Abstract
In this study, the applicability of waste concrete as a sorbent material for the liquid radioactive waste management was considered. The sample was properly characterized in terms of mineralogical and surface composition, particles morphology, radioactivity, and of the behavior in aqueous solutions at different solid/liquid ratios and pH values. Since radioactive isotopes of Sr, Co and Ni are significant components of the liquid radioactive waste, sequestering of their ions from aqueous media was studied in single and multi-component batch sorption systems. The capacity of waste concrete decreased in the order Ni2+ (0.54 mmol/g) > Co2+ (0.32 mmol/g) > Sr2+ (0.25 mmol/g). Concurrent sorption was analyzed using Simplex Centroid Experimental Design and the coefficients that correspond to the linear and interaction terms were obtained using a special cubic model. The presented results demonstrate the potential of waste concrete in the radioactive waste treatment and conditioning, due to its high sorption capacity and compatibility with the solidification matrices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concrete is a composite material made of aggregates and fluid cement which is, after water, the second most utilized substance in the world. The overall world production of concrete is roughly 3.8 billion cubic meters, which is approximately 1 tone per person per year (ECOSMART). Summarizing the consumption of raw materials and energy for the production of new concrete, growing amounts of the waste concrete and the areas required for its disposal, the need for the concrete recycling was highlighted (Oikonomou 2005).
The utilization of crushed concrete as a component in the production of construction materials was explored extensively, with an emphasis on the mechanical properties of freshly prepared concrete (Verian et al. 2018). However, considering the results achieved by implementation of the European Union strategy on waste recycling (EU Commission 2014), it was noticed that the utilization of the construction and demolition waste (C&DW) was not explored enough in other sectors, such as in the development of sustainable and effective water treatment technologies (Grace et al. 2016). The research focus in sorption technologies is nowadays notably shifted from natural to waste materials, which is justified in terms of reducing, reusing and recycling the accumulated waste, and minimizing the exploitation of natural raw materials. Various waste materials were tested as sorbents for the purification of heavy metal laden water. The usability of industrial sludge, ash, slag, bauxite residue, etc., was elaborated in the recent review paper (Ahmed and Ahmaruzzaman 2016). Furthermore, the applicability of agricultural waste, such as rice husk (Elhafez et al. 2017), rice straw, maize stalks, sugarcane bagasse (Abdel-Tawwab et al. 2017), functionalized sugarcane bagasse (Sarker et al. 2017) is also well documented. In contrast, C&DW has gained considerably less attention. Since the crushed concrete from the demolition of buildings and concrete structures comprise the largest fraction of C&D waste (Monier et al. 2011), its reuse has the greatest effect on reduced environmental stress. The studies of waste concrete applicability so far referred to phosphorous removal from wastewaters (Liu et al. 2014), neutralization of acid mine drainage (Raclavská and Škrobánková 2008), and adsorption of Cu, Zn and Pb (Coleman et al. 2005). There are indications that crushed concrete may be an effective sorbent or filter media for the removal of contaminants from aqueous solutions, based on its alkaline nature and mineral components (oxides, carbonates, silicates) with already recognized sorption potential (Uddin 2017). Hovewer, in order to fully assess its potential, a broad spectrum of contaminants should be included in further work (Grace et al. 2016).
One of the options of using crushed concrete could be linked to the treatment of liquid radioactive waste (LRW). LRW treatments include evaporation, ion exchange, sorption, precipitation and other methods to concentrate the radioactivity from large volumes of liquid phase to small amounts of solid waste, suitable for further solidification and disposal. The solidification of concentrated waste in matrices, such as concrete (Koťátková et al. 2017), glass, plastics or bitumen (Ojovan and Lee 2005), is carried out in order to stabilize the radionuclide and to prevent their leaching at the disposal site. Accordingly, the materials used in LRW treatments should be compatible with the solidification matrices. Given that the concrete, as well as other construction and demolition waste components, (like bricks, roof and ceramic tiles, asphalt, etc.) are mixable and compatible with several solidification matrices, the sorption properties of aged concrete sample deserve to be tested with respect to metal cations of the radioactive isotopes that are important pollutants in the LRW. In contrast to the intensive research on heavy metal removal, the sorption of radionuclides onto waste and by-products was rarely investigated, although several studies have indicated that a high rate of decontamination can be achieved by the use of such economical materials. Treated animal bones and bauxite residue were found to remove Sr2+, Co2+ and Cs+ ions (Šljivić-Ivanović et al. 2015), sorption of Cs+ was achieved using low cost oxidized charcoal prepared from bamboo (Khandaker et al. 2018), while activated carbon, prepared from coconut shells was effective for the decontamination of effluents that contained Eu3+, Ce3+, Sr2+ and Cs+ ions (Moloukhia et al. 2016). Recent studies on Sr2+, Co2+ and Ni2+ removal by various C&D components such as facade, different types of bricks, concrete and asphalt pavements (Jelić et al. 2018), ceramic and roof tiles (Jelić et al. 2017), have indicated the highest sorption affinity of cement-based materials.
