores in adipocytes which stimulates lipolysis. Furthermore, it has been suggested that LLLT activates a cascade of activities, which could cause the induction of adipocyte apoptosis leading to a release of lipids [31].
Interestingly, it has been shown that the application of phototherapy can promote biochemical adaptation of the mitochondria with changes in the redox state, leading to a conversion of electromagnetic to biochemical energy and consequently increasing the oxygen binding, production of ATP, respiration rate, and formation of giant mitochondria [32, 33]. Also, LLLT may activate enzymatic processes in cells to improve metabolism and lipid profile [16, 34]. Recent evidence in experimental investigations suggests that LLLT promotes skeletal muscle regeneration by reducing the duration of acute inflammation and accelerating tissue repair. This would happen through modulated cytokine expression during short-term muscle remodeling, inducing a decrease in TNF-α, TGF-β, and IL-1β without cytotoxic effects [29, 30].
Our results support these findings since the significant reduction in IL-6 was only observed in the phototherapy group compared with the SHAM group. These results confirm our hypothesis that LLLT can modulate a cascade of reactions to change body homeostasis leading to better association between proinflammatory/anti-inflammatory adipokines, improving health in obese women. LLLT also acts on the markers of oxidative stress, such as protein carbonyls and superoxide dismutase [35, 36], in addition to yielding clinical signs of improvement, delayed muscle fatigue, and improved physical performance [36–39]. Corroborating this, it is known that IL-6 is an important interleukin produced by myocytes to improve muscle repair, and its production is stimulated by resistance training [36]. In fact, it was recently suggested that LLLT in conjunction with aerobic training may provide a therapeutic approach to intensely reduce proinflammatory markers in an experimental study, including a reduction in IL-6 and TNF-alpha. However, LLLT without exercise was not able to improve physical performance in elderly animals [36].
Altogether, these results reinforce the potential effects of LLLT associated with combined training to promote some amelioration in the inflammatory process related to obesity in both animals and humans. Corroborating these findings, in the present study, we observed an increase in adiponectin concentration, a reduction in IL-6 and insulinemia, and a significant improvement in the adiponectin/interleukin-6 ratio, important markers of inflammatory state only in the phototherapy group.
Additionally, we were able to show an increase in the adiponectin/fat mass (kg and %) ratio in the phototherapy group. In fact, results published in the current year suggest that the use of LLLT may enhance cellular homeostasis, promoting an increase in the concentration of active mitochondria in irradiated cells through an upregulation of the genes involved in the mitochondrial complexes [40, 41]. In addition, physical exercise promotes biochemical and structural changes in the mitochondria [42]. Collectively, these results might suggest the hypothesis that phototherapy may help enhance the effects of physical exercise training proposed in the present investigation. The exact mechanisms of action following LLLT are not yet well understood; however, it has been proposed that the chronic application of LLLT can activate or inhibit enzymes. In this context, it was previously suggested that LLLT intensifies the transfer of electrons within cytochrome-c oxidase by making more electrons available. Thus, it may accelerate oxidative metabolism leading to an increase in ATP synthesis [43].
There has been a recent discovery of irisin which is predominantly secreted by muscle tissue acting as an endocrine organ in response to physical exercise. During physical exercise, peroxisome proliferator-activated receptor ɣ coactivator 1α (PGC1α) is activated, inducing the release of fibronectin domain-containing protein 5 (FNDC5) which is then cleaved to irisin. This hormone may improve the transdifferentiation of white adipocyte to beige and brown, in both white and brown adipose tissues, by enhancing the lipid mobilization and activating the uncoupling protein 1 (UCP1) in mitochondria, resulting in the synthesis of adenosine triphosphate (ATP) and the dissipation of energy in the form of heat. This process could promote reductions in body weight and improvements in metabolic profile, suggesting that irisin could be a possible novel treatment for diabetes and obesity [44]. Therefore, it is possible to hypothesize that the combination of LLLT associated with combined exercise in the present study may accentuate the response in the control of fat mass correspondent to a reduction in the inflammatory state, since we were able to show an increase in the adiponectin/fat mass (% and kg) in the phototherapy group. However, this needs to be confirmed in future research with a focus on investigations into the possible influences of LLLT intervention in the transdifferentiation process of white adipocyte to beige and brown associated with exercise training.
