Abstract
The literature has shown that low-level laser therapy accelerates the repair of cutaneous wounds. However, there is a scarcity of scientific studies that characterise the possible systemic interference of laser photobiomodulation. The aim of this research was to quantitatively evaluate blood corticosterone levels and tissue cytokine expression in cutaneous wounds of rats treated with low-level laser therapy (semiconductor diode AsGaAl, continuous emission, 9 mW, 670 nm, 0.031 W/cm2, beam with an output area of 0.28 cm2) and normal controls. A total of 36 male Wistar rats were used and randomly divided into two groups of 18 rats each. A standardised circular 6-mm-diameter wound was made in the dorsal skin region of each rat, and they were euthanised at 1, 6 and 12 h after cutaneous surgery. The blood was collected, and portions of cutaneous tissue and subcutaneous muscle were removed and cryopreserved. Corticosterone levels in the blood were measured by a radioimmunoassay technique; histological sections were submitted to the ELISA technique for analysis of tissue cytokine expression levels. At 6 h after surgery, a significant increase in corticosterone and a significant reduction in the levels of IL-1β and IL-6 in tissues of irradiated wounds were observed when compared to controls (p < 0.05). The levels of TNF-α and IL-10 expression were not significantly different between the groups at different time intervals. Thus, this study strongly suggests a systemic and local biomodulation of low-level laser therapy as indicated by the blood levels of corticosterone and the tissue expression of IL-1β and IL-6, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photobiomodulatory therapies are considered important alternatives for the treatment of cicatricial processes because they can regulate the inflammatory response, reduce painful symptoms and stimulate tissue repair without the adverse effects commonly caused by drugs [1].
Low-level laser therapy (LLLT) mediates photochemical, photophysical and photobiological effects at the site of application as well as in neighbouring regions. Therefore, laser action is not limited to the area of optical diffusion [2], and the chemical mediators stimulated by lasers may reach distant areas of the body via the blood and lymphatic vessels, thereby generating systemic effects [3]. Previous studies have demonstrated that LLLT can alter the cytokine profiles in tissues as well as the blood [4, 5].
IL-1β and TNF-α are key mediators of inflammation, and studies have shown that laser phototherapy may reduce the production of these mediators, thereby interfering with the inflammatory response [4]. Cytokines are involved in pro-inflammatory biological activities, such as fever, stimulation of neutrophil migration into tissues, induction of vascular adhesion molecules and stimulation of acute phase protein synthesis [6]. TNF-α plays an essential role in the cytokine cascade by stimulating IL-6 secretion [2], which has both pro- and anti-inflammatory effects [5, 7] and is frequently used as a marker of systemic pro-inflammatory activation [8]. Experimental evidence has also indicated that IL-6 inhibits the regulation of IL-1β and TNF-α synthesis [9]. IL-10 is also known to be a critical anti-inflammatory cytokine involved in the human immune response [10]. The immunosuppressive effects of IL-10 are mediated by the inhibition of pro-inflammatory cytokines, such as TNF-α and IL-1β [11–13].
In addition to stimulating the immunoregulatory actions of cytokines, low-level lasers may modulate inflammation by promoting the release of endogenous cortisol, a hormone that acts as a natural anti-inflammatory agent [14]. Cytokines stimulate the hypothalamic–hypophyseal axis to produce glucocorticoids via the activity of signalling factors [15], and endogenous glucocorticoids released in response to stress have been the subject of extensive study because these hormones act on numerous cell types [16]. However, little is known about the influence of laser phototherapy on glucocorticoid levels.
The purpose of this study was to evaluate the corticosterone levels in the blood and the inflammatory cytokine expression in tissues immediately after the formation of standardised cutaneous wounds and treatment with 670 nm LLLT in Wistar rats.
Null hypothesis
The 670-nm diode laser will not modulate corticosterone levels in the blood and will have no effect on cytokine expression during the early phase of wound repair.
Materials and methods
This study was approved by the Ethics Committee on the Use of Animals in Research of the Bahian School of Medicine and Public Health, under report no. 015/2009. All animal handling procedures were performed in accordance with the rules and guidelines set by this committee.
Animals
Thirty-six Wistar rats, weighing between 150 and 200 g, were kept in individual cages and were given free access to water and a balanced diet. The animals were randomly divided into two experimental groups containing 18 rats each. Within each experimental group, a further random division was made to produce three subgroups composed of six animals each, and each group was subjected to euthanasia at different times (1, 6 or 12 h) after the surgical procedure.