Considering the increasing interest in the utilization of the crushed waste concrete, and the gap in knowledge in terms of its applicability to the LRW treatment processes, the aim of this study was to investigate the sorption potential of a concrete sample collected from ruined buildings towards common constituents of LRW (Sr2+, Co2+, Ni2+). The literature survey showed that the utilization of such construction material, aged for a few decades, was not considered in the water treatment so far.
Prior to sorption experiments, the proper characterization of the sorbent was performed, including the determination of crystalline phases, surface active groups, morphology of the particles and their elemental composition, radioactivity and chemical behavior of the material in the aquatic systems. The sorption of Sr2+, Co2+ and Ni2+ was investigated in single metal solutions, as well as in multi-component systems with variable cations concentrations and proportions, using experimental design methodology. Finally, the stability of loaded sorbent was explored using extraction methods.
Materials and methods
Collection, preparation and characterization of waste concrete
The concrete samples, used for walls construction, were collected in the city of Belgrade (Serbia) from the ruins of 5 different buildings dating back to the 1970s. To obtain a representative sample, sub-samples were mixed, and the total amount of 1 kg was crushed, homogenized, milled and sieved to particle size between 0.3 and 0.6 mm. The material was spread over the area of 1 m2 and 50 g was taken randomly from different points. Prior to characterization, this sample was washed with deionized water and dried at 373 K. The sample of waste concrete was characterized using X-ray diffraction (XRD), Fourier transformed infrared (FT-IR) spectroscopy, scanning electron microscopy with energy dispersive spectroscopy (SEM–EDS), and γ-spectrometry analysis. Furthermore, the waste pH was evaluated, as well as the stability of the material in the solutions of different pH. The following equipment and characterization procedures were used:
-
XRD analysis was performed using Ultima IV Rigaku diffractometer in the 2θ range between 4° and 65°, with a scanning step size of 0.02°, and at a scan rate of 5°/min. The present mineral phases were identified using ICDD data base (The International Centre for Diffraction Data (ICDD) 2012).
-
FT-IR spectra were recorded using Nicolet IS 50 FT-IR Spectrometer, operating in the ATR mode (resolution of 4/cm with 32 scans).
-
The surface morphology and the composition were analyzed using the JEOL 5800 SEM coupled with EDS Oxford Inca. Before the analysis, the samples were coated by a thin gold film.
-
The content of radionuclides was determined by the spectrometry of γ-emitters, using two HPGe p-type detectors, with relative efficiencies of 18 and 20%. The measurement time was 3000 s. The obtained output was analyzed using the GENIE 2000 program, and the results were given at the level of significance of 95%.
-
Waste pH was determined using the modified US EPA 9045D method for the determination of soil and waste pH (US EPA 2002). The pH values of investigated waste materials were determined for the different mass ratios of the powders in deionized water, namely 1:1, 1:10, 1:20, 1:100 and 1:200. The suspension pH values were measured, as well as the pH values of corresponding filtrates. In addition, the electrical conductivity of the filtrates was measured.
-
The effect of solution pH onto Ca2+, Sr2+, Co2+ and Ni2+ ions leaching from the waste concrete sample was defined, based on the reaction with deionized water, whose pH was previously adjusted to 2, 6 and 8. This experiment was conducted at solid/liquid ratio of 1:200, on an orbital shaker, for 24 h at the ambient temperature. Afterwards, the suspensions were filtered and pH values of the clear solutions were measured as well as the concentrations of the released ions.
Sorption experiments
Sorption isotherms from single metal solution
Sorption isotherms were defined for Sr2+, Co2+ and Ni2+ ions, at 293 K. 20 cm3 of appropriate Sr2+, Co2+ and Ni2+ solutions were shaken with 0.1 g of sorbent at 10 rotations per minute for 48 h. Solutions were prepared using the nitrate salts of investigated cations (Merck, p.a.). The initial cation concentrations were varied in the range 10−4–7 × 10−3 mol/dm3. Initial pH values of the solutions were in the range 5–5.6 and they were applied without any additional pH adjustments.
Sorption from multi-component solutions
Concurrent sorption of Sr2+, Co2+ and Ni2+ ions from two and three-component solutions was investigated. These experiments aimed to explore the sorbent performance in systems that correspond better to the real wastewater, which is commonly a mixture of different pollutants. Proportions of cations in the mixtures were considered as independent variables, whereas the sorption capacities, expressed as sorbed amounts of cations per 1 g of sorbent, were chosen as the system responses. The mixture simplex centroid experimental design methodology was applied to define the mathematical relationships between the mixtures’ composition and the sorption capacities. The minimum number of runs for this experimental design is 2q–1 = 7, where q is the number of components. These runs correspond to 3 one-component solutions, 3 equimolar two-component solutions and 1 equimolar three-component solution (in the center of the simplex). The design can be expanded with axial points, which represent three-component solutions with the coordinates (1/6, 1/6, 2/3), (1/6, 2/3, 1/6), and (2/3, 1/6, 1/6), giving the total of 10 experimental runs. The increase of the number of experimental runs enables more accurate data description and more reliable conclusions regarding multi-component sorption.