Our findings present some limitations, including the absence of experiments with different kinds of laser irradiation. The effects of laser irradiation are highly dependent on characteristics such as wavelength, power density, and fluency [40, 45–47].
However, to our knowledge, the current study is the first investigation to show that LLLT improves the inflammatory process related to obesity in obese women, particularly by promoting a reduction in the proinflammatory IL-6 and an increase in the anti-inflammatory adiponectin, adiponectin/interleuckin-6, and adiponectin/fat mass ratio (kg and %). In addition, the LLLT showed higher changes in the control of neck circumference compared with the SHAM group. Interestingly, it was recently suggested that there is growing evidence that neck circumference is considered an interesting marker of inflammation and cardiovascular diseases [48, 49]. Nevertheless, it is important to note that in both analyzed groups, the BMI, body mass, and body fat presented similar changes, considering delta values after 20 weeks of LLLT associated with physical exercise training.
Finally, there are a limited number of clinical studies with the application of LLLT in obesity. However, a number of clinical applications have been found for the lasers in a variety of medical specialties [33]. Therefore, this needs to be confirmed in a large cohort study of the obesity population.
Conclusions
In the present investigation, we were able to show that the association of physical exercise training with low-level laser (light) therapy applied in obese women during a 4-month period promotes an improvement in the inflammatory framework and body composition. These are important results that suggest that phototherapy can be an important tool in the treatment of obesity, principally considering its potential effects associated with physical exercise training in attenuating inflammation in women, and as such, these results are applicable in the clinical practices to control the related risks associated with obesity.
References
Gasparyan VC (2000) Method of determination of aortic valve parameters for its reconstruction with autopericardium: an experimental study. J Thorac Cardiovasc Surg 119:386–387
Ucero AC, Sabban B, Benito-Martin A, Carrasco S, Joeken S, Ortiz A (2013) Laser therapy in metabolic syndrome-related kidney injury. Photochem Photobiol 89(4):953–960
Mitu F, Cobzaru R, Leon MM (2013) Influence of metabolic syndrome profile on cardiovascular risk. Rev Med Chir Soc Med Nat Iasi 117(2):308–314
Padwal RS (2013) Obesity, Diabetes, and the Metabolic Syndrome: The Global Scourge. Can J Cardiol 8. pii: S0828-282X(13)01635-8.
Correia F, Poínhos R, Freitas P, Pinhão S, Maia A, Carvalho D, Medina JL (2006) Prevalence of the metabolic syndrome: comparison between ATPIII and IDF criteria in a feminine population with severe obesity. Acta Med Port 19(4):289–293
Caranti DA, Lazzer S, Dâmaso AR, Agosti F, Zennaro R, de Mello MT, Tufik S, Sartorio A (2008) Prevalence and risk factors of metabolic syndrome in Brazilian and Italian obese adolescents: a comparison study. Int J Clin Pract 62(10):1526–1532
Phillips CM, Perry IJ (2013) Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab 98(10):E1610–1619
Matos MF, Lourenço DM, Orikaza CM, Gouveia CP, Morelli VM (2013) Abdominal obesity and the risk of venous thromboembolism among women: a potential role of interleukin-6. Metab Syndr Relat Disord 11(1):29–34
Mirza S, Qu HQ, Li Q, Martinez PJ, Rentfro AR, McCormick JB, Fisher-Hoch SP (2011) Adiponectin/leptin ratio and metabolic syndrome in a Mexican American population. Clin Invest Med 34(5), E290
Masquio DC, de Piano A, Sanches PL, Corgosinho FC, Campos RM, Carnier J, da Silva PL, Caranti DA, Tock L, Oyama LM, Oller do Nascimento CM, de Mello MT, Tufik S, Dâmaso AR (2013) The effect of weight loss magnitude on pro-/anti-inflammatory adipokines and carotid intima-media thickness in obese adolescents engaged in interdisciplinary weight loss therapy. Clin Endocrinol (Oxf) 79(1):55–64
Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, Milicic T, Seferovic JP, Macesic M, Gajovic JS (2014) Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. Int J Environ Res Public Health 11(4):3586–3598
Corgosinho FC, de Piano A, Sanches PL, Campos RM, Silva PL, Carnier J, Oyama LM, Tock L, Tufik S, de Mello MT, Dâmaso AR (2012) The role of PAI-1 and adiponectin on the inflammatory state and energy balance in obese adolescents with metabolic syndrome. Inflammation 35(3):944–951
Su SC, Pei D, Hsieh CH, Hsiao FC, Wu CZ, Hung YJ (2011) Circulating pro-inflammatory cytokines and adiponectin in young men with type 2 diabetes. Acta Diabetol 48(2):113–119
Lira FS, Rosa JC, Dos Santos RV, Venancio DP, Carnier J, Sanches Pde L, do Nascimento CM, de Piano A, Tock L, Tufik S, de Mello MT, Dâmaso AR, Oyama LM (2011) Visceral fat decreased by long-term interdisciplinary lifestyle therapy correlated positively with interleukin-6 and tumor necrosis factor-α and negatively with adiponectin levels in obese adolescents. Metabolism 60(3):359–365
Hrnjak M, Kuljic-Kapulica N, Budisin A, Giser A (1995) Stimulatory effect of low-power density He-Ne laser radiation on human fibroblasts in vitro. Vojnosanit Pregl 52:539–546
Aquino AE Jr, Sene-Fiorese M, Paolillo FR, Duarte FO, Oishi JC, Pena AA Jr, Duarte AC, Hamblin MR, Bagnato VS, Parizotto NA (2013) Low-level laser therapy (LLLT) combined with swimming training improved the lipid profile in rats fed with high-fat diet. Lasers Med Sci 28(5):1271–1280
Ryan AS, Ge S, Blumenthal JB, Serra MC, Prior SJ, Goldberg AP (2014) Aerobic Exercise and Weight Loss Reduce Vascular Markers of Inflammation and Improve Insulin Sensitivity in Obese Women. J Am Geriatr Soc 62(4):607–614
Dobrosielski DA, Barone Gibbs B, Chaudhari S, Ouyang P, Silber HA, Stewart KJ (2013) Effect of exercise on abdominal fat loss in men and women with and without type 2 diabetes. BMJ Open 3(11):e003897
Geloneze B, Repetto EM, Geloneze SR, Tambascia MA, Ermetice MN (2006) The threshold value for insulin resistance (HOMA-IR) in an admixtured population IR in the Brazilian Metabolic Syndrome Study. Diabetes Res Clin Pract 72(2):219–220
Shan W, Ning C, Luo X, Zhou Q, Gu C, Zhang Z, Chen X (2014) Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: a prospective cross-sectional study. Gynecol Oncol 32(3):606–610
Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S (2009) Validity of multiplex-based assays for cytokine measurements in serum and plasma from "non-diseased" subjects: comparison with ELISA. J Immunol Methods 350:125–132
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American College of Sports Medicine (2009) American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41:459–471
Kraemer WJ, Ratamess NA, French DN (2002) Resistance training for health and performance. Curr Sports Med Rep 1:165–171
Jenkins PA, Carroll JD (2011) How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg 29(12):785–787
Matsuda M, Shimomura I (2014) Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord 15(1):1–10
Alberti KG, Zimmet P, Shaw J (2006) Metabolic syndrome: a new world-wide definition: a consensus statement from the International Diabetes Federation. Diabet Med 23(5):469–480
Rubio-Guerra AF, Cabrera-Miranda LJ, Vargas-Robles H, Maceda-Serrano A, Lozano-Nuevo JJ, Escalante-Acosta BA (2013) Correlation between levels of circulating adipokines and adiponectin/resistin index with carotid intima-media thickness in hypertensive type 2 diabetic patients. Cardiology 125(3):150–153
Wu JY, Chen CH, Wang CZ, Ho ML, Yeh ML, Wang YH (2013) Low-power laser irradiation suppresses inflammatory response of human adipose-derived stem cells by modulating intracellular cyclic AMP level and NF-κB activity. PLoS One 8(1), e54067
Mesquita-Ferrari RA, Martins MD, Silva JA Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26(3):335–340
Lima AA, Spínola LG, Baccan G, Correia K, Oliva M, Vasconcelos JF, Soares MB, Reis SR, Medrado AP (2014) Evaluation of corticosterone and IL-1β, IL-6, IL-10 and TNF-α expression after 670-nm laser photobiomodulation in rats. Lasers Med Sci 29(2):709–715
