Abstract
In this study, biodiesel was produced using waste cooking oil that was discarded as a waste in the environment. The properties of the feedstock were determined using standard ASTM methods. The transesterification process was implemented to extract the biodiesel, and this process was optimized and standardized by selecting three different parameters: molar ratio (methanol:oil), catalyst concentration (KOH) and reaction temperature. The physicochemical properties of the biodiesel so produced were tested and analyzed using gas chromatography. Biodiesel and diesel were mixed in different volumetric ratios, and the exhaust emission characteristics of the blends were determined by testing the blends on a variable compression ratio engine. The study concluded that waste cooking oil has a great potential for waste to energy process. The highest yield of 93.8% was obtained by optimizing the process. Emission characteristics of CO for B50 blend showed a downward trend while NO x emission was found to be greater for blending ratios above 10%. B10 showed the best results pertaining to lower NO x and CO emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy is an essential constituent of human life which enables the mankind to function and carry out their daily activities. It is considered as a vital element which brings about the socioeconomic development on a global level. With the advent of expeditious urbanization and modernization, there is an intense growth in the fossil fuel demand which is the vital element of the energy sector. The fossil fuels are non-renewable in nature and are exhausting as these are being utilized at a very fast pace. Its continuous use has resulted serious environmental threats such as emission of the greenhouse gases which eventually results in global warming. This underlines the fact that an alternative fuel is required which is eco-friendly in nature, economical, available throughout the time span and has a high feasibility rate. Hence, interest in research for an effective substitute for petroleum diesel is increasing and biodiesel has many benefits over petroleum diesel (Sunthitikawinsakul and Sangatith 2012). It is superior to fossil diesel fuel (Mahesh et al. 2015).
The use of edible oil and non-edible sources as a raw material had few technical and financial limitations. The limited feedstock available for extracting biodiesel further hindered the accomplishment of the final objective. Thus, the government focuses on promoting an alternative source that can be used for producing biodiesel.
Oil is used for frying in Indian kitchens to a large extent. During deep frying, a number of chemical reactions take place—hydrolysis, oxidation, thermal decomposition and polymerization. It increases the polar materials and decreases the unsaturated fatty acids (Kabir et al. 2014). It is not good for human health. So it is usually dumped as a waste. Improper disposal of these oils in the drainage system might lead to the blockage of the system, and huge investment has to be made in order to ensure proper cleanliness of the same. It may also prove to be disastrous if dumped onto the soil or is discarded into the nearest water body. Waste cooking oil has a great potential for the waste to energy conversion process (Mata et al. 2012). According to reports, biodiesel production from used cooking oil is 60 million liters from 1000 t of feedstock use and 115–130 million liters of biodiesel is produced from multiple feed stocks such as to include crude vegetable oil, used cooking oil, animal fat and other (Aradhey 2014).
Waste cooking oil parameters can be adjusted and can be brought in the range on standard fuel values by transesterification process. So knowledge of different parameters of waste cooking oil is a must (Knothe 2006). Transesterification process is the most common and economical process of producing biodiesel (Reed et al. 1993) though during this process the presence of water in waste cooking oil sample often leads to hydrolysis, whereas high FFA content and high saponification number can lead to saponification reaction, which are responsible for low biodiesel yield and high catalyst consumption (Carlinia et al. 2014). But still waste cooking oil is used to produce biodiesel because economic feasibility of biodiesel depends on availability of low-cost feedstock (Cunha et al. 2013). The use of waste cooking oil as feedstock can effectively reduce the cost of biodiesel by 60–70%. Biodiesel is used as a mixture constituent of petroleum diesel in proportions for running a diesel engine, since using neat biodiesel has some engine issues (Valente et al. 2011). It has been reported that mixing of 5% of biodiesel