The design matrix generation, data interpretation and analysis of the obtained results were performed using the Minitab software.
Stock solutions for these experiments were prepared by dissolving the adequate masses of nitrate salts in deionized water, in order to obtain the solutions with the total cation concentration of 3 × 10−3 mol/dm3 and the molar ratios of cations specified by the matrix (Table 1). Other experimental conditions were the same as in the isotherm sorption study. After the equilibration, the suspensions were filtered and pH values of filtrates were measured as well as the residual metal concentrations. All sorption experiments were run in duplicate.
Characterization of the loaded sorbent
Possible changes in the surface morphology and composition of the sorbent induced by metal sorption were explored, and the strength of bonds established with different metal cations. For such analyses, loaded sample was prepared by equilibrating 1 g of sorbent with 20 cm3 of equimolar three-component solution, with the total concentration of 3 × 10−3 mol/dm3. The other conditions and solid/liquid separation were described previously.
The solid residue was examined by SEM–EDS analysis in the same way as the raw material, and additionally subjected to an extraction analysis, to evaluate and compare the stability of retained cations. 1 g of loaded sorbent was initially mixed with 8 cm3 of 1 mol/dm3 MgCl2 for 1 h, to separate the cations bonded by ion-exchange (F1). After the centrifugation, the solid residue was treated with 8 cm3 of 1 mol/dm3 sodium acetate (CH3COONa) solution which pH was adjusted to pH = 5 with acetic acid (CH3COOH). Following 5 h of agitation at room temperature, the suspension was centrifuged again to obtain the carbonate contained/acid soluble fraction of metal cations (F2). These two extraction steps were adopted from Tessier sequential extraction procedure (Tessier et al. 1979), commonly used for the estimation of metal distribution in soils and sediments. The leached amounts of metals were measured in separated liquid phases.
Measurement techniques
The pH measurements were performed using a WTW InoLab pH-meter, while electrical conductivities of filtrates were measured by a WTW InoLab Cond 7110. Before the measurements, both devices were calibrated using the original standard solutions purchased from the manufacturer.
The concentrations of Sr2+, Co2+, Ni2+ and Ca2+ ions were determined using a Perkin Elmer 3100 Atomic Absorption Spectrometer (AAS). For the instrument calibration, standard solutions were prepared from a single element Perkin Elmer standard (1000 mg/dm3). The calibration was re-checked after each 10 samples. To diminish the interfering effects of coexisting cations, matrix matched standard solutions were prepared for the analyses of multi-component solutions. The initial concentrations of Sr2+, Co2+, Ni2+ in starting solutions were measured, as well as their concentrations after the sorption experiments. Sorbed amounts of cations were calculated as the differences between the initial and the residual concentrations.
Results and discussion
Characteristics of waste concrete
The crystalline mineral phases in waste concrete that were identified using the XRD technique were quartz and calcite (Fig. 1a). Furthermore, FT-IR spectroscopy was applied for the recognition of functional groups on the sorbent surfaces which are the potential sorption sites. In the vicinity of 3500 1/cm, the –OH vibrations can be observed. Detected Si–O vibration bands at 970, 770, 690 and 525 1/cm, and Si–O–Si and O–Si–O vibrations at 460 1/cm are in agreement with the SiO2 phase detected by the XRD (Nasrazadani et al. 2010). In addition, the vibration peaks characteristic for carbonates was found at 870 1/cm and 1420 1/cm (Dimović et al. 2009), while the features of AlO6 groups (Fernndez-Carrasco et al. 2012) were observed at 680, 640 and 450 1/cm.
SEM–EDS analysis has provided an insight into morphology and elemental composition of the sample (Fig. 2). At lower magnifications, the irregularly shaped aggregate particles can be observed. The previous SEM investigation of fresh concrete sample showed that hydration products make the network structure which is porous and tortuous (Cui et al. 2015). In contrast, the images obtained at higher magnifications showed diminished porosity of aged concrete, which can be attributed mainly to the carbonation process. Namely, the diffusion of atmospheric CO2 induces precipitation of carbonates near the pore surface, causing their obstruction. The influence of the carbonation level (Cui et al. 2015) and acids deposit (Fan and Luan 2013) onto the morphology of concrete particles was previously investigated. Both studies, conducted to explain the process of aging under different environmental conditions, have asserted that the surface of aged samples became smoother and less porous with the increasing exposure time.
The analysis of surface composition by EDS technique has revealed high oxygen content followed by silica and calcium, and minor amounts of aluminum, iron, potassium, sulphur, sodium and magnesium (Fig. 2d).