Experimental groups
Control group
Following cutaneous surgery, the tip of the inactivated laser therapy system was placed in contact with the wound. This placebo group was divided into three subgroups, containing animals undergoing euthanasia 1, 6 or 12 h after cutaneous surgery.
Laser group
A semiconductor diode, with an AsGaAl continuous emission (9 mW, 670 nm, 0.031 W/cm2) beam and an output area of 0.28 cm2 (Laser VR-KC-610; Dentoflex, Brazil), was used for laser treatment. The laser was applied to the rats at a dose of 1 J/cm2, immediately following cutaneous surgery. The laser was administered for 31 s in four equidistant points at the borders of the circular wound.
Surgical procedures
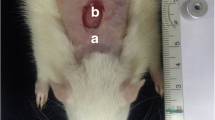
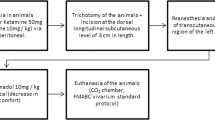
The animals were anaesthetised with an intraperitoneal dose (0.2 ml/100 g) of 5 % ketamine (2.5 ml) (Vetanarcol – Konig – lot 007 07, Brazil) and 2 % xylazine (0.5 ml) (Sedomin – Konig – lot 002 08, Brazil) diluted in saline (1.0 ml). Next, the dorsum of each rat was shaved, and a circular wound was made in the cutaneous tissue between the animal’s front legs near the cervical region (Fig. 1). To standardise the wound, a punch 6 mm in diameter (Stiefel Tabe, São Paulo, Brazil) was used as the surgical instrument and was applied perpendicular to the shaved skin. Representative tissue was removed from the ulcerated border of all experimental animals in both experimental groups (Fig. 2) 1, 6 and 12 h post-surgery. These tissues were immediately subjected to the procedure described below.
Quantification of cytokines extracted from the total skin protein
Total protein was extracted, and inflammatory cytokines were measured using 100 mg of tissue/ml in PBS buffer supplemented with 0.4 M NaCl, 0.05 % Tween 20 and protease inhibitors (0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A/100 ml). The samples were centrifuged for 10 min at 3,000×g, and the supernatant was frozen at −70 °C until quantification was performed. IL-1β, IL-6, IL-10 and TNF-α levels were estimated using a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s guidelines. Briefly, 96-well plates were coated with the capture antibody (1:200) overnight and incubated with blocking buffer at room temperature for 1 h. Samples were added in duplicate and incubated overnight at 4 °C. Biotinylated antibodies (1:200) were added, and the plates were incubated for 2 h at room temperature. A 30-min incubation with streptavidin-HRP (1:200) was followed by detection using 3,5,3′,5′-tetramethylbenzidine and H2O2. The plates were read using a 450-nm wavelength laser.
The optical density was analysed, and a standard curve was constructed using SoftMax® Pro software (Molecular devices, CA, USA). The average of the blank optical density, which was generated in the wells containing only diluent solution as the sample, was subtracted from the duplicate readings for each standard point and sample, and then a standard curve was created by reducing the data and generating a four-parameter logistic (4-PL) curve fit.
Blood collection and determination of plasma corticosterone levels
Blood was collected by decapitation without anaesthesia at 8:00 a.m. on the day corresponding to the experimental subgroup. Plasma was separated by centrifugation and stored at −70 °C until further use. Steroids were extracted from the plasma with 1 ml ethanol. Corticosterone concentrations were determined by radioimmunoassay, as described by Vecsei [17], and anti-corticosterone rabbit antibodies and 3 H-corticosterone were used as the competitor.
Statistical analysis
The mean and median values of all continuous variables are presented. Corticosterone and cytokine levels were analysed using the Kruskal Wallis test followed by a Mann–Whitney post-test with the Bonferroni correction. P values ≤ 0.05 were considered significant.
Results
Blood corticosterone levels measured at 1 and 6 h after surgery were increased in the laser-treated group compared to the control group, although statistical significance was achieved only at 6 h after surgery (p = 0.026). At 12 h after surgery, a decrease in hormone levels was observed in both groups (Table 1; Fig. 3).
At 1 h after surgery, the levels of TNF-α and IL-1β were increased in tissues treated with the laser compared to the control tissues, although this difference was not statistically significant. However, a statistically significant reduction in IL-1β and IL-6 expression was observed at 6 and 12 h after surgery in the treated animals compared to the controls (p = 0.039 and p = 0.001; p = 0.002 and p = 0.005, respectively) (Tables 2 and 3; Fig. 4). The observed reduction in tissue levels of IL-10 was not statistically significant (Table 3; Fig. 4).