Avci P, Nyame TT, Gupta GK, Sadasivam M, Hamblin MR (2013) Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med 45(6):349–357
Amat A, Rigau J, Waynant RW, Ilev IK, Tomas J, Anders JJ (2005) Modification of the intrinsic fluorescence and the biochemical behavior of ATP after irradiation with visible and near-infrared laser light. J Photochem Photobiol B 81:26–32
Bakeeva LE, Manteifel VM, Rodichev EB, Karu TI (1993) Formation of gigantic mitochondria in human blood lymphocytes under the effect of an He-Ne laser. Mol Biol (Mosk) 27:608–617
Ferraresi C, de Brito OT, de Oliveira ZL, de Menezes Reiff RB, Baldissera V, de Andrade Perez SE, Matheucci Junior E, Parizotto NA (2011) Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci 26(3):349–358
Leal Junior EC, Lopes-Martins RA, Frigo L, De Marchi T, Rossi RP, de Godoi V, Tomazoni SS, Silva DP, Basso M, Filho PL, de Valls CF, Iversen VV, Bjordal JM (2010) Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports Phys Ther 40(8):524–532
Amadio EM, Serra AJ, Guaraldo SA, Silva JA Jr, Antônio EL, Silva F, Portes LA, Tucci PJ, Leal-Junior EC, de Carvalho PT (2015) The action of pre-exercise low-level laser therapy (LLLT) on the expression of IL-6 and TNF-α proteins and on the functional fitness of elderly rats subjected to aerobic training. Lasers Med Sci Feb 3.
Leal Junior EC, Lopes-Martins RA, Dalan F, Ferrari M, Sbabo FM, Generosi RA, Baroni BM, Penna SC, Iversen VV, Bjordal JM (2008) Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg 26(5):419–424
Leal-Junior EC, Vanin AA, Miranda EF, de Carvalho PT, Dal Corso S, Bjordal JM (2015) Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci 30(2):925–939
Lopes-Martins RA, Marcos RL, Leonardo PS, Prianti AC Jr, Muscará MN, Aimbire F, Frigo L, Iversen VV, Bjordal JM (1985) Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol 101(1):283–288
Houreld NN (2014) Shedding light on a new treatment for diabetic wound healing: a review on phototherapy. ScientificWorldJournal 6:398412
Masha RT, Houreld NN, Abrahamse H (2013) Low-intensity laser irradiation at 660 nm stimulates transcription of genes involved in the electron transport chain. Photomed Laser Surg 31(2):47–53
Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA (2003) Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med 33:783–793
Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R (2009) Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B 95(2):89–92
Novelle MG, Contreras C, Romero-Picó A, López M, Diéguez C (2013) Irisin, two years later. Int J Endocrinol 2013:746281
Houreld N, Abrahamse H (2007) In vitro exposure of wounded diabetic fibroblast cells to a helium-neon laser at 5 and 16 J/cm2. Photomed Laser Surg 25(2):78–84
Houreld NN, Abrahamse H (2007) Effectiveness of helium-neon laser irradiation on viability and cytotoxicity of diabetic-wounded fibroblast cells. Photomed Laser Surg 25(6):474–481
Hawkins DH, Abrahamse H (2006) The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation. Lasers Surg Med 38(1):74–83
Preis SR, Pencina MJ, D’Agostino RB Sr, Meigs JB, Vasan RS, Fox CS (2013) Neck circumference and the development of cardiovascular disease risk factors in the Framingham Heart Study. Diabetes Care 36(1), e3
Jamar G, Pisani LP, Oyama LM, Belote C, Masquio DC, Furuya VA, Carvalho-Ferreira JP, Andrade-Silva SG, Dâmaso AR, Caranti DA (2013) Is the neck circumference an emergent predictor for inflammatory status in obese adults? Int J Clin Pract 67(3):217–224
Acknowledgments
Support Foundation of São Paulo Research-FAPESP (2013/041364; 2013/19046-0; 002804928-41), National Council for Scientific and Technological Development–CNPq (150177/2014-3), and Coordination of Higher Education Personnel Training–CAPES.
Conflict of interest
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
da Silveira Campos, R.M., Dâmaso, A.R., Masquio, D.C.L. et al. Low-level laser therapy (LLLT) associated with aerobic plus resistance training to improve inflammatory biomarkers in obese adults. Lasers Med Sci 30, 1553–1563 (2015). https://doi.org/10.1007/s10103-015-1759-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1759-9