Summarizing the results obtained by different techniques, it can be concluded that the investigated waste concrete represents the mixture of Si, Al, Fe oxides and carbonate compounds, predominantly calcium carbonate. This chemical composition is in compliance with the composition of components in cement production, i.e., oxides of calcium, silica, iron and aluminum and the sulphate carrier (usually gypsum CaSO4·2H2O) (Achternbosch et al. 2003). The main source of quartz is sand added in the concrete preparation process. Furthermore, the hydration products of cement paste are different silicates, which under atmospheric conditions undergo decalcification and silicate polymerization (Black et al. 2008), while the detected carbonates are products of the portlandite carbonization (Kontoleontos et al. 2013). The other elements are components of used raw materials.
Building materials may contain significant amounts of natural radionuclides 238U, 232Th and 40K, due to their presence in raw minerals. The activity of 226Ra and 232Th in the waste concrete was determined by their decay products: 214Bi (609, 1120 and 1764 keV), 214Pb (295 and 352 keV) and 228Ac (338 and 911 keV), respectively. The activities of 40K and 137Cs were determined from their 1460 and 661 keV c-energy, respectively.
The radiological analysis has revealed 18 ± 4, 17 ± 6 and 290 ± 50 Bq/kg of 226Ra, 232Th and 40K. Additionally, a very small activity came from 137Cs (3 ± 1 Bq/kg). The obtained activities are close, even lower, than those reported for concrete samples used in Sweden (Döse et al. 2016) and Israel (Kovler 2017). Moreover, the average activity concentrations of Ra, Th and K are higher in concrete samples produced in EU countries (60, 35 and 392 Bq/kg, respectively) (Trevisi et al. 2012). Consequently, the waste concrete sample does not pose a radiological risk for the environment.
Waste concrete pH values, measured in the suspensions and clear filtrates, as well as the electrical conductivities of filtrates, were highly dependent on the solid to liquid ratio (Fig. 3). The increase in measured parameters with the increase in solid content was observed. Suspensions with the solid/liquid ratio higher than 1:20 (i.e., solid concentration, > 50 g/dm3) had pH > 12 and can be classified as corrosive according to the EPA recommendations (US EPA 2002), while less concentrated suspensions were alkaline (pH > 11), but non-corrosive.
The tests of waste concrete stability in the aquatic environment showed that excessive amounts of Ca2+ ions were released in the acidic solution of initial pH 2 (approximately 4–5 times higher in respect to the amounts released at pH 6 and pH 8). Relatively low and similar amounts of Ca2+ released in near neutral pH range indicate high stability of the sample under such pH conditions. Following waste concrete mixing with the aqueous solutions, the concentrations of Sr2+, Co2+ and Ni2+ were below their detection limits, regardless of the initially adjusted pH. Furthermore, the final pH of 6.7 was recorded after the reaction of the waste material with solution having initial pH 2, while initial pH values of 6 and 8 have increased to 10.5. These experiments signify the alkaline nature and buffering properties of the material.
Sorption isotherms
The effect of initial metal concentration onto amounts sorbed at equilibrium was investigated in batch experiments. The constructed sorption isotherms signify different affinity and capacity of the waste material for investigated sorbates (Fig. 4). Considering the highest initial metal concentration, the sorption capacities were found to increase in the sequence: 0.25 mmol Sr2+/g > 0.32 mmol Co2+/g > 0.54 mmol Ni2+/g.
The sorption of Sr2+ ions was negligible until the initial concentration was higher than 10−3 mol/dm3, and such low affinity is most probably a result of the competitive effect of Ca2+ ions released from the sorbent material. On the other hand, with further increase in initial Sr2+ concentration, sorption efficiency of waste concrete continuously increased in the investigated concentration range. The Sr2+ isotherm corresponds to the shape of the S-type isotherm, for which the alteration of sorption mechanism is anticipated (Giles et al. 1974). In this case, the isotherm can be divided into the first horizontal section with very low Sr2+ sorption due to the competition with Ca2+ ions, and the second part where Sr2+ concentration became high enough to effectively compete with Ca2+ ions for the sorption sites.
Quite the opposite, Co2+ and Ni2+ sorption isotherms had defined plateaus, which correspond to the saturation point of the material. The characteristic sharp increase in Ni2+ and Co2+ sorption in a lower concentration range indicates the H-type isotherm shape, i.e., high affinity sorption (Giles et al. 1974). The presence of Ca2+ ions in the solution did not affect the sorption of Co2+ and Ni2+ species, which is in accordance with previous studies (Šljivić-Ivanović et al. 2015).