Expression of inflammatory cytokines in the tissue, including IL-1β, IL-6, IL-10 and TNF-α, represented as the median values detected in the control and laser groups. IL-1β *p = 0.039, statistically significant between control and laser groups after 6 h. *p = 0.001, statistically significant between control and laser groups after 12 h. IL-6 *p = 0.002, statistically significant between control and laser groups after 6 h. *p = 0.005, statistically significant between control and laser groups after 12 h
Discussion
Photobiomodulation therapies have been studied for over 40 years, and their capacity to modulate inflammation has been demonstrated by numerous authors [2, 5, 18, 19]. Of the studied photobiomodulation therapies, LLLT has demonstrated outstanding results, and its ability to accelerate the healing process is well documented in the literature. In addition to the local effects of LLLT, some authors have suggested that the potential systemic effects caused by the laser act as co-adjuvants in tissue repair [14, 20, 21]. In the present study, LLLT promoted alterations in pro-inflammatory cytokine levels in tissues and corticosterone levels in the blood, suggesting both local and systemic effects of LLLT within the first 12 h after surgery.
Although experimental models have limitations and their results cannot always be extrapolated to humans, countless studies have demonstrated the effects of in vivo LLLT in animals [1, 6, 14]. The results of our initial statistical analysis indicated that a very large number of animals would be necessary for this study (approximately 40 animals per group). Due to ethical considerations, particularly the need to avoid the overuse of animals, a new sample size calculation was performed that would enable the detection of significant differences while taking into consideration the ethical use of animals. Thus, each experimental group (control and laser-treated) consisted of 18 animals.
Inflammation is mediated by responses generated in the tissues adjacent to a wound. During an inflammatory response, blood vessels supply immune factors to the injured tissue, where they act to combat infection or tissue injury. This process occurs as a cascade of cellular and molecular events that is initiated immediately following injury. In the early phase of inflammation, neutrophilic infiltrates predominate, which results in intense exudation. However, these polymorphonuclear cells are gradually replaced by a monomorphonuclear infiltrate consisting mainly of macrophages. The proliferative phase is then established and is characterised by angiogenesis and fibroplasia. Macrophages together with fibroblasts produce complementary chemical signals to attract circulating inflammatory cells and pro-inflammatory cytokines [18]. The combination of these processes and events mediates the characteristic signs and symptoms of inflammation, such as oedema, pain and functional debility [10]. Photobiomodulation therapies, such as LLLT, are considered adjuvant therapies and have been used to minimise the deleterious effects of the inflammatory process.
Cytokines have been extensively studied in both clinical situations and animal studies and are known to mediate inflammation, immunity and haematopoiesis [4, 5, 10, 18]. The present study evaluated the expression of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α and the anti-inflammatory cytokine IL-10 following LLLT. Statistically significant differences in IL-1β and IL-6 expression were observed in treated tissues in comparison to control tissues at 6 and 12 h after surgery, and these results support previously demonstrated effects of LLLT on cytokine expression. For example, the study by Pires et al. in 2010 [5] also demonstrated reductions in pro-inflammatory cytokine expression post-treatment. In this previous study, tendonitis was induced in 42 male Wistar rats, which were divided into seven groups. The treated animals were irradiated with LLLT (λ = 780 nm for 75 s at a dose of 7.7 J/cm2) at 12 h and 7 days after the induction of experimental tendinitis, and at both time points, irradiation resulted in diminished IL-6, COX-2 and TGF-β expression. Furthermore, the studies by Albertini et al. [18, 22] reported reductions in IL-1β and TNF-α using other experimental models.
During the initial stage of an inflammatory process, the pro-inflammatory cytokines TNF-α and IL-1β are released following the activation of macrophages and monocytes at the site of injury [5]. IL-1β mediates host inflammation in response to infections and other inflammatory stimuli and works in conjunction with TNF-α to mediate the innate immune response to tissue damage, which is accomplished via the modulation of cell migration and the release of chemical mediators. In addition, these pro-inflammatory factors are responsible for inducing the biosynthesis of other cytokines and are important inducers of the acute inflammatory response [18–23].