The equilibrium pH values were found to decrease with the increase in initial metal concentration, and ranged between 10.3 and 6.7 for Sr2+, 10.9 and 6.6 for Co2+, and between 10.8 and 6.5 for Ni2+. Considering the experiments conducted with lower initial concentrations of Co2+ and Ni2+ (lower than 10−3 mol/dm3), pH values at equilibrium were ≥ 9. The hydrolysis of Ni2+ ions starts at pH 8, whereas the precipitation of insoluble hydroxide reaches its maximum at pH 10. Similarly, the hydrolysis and precipitation of Co(OH)2 occur in the pH range 8–12. The initial, almost vertical section of Co2+ and Ni2+ isotherms coincides with equilibrium pH values above the precipitation threshold, which indicates that the removal of Co2+ and Ni2+ ions by waste concrete under these conditions was primarily carried out through the precipitation. With a further increase in initial cation concentration, the equilibrium pH values were 6–7. The decrease in pH with an increase in sorption is an indication of the specific cation sorption, i.e., the formation of inner-sphere complexes between a hydrated cation from the solution and active surface centers, accompanied with the H+ release.
In terms of the process efficiency, the overall Co2+ and Ni2+ removal was 23.8–100%, and 40.7–100%, respectively. It is important to note the high efficiency of waste concrete (more than 90%) in removing these ions up to the initial concentrations of 10−3 mol/dm3. On the other hand, the efficiency of Sr2+ removal was considerably lower (< 30%).
The sorption capacities of investigated waste concrete were reasonably high and comparable with the capacities of other investigated inorganic waste materials and mineral sorbents (Table 2).
Multi-component sorption
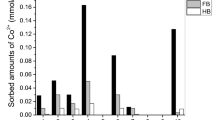
The coexisting chemical species can hinder or promote the sorption of the particular ion. Accordingly, the performance of the waste concrete was explored in more complex, multi-component systems, using experimental design methodology. Sorbed amounts of the investigated cations are presented in Fig. 5, as a function of solution composition defined in Table 1.
Sorbed amounts of Sr2+, Co2+ and Ni2+ ions from multi-component solutions by waste concrete. The numbers on X-axis refer to the specific composition of the solution given in Table 1
Special cubic model can be used for the description of data obtained by the simplex centroid design. For three-component solution, this general model can be represented as:
where \(Y\) is the system response, \({x_1}\), \({x_2}\) and \({x_3}\) are the initial metal concentrations (independent variable), \({x_1}{x_2}\), \({x_1}{x_3}\), \({x_2}{x_3}\) and \({x_1}{x_2}{x_3}\) are interactions terms, \({\beta_{1\;}}\), \({\beta_{2\;\;}}\) i \({\beta_{3\;\;}}\) are regression coefficients, \({\beta_{12\;}}\), \({\beta_{13\;}}\), \({\beta_{23\;}}\) i \({\beta_{123\;\;}}\) are coefficients that correspond to quadratic and ternary terms, \(\varepsilon\) is residual.
By solving the Eq. 1, the unknown values of the coefficients that correspond to the linear and interaction terms can be obtained. After testing the general model (Eq. 1), as well as the models derived from Eq. 1, like linear and quadratic model, it was obtained that Sr2+ and Ni2+ sorption were best described by the linear models, while Co2+ sorption model included two-way interaction terms. The chosen confidence interval was 0.95, giving that statistically significant terms were those with p < 0.05.
Including only statistically significant terms, the following equations were derived:
These equations imply that sorption of investigated cations is directly proportional and principally affected by their own concentration in the multi-component solution. Significant interaction was found only in the case of Co2+ sorption from the mixture, i.e., the interaction between Sr2+ and Co2+ ions enhanced the efficiency of Co2+ removal. Otherwise, the presence of Sr2+ diminished Co2+ sorption, Ni2+ negatively influenced Sr2+ sorption, but also Co2+ affected Ni2+ sorption. According to the numerical values of the coefficients in the Eqs. 2–4, both positive and negative effects of coexisting cations were at least one order of magnitude lower in respect to the most dominant effect.
An analysis of variance (ANOVA) was used for the determination of statistically significant parameters, based on correlation coefficients, Fisher and p-test values (Table 3). The proposed Eqs. 2–4 explain about 58.3% of Sr2+, 91.0% of Co2+ and 69.0% of Ni2+ sorption data. These equations can be graphically presented by the construction of ternary contour plots (Fig. 6).
Competing effects between different cations are dependent on their bonding mechanisms, which rely on both the cation and the sorbent properties. Negative influence of Co2+ ions onto Ni2+ sorption and the suppressed Co2+ sorption in the presence of Sr2+ ions were also detected using waste roof tiles as their sorbent (Jelić et al. 2017). In addition, Co2+ sorption by waste ceramic tiles was diminished in the presence of Ni2+ (Jelić et al. 2017).
The polyacrylic acid grafted and non-grafted activated carbon felts were tested as sorbents for the purification of simulated wastewater containing equimolar amounts of Sr2+, Ni2+ and Co2+ ions (Ferreira Esmi et al. 2014). Taking into consideration the calculated values of competition coefficients, the authors concluded that the presence of Sr2+ ions did not affect the sorption of Co2+ and Ni2+ ions, while coexistence of Co2+ and Ni2+ influenced their sorption behavior due to the competition for similar sorption sites. Many other materials, such as red mud, calcined animal bones (Šljivić-Ivanović et al. 2015) and synthetic hydroxyapatite (Ma et al. 2010), exhibited higher affinity for Co2+ species in reaction with the mixture of Co2+ and Sr2+ ions.
Previously reported analyses of the sorption processes based on the mixture design methodology for the mixtures of Pb(II), Cu(II) and Ni(II) ions (Lei et al. 2014), and the results presented in this paper, signify that the competition in multi-component solutions can be satisfactorily described and modeled using the statistical approach.
Characterization of the loaded sorbent
The loaded sorbent was prepared using solid/liquid ratio 1:20 and equimolar three-component solution with the total initial concentration of 3 × 10−3 mol/dm3. Under these experimental conditions, due to the applied high sorbent mass, 100% of Ni2+ and Co2+ ions and 61.5% of Sr2+ ions were sorbed. Calculated sorbed amounts were 0.012 mmolSr2+/g, and 0.02 mmol/g for Ni2+and Co2+ ions. The values were lower than those obtained using the same solution of metals but different solid/liquid ratio 1:200 (Section “Multi-component sorption”). The sorbed amounts expressed per gram of a sorbent material are generally lower when higher mass of sorbent is used, due to the dissipation of concentration gradient (Kumar and Porkodi 2007). SEM analysis (Fig. 7) of waste concrete sample after metal sorption showed the absence of morphological changes. The agglomerates were of the irregular shape, while under higher magnification micropores were observable.
The peaks of Co and Ni have appeared in the EDS spectrum of loaded waste concrete, revealing their similar concentrations of 0.27 and 0.33%, respectively. The presence of Sr in the sample could not be confirmed by the EDS analysis, probably due to a lower sorption capacity and overlapping of its characteristic maximum at 1.806 keV with more intense peak of Si at 1.739 keV.
Sr2+, Co2+ and Ni2+ loaded concrete sample was subjected to extraction analysis which is a practical method to assess the leachability of metals under different conditions. Subsequent utilization of selected reagents allows separation of metals into operationally defined fractions: F1-ion-exchangeable and F2-acid soluble (carbonate and specifically sorbed ions).
The tests of waste concrete stability in aqueous media (Section “Characteristics of waste concrete”) showed that Sr2+, Co2+ and Ni2+ ions potentially contained in the sample do not dissolve even at initial pH 2. The sequential extraction of cement clincker also confirmed the high stability of contained Co i Ni ions (Lu et al. 2016). Therefore, the release of metals from the loaded sorbent within the extraction phases F1 and F2 results from the applied sorption process and the percentages of desorption are calculated with respect to the sorbed amounts.
The distribution of metals presented in Fig. 8 illustrates their association with different waste concrete fractions. 7.1% of Sr2+ and even less quantities of sorbed Co2+ and Ni2+ (2.9 and 4.9%, respectively) were extracted in the scope of the ion-exchangeable fraction. Furthermore, in F2 phase 36.8% of Sr2+ was extracted simultaneously with equivalent amounts of sorbed Co2+ and Ni2+ ions (15.0%).
Distribution of metal cations sorbed by waste concrete from equimolar tree-component solution (total concentration of the mixture 3 × 10−3 mol/dm3, solid/solution ratio 1/20). Black bars—ion-exchangeable fraction (F1), white bars—acid-soluble fraction (F2), in relation to the total sorbed quantities
Between investigated cations, Sr2+ displayed the highest tendency for both F1 and F2 fractions, in accordance with its general affinity for the ion-exchange, incorporation in carbonate and formation of outer-sphere complexes with silica surface (Brown and Parks 2001). Furthermore, Ni2+ and Co2+ ions have high tendency to form stronger chemical bonds with Fe, Mn oxides or organic compounds (Bradl 2004), resulting in lower relative amounts in F1 and F2 phases. The results obtained imply the highest mobility of Sr2+ ions in conditions of increased ionic strength and acidity; however, as the total amounts of sorbed Sr2+ ions were lower compared to Co2+ and Ni2+, the absolute amounts of metals that could potentially be leached are similar.
Future investigations and the fate of the spent sorbent
The characterization of raw and metal loaded aged concrete sample and the investigation of equilibrium of the sorption process have revealed a high potential of investigated waste material for the removal of contaminants. Therefore, waste concrete can be considered as a cost-effective alternative to ion-exchange resins and other commercial sorbents used for the separation and concentration of the contaminants. Following the LRW treatment, the conditioning of the loaded waste concrete can be done via solidification in cement or glass matrices (Koťátková et al. 2017). Solidified forms are candidates for permanent disposal; however, it is necessary to explore the effect of added spent waste concrete onto mechanical properties of the final products and to apply leaching tests for the assessment of possible long-term environmental risks.