IL-6, in association with IL-1β and TNF-α, induces the secretion of C-reactive protein from hepatocytes, and it has been suggested that the main immunological function of IL-6 is to potentiate the effects of other cytokines [1, 4]. In this study, the reduced levels of IL-1β and IL-6 at 6 and 12 h after surgery demonstrated the anti-inflammatory properties of LLLT. Furthermore, due to its effects on the migration of phagocytes and other inflammatory cells, IL-6 plays an important role in the initial stage of healing [7].
IL-10 is an important anti-inflammatory cytokine that can suppress TNF-α produced during an immunological response [7]. However, there are few reports regarding the influence of LLLT on the expression of IL-10. In the present study, in addition to the observed reduction in pro-inflammatory cytokine levels, a reduction in IL-10 was observed at 6 and 12 h after surgery, although these differences were not statistically significant. IL-10 is one of the main late-acting anti-inflammatory cytokines released by activated macrophages and serves to prevent the excessive activation of macrophages during periods of inflammation. It would be expected that a significant increase in IL-10 expression would justify the anti-inflammatory actions of laser therapy. However, although not observed in the present study, it is possible that laser photobiomodulation may be acting via a different mechanism. The molecular pathways that evoke IL-10 production by macrophages are poorly understood and have not been completely elucidated. Furthermore, it is known that the release of pro-inflammatory cytokines at the onset of wound healing may prevent the release of other inhibitory chemical mediators such as IL-10.
The beneficial effects of LLLT have been attributed not only to cytokine modulation but also to the synergistic and modulatory affects on glucocorticoids. One anti-inflammatory mechanism of action for LLLT may be its stimulation of endogenous corticosterone secretion, which acts as a natural anti-inflammatory agent against various types of stress, such as infection and injury [14, 24]. Corticosterone, the main hormone involved in tissue repair, has anti-inflammatory effects and alters carbohydrate and protein metabolism. It is known that IL-1β and IL-6 activate the hypothalamic–hypophyseal axis and the release of corticosterone, which in turn regulates the immune response [15]. In the present study, the systemic effect of LLLT was demonstrated by the significant increase in corticosterone levels in the laser-treated group at 6 h after surgery. During this same period after treatment, a reduction in the levels of pro-inflammatory cytokines was also observed. Considering the clinical implications of laser therapy, the results of the present study strongly suggest that LLLT has a significant anti-inflammatory effect during the first hours after tissue damage, and its clinical use should therefore be indicated early in several situations, such as during the immediate post-operative period and in chronic inflammatory diseases.
Although not investigated in the current study, previous authors have shown that LLLT interferes with the biological actions of tissue mast cells and can intensify their degranulation during the first hours after tissue damage. For example, Pereira et al. [25] found an increase in the number of degranulated tissue mast cells in rats treated with laser therapy in a model of skin healing identical to the one used in the present study. This increase occurred 12 h after the experimental wound and may represent a compensatory immune defence mechanism, given that other inflammatory pathways could be inhibited by the increased production of cortisol stimulated by laser therapy, as shown in our study.
In the present study, the authors were unable to confirm the positive effects of LLLT in the chronology of skin repair because the period analysed corresponded to the first few hours after the surgical wound was made and laser treatment was administered. Therefore, only the primary effects of LLLT were analysed. Previous studies published by our research group have demonstrated the beneficial effects of LLLT during different wound healing periods, and Medrado et al. [26] studied the effects of LLLT on wound healing until 60 days after cutaneous surgery.
Together, the results of the current study demonstrate that LLLT (at 670 nm) increased the corticosterone level in the blood during earlier time points following tissue damage, indicating a potential systemic effect of the laser. In addition, the local tissue responses evaluated in situ showed evidence of the anti-inflammatory effects of LLLT during the first 12 h after cutaneous surgery, as revealed by the decreased expression of IL-1β and IL-6. However, additional studies are needed to confirm the positive effects of photobiomodulation on the wound healing inflammatory process, and such studies should evaluate serum and tissue cytokine expression in relation to the chronological effects of laser therapy.