Conclusion
Utilization of waste from the construction and demolition sector in the removal of contaminants from water has a great significance in implementing the concept of circular economy, fostered by the European legislation. In the present study, the suitability of crushed waste concrete as a sorbent material for decontamination of LRW was assessed, through its physicochemical characterization and quantifying sorption potential of common radioactive contaminants. As a result of concrete aging, quartz and calcium carbonate were found to be the main crystalline phases in the investigated sample, taken from the demolished buildings sites. Alkaline nature and mineral composition of aged concrete proved to be a good basis for Ni2+, Co2+ and Sr2+ ions separation. Out of investigated species, Sr2+ ions exhibited least effective and least competitive sorption, and the highest potential to be leached from the loaded sorbent. Given that the waste needs to be in solid form and resistant to leaching before disposal, crushed concrete saturated with radionuclides can be mixed and solidified in several matrix materials, such as cement or glass. The chemical compatibility of waste concrete with solidification matrices must be emphasized as an advantage over other high capacity sorbents, which permanent disposal may be problematic. Testing the removal efficiency of radionuclides from the real LRW in order to optimize the process conditions, assessment of the performance of solidification matrices and safety of disposal should be in the focus of future research.
References
Abdel-Tawwab M, El-Sayed GO, Shady SHH (2017) Capability of some agricultural wastes for removing some heavy metals from polluted water stocked in combination with Nile tilapia, Oreochromis niloticus (L.). Int Aquat Res 9:153–160. https://doi.org/10.1007/s40071-017-0166-1
Achternbosch M, Bräutigam K-R, Hartlieb N, et al (2003) Heavy metals in cement and concrete resulting from the co-incineration of wastes in cement kilns with regard to the legitimacy of waste utilisation. Karlsruhe
Ahmed MJK, Ahmaruzzaman M (2016) A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J Water Process Eng 10:39–47. https://doi.org/10.1016/j.jwpe.2016.01.014
Black L, Garbev K, Gee I (2008) Surface carbonation of synthetic C–S–H samples: a comparison between fresh and aged C–S–H using X-ray photoelectron spectroscopy. Cem Concr Res 38:745–750
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18. https://doi.org/10.1016/j.jcis.2004.04.005
Brown G, Parks G (2001) Sorption of trace elements onto mineral surfaces: modern perspectives from spectroscopic studies, and comments on sorption in the marine environment. Int Geol Rev 43:963–1073
Coleman NJ, Lee WE, Slipper IJ (2005) Interactions of aqueous Cu2+, Zn2+ and Pb2+ ions with crushed concrete fines. J Hazard Mater 121:203–213. https://doi.org/10.1016/j.jhazmat.2005.02.009
Cui H, Tang W, Liu W et al (2015) Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Constr Build Mater 93:522–527. https://doi.org/10.1016/j.conbuildmat.2015.06.007
Dimović S, Smičiklas I, Plećaš I et al (2009) Comparative study of differently treated animal bones for Co2+ removal. J Hazard Mater 164:279–287
Döse M, Silfwerbrand J, Jelinek C et al (2016) Naturally occurring radioactivity in some Swedish concretes and their constituents: assessment by using I-index and dose-model. J Environ Radioact 155–156:105–111. https://doi.org/10.1016/j.jenvrad.2016.02.012
ECOSMART A concrete contribution to the environment. http://ecosmartconcrete.com/?page_id=208. Accessed 1 Jan 2016
Elhafez SEA, Hamad HA, Zaatout AA, Malash GF (2017) Management of agricultural waste for removal of heavy metals from aqueous solution: adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis. Environ Sci Pollut Res 24:1397–1415. https://doi.org/10.1007/s11356-016-7891-7
EU Commission (2014) Towards a circular economy: a zero waste programme for Europe. COM(2014) 398 Final. https://doi.org/10.1017/cbo9781107415324.004
Fan Y, Luan H (2013) Pore structure in concrete exposed to acid deposit. Constr Build Mater 49:407–416. https://doi.org/10.1016/j.conbuildmat.2013.08.075
Fernndez-Carrasco L, Torrens-Martn D, Morales LM, Martnez-Ramrez S (2012) Infrared spectroscopy in the analysis of building and construction materials. In: Theophile T (ed) Infrared spectroscopy: materials science, engineering and technology. InTech, London
Ferreira Esmi C, Guevar C, Dhoury M et al (2014) Evaluating the use of activated carbon felts to remove Co2+, Ni2+ and Sr2+ from wastewater. J Environ Chem Eng 2:1705–1712. https://doi.org/10.1016/j.jece.2014.06.009
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J Colloid Interface Sci 47:755–765. https://doi.org/10.1016/0021-9797(74)90252-5
Grace MA, Clifford E, Healy MG (2016) The potential for the use of waste products from a variety of sectors in water treatment processes. J Clean Prod 137:788–802. https://doi.org/10.1016/j.jclepro.2016.07.113
Jelić I, Šljivić-Ivanović M, Dimović S et al (2017) Utilization of waste ceramics and roof tiles for radionuclide sorption. Process Saf Environ Prot 105:348–360. https://doi.org/10.1016/j.psep.2016.11.021
Jelić I, Šljivić-Ivanović M, Dimović S et al (2018) The applicability of construction and demolition waste components for radionuclide sorption. J Clean Prod 171:322–332. https://doi.org/10.1016/j.jclepro.2017.09.220
Khandaker S, Toyohara Y, Kamida S, Kuba T (2018) Adsorptive removal of cesium from aqueous solution using oxidized bamboo charcoal. Water Resour Ind 19:35–46. https://doi.org/10.1016/J.WRI.2018.01.001
Kontoleontos F, Tsakiridis P, Marinos A et al (2013) Dry-grinded ultrafine cements hydration. Physicochemical and microstructural characterization. Mater Res 16:404–416
Koťátková J, Zatloukal J, Reiterman P, Kolář K (2017) Concrete and cement composites used for radioactive waste deposition. J Environ Radioact 178–179:147–155. https://doi.org/10.1016/J.JENVRAD.2017.08.012
Kovler K (2017) The national survey of natural radioactivity in concrete produced in Israel. J Environ Radioact 168:46–53. https://doi.org/10.1016/j.jenvrad.2016.03.002
Kumar KV, Porkodi K (2007) Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum. J Hazard Mater 146:214–226. https://doi.org/10.1016/j.jhazmat.2006.12.010
Lei DY, Liu Z, Peng YH et al (2014) Biosorption of copper, lead and nickel on immobilized Bacillus coagulans using experimental design methodologies. Ann Microbiol 64:1371–1384. https://doi.org/10.1007/s13213-013-0782-y
Liu C, Yang Y, Wan N (2014) Kinetic studies of phosphate adsorption onto construction solid waste (CSW). Water Qual Res J Can 49:307–318
Lu H, Wei F, Tang J, Giesy JP (2016) Leaching of metals from cement under simulated environmental conditions. J Environ Manage 169:319–327. https://doi.org/10.1016/j.jenvman.2015.12.008
Ma B, Shin WS, Oh S et al (2010) Adsorptive removal of Co and Sr ions from aqueous solution by synthetic hydroxyapatite nanoparticles. Sep Sci Technol 45:453–462. https://doi.org/10.1080/01496390903484941
Moloukhia H, Hegazy WS, Abdel-Galil EA, Mahrous SS (2016) Removal of Eu3+, Ce3+, Sr2+, and Cs+ ions from radioactive waste solutions by modified activated carbon prepared from coconut shells. Chem Ecol 32:324–345. https://doi.org/10.1080/02757540.2016.1139089
Monier V, Mudgal S, Hestin M, Trarieux M, Mimid S (2011) Service contract on management of construction and demolition waste. Paris, p 120. Retrieved from http://ec.europa.eu/environment/waste/pdf/2011_CDW_Report.pdf
Nasrazadani S, Mielke D, Springfield T, Ramasamy N (2010) Practical application of FTIR to characterize paving materials. University of North Texas, Denton
Oikonomou ND (2005) Recycled concrete aggregates. Cem Concr Compos 27:315–318
Ojovan MI, Lee WE (2005) An introduction to nuclear waste immobilisation. Elsevier, London
Raclavská H, Škrobánková DM (2008) Potential utilization of crushed concrete in the acid mine drainage treatment. In: 12th Conference on environment and mineral processing. Part III. VŠB-Technical University, Ostrava, pp 147–154
Sarker TC, Azam SMGG, El-Gawad AMA et al (2017) Sugarcane bagasse: a potential low-cost biosorbent for the removal of hazardous materials. Clean Technol Environ Policy 19:2343–2362. https://doi.org/10.1007/s10098-017-1429-7
Šljivić-Ivanović M, Smičiklas I, Dimović S et al (2015) Study of simultaneous radionuclide sorption by mixture design methodology. Ind Eng Chem Res 54:11212–11221. https://doi.org/10.1021/acs.iecr.5b03448
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851. https://doi.org/10.1021/ac50043a017
The International Centre for Diffraction Data (ICDD) (2012) Powder Diffraction File, PDF-2 Database, announcement of new database release
Trevisi R, Risica S, D’Alessandro M et al (2012) Natural radioactivity in building materials in the European Union: a database and an estimate of radiological significance. J Environ Radioact 105:11–20. https://doi.org/10.1016/j.jenvrad.2011.10.001
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462. https://doi.org/10.1016/J.CEJ.2016.09.029
US EPA (2002) Reference methodology: method 9045D, soil and waste pH. US EPA, Washington
Verian KP, Ashraf W, Cao Y (2018) Properties of recycled concrete aggregate and their influence in new concrete production. Resour Conserv Recycl 133:30–49. https://doi.org/10.1016/j.resconrec.2018.02.005
Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project III 43009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Šljivić-Ivanović, M., Jelić, I., Dimović, S. et al. Exploring innovative solutions for aged concrete utilization: treatment of liquid radioactive waste. Clean Techn Environ Policy 20, 1343–1354 (2018). https://doi.org/10.1007/s10098-018-1563-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-018-1563-x