References
Lee GY, Kim WS (2012) The systemic effect of 830-nm LED phototherapy on wound healing of burn injuries: a controlled study in mouse and rats models. Cosmet Laser Ther 14(2):107–10
Fukuda TY, Tanji MM, Jesus JF, Sato MN, Duarte AJS, Plapler H (2010) Single session to infrared low level diode laser on TNF-a and IL-6 cytokines release by mononuclear spleen cells in mice: a pilot study. Lasers Surg Med 42:584–588
Hawkins D, Abrahamse H (2007) Phototherapy—a treatment modality for wound healing and pain relief. Afr J Biomed Res 10:99–109
Safavi SM, Kazemi B, Esmaeili M, Fallah M, Fallah A, Modarresi A et al (2008) Effects of low-level He–Ne laser irradiation on the gene expression of IL-1β, TNF-α, IFN-γ, TGF-β, bFGF, and PDGF in rat’s gingiva. Lasers Med Sci 23:331–335
Pires D, Xavier M, Araújo T, Silva JÁ, Aimbire F, Albertini R (2010) Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94
Utsunomiya I, Misa I, Sachiko O (1998) Generation of inflammatory cytokines in zymosan-induced pleurisy in rats: TNF induces IL-6 and cytokine-induced neutrophil chemoattractant (CINC) in vivo. Cytokine 10(12):956–963
Opal SM, DePalo VA (2000) Anti-inflammatory cytokines. Chest 117:1162–1172
Frode TS, Souza GEP, Calixto JB (2002) The effects of IL-6 and IL-10 and their specific antibodies in the acute inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine 17(3):149–156
Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF (1998) IL-6 Is an Antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101(2):311–320
Boschi ES, Leite CE, Saciura VS, Caberlon E, Lunardelli A, Bitencourt S et al (2008) Anti-inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg Med 40:500–508
Simi A, Tsakiri N, Wang P, Rothwell NJ (2007) Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans 35(5):1122–1126
Kremlev SG, Palmer C (2005) Interleukin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. J Neuroimmunol 162:71–80
Morganti-Kossman MC, Rancan M, Otto VI, Stahel PF, Kossman T (2001) Role of cerebral inflammation after traumatic brain injury: A revisited concept. Shock 16(3):165–177
Lopes-Martins RAB, Albertini R, Martins PSLL et al (2005) Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomed Laser Surg 23:377–381
Webster JI, Sternberg EM (2004) Role of the hypothalamic–pituitary–adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol 181:207–221
Baccan GC, Oliveira RDR, Mantovani B (2004) Stress and immunological phagocytosis: possible nongenomic action of corticosterone. Life Sci 75:1357–1368
Vecsei P (1979) Glucocorticoids: cortisol, corticosterone and compound S. In: Jaffe BM, Behrman HR (eds) Methods of Hormone Radioimmunoassay. Academic, New York, pp 393–415
Ferrari RAM, Martins MD, Silva JA Jr, Silva TD, Piovesan RF, Pavesi VCS (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26:335–340
Medrado ARAP, Pugliese LS, Reis SRA, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Med Sci 32:239–244
Rodrigo SM, Cunha A, Pozza DH, Blaya DS, Moraes JF, Weber JBB (2009) Analysis of the systemic effect of red and infrared laser therapy on wound repair. Photomed Laser Surg 27(6):929–935
Gal P, Vidinsky B, Toporcer T et al (2006) Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed Laser Surg 24:480–488
Albertini R, Villaverde AB, Aimbire F, Bjordal J, Brugnera AJ, Mittmann J et al (2008) Cytokine mRNA expression is decreased in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low-level laser therapy. Photomed Laser Surg 26(1):19–24
Pesevska S, Nakova M, Gjorgoski I, Angelov N, Ivanovski K, Nares S et al (2012) Effect of laser on TNF-alpha expression in inflamed human gingival tissue. Lasers Med Sci 27(2):377–81
Gonçalves WLS, Souza FM, Conti CL, Cirqueira JP, Rocha WA, Pires JGP et al (2007) Influence of He–Ne laser therapy on the dynamics of wound healing in mice treated with anti-inflammatory drugs. Braz J Med Biol Res 40(6):877–884
Pereira MC, Pinho CB, Medrado ARP, Andrade ZA, Reis SRA (2010) Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B: Biol 98:188–192
Medrado ARAP, Soares AP, Santos E, Reis SRA, Andrade Z (2008) Influence of laser photobiomodulation upon connective tissue remodeling during wound healing. J Photochem Photobiol B: Biol 92:144–152
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lima, A.A.M., Spínola, L.G., Baccan, G. et al. Evaluation of corticosterone and IL-1β, IL-6, IL-10 and TNF-α expression after 670-nm laser photobiomodulation in rats. Lasers Med Sci 29, 709–715 (2014). https://doi.org/10.1007/s10103-013-1356-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1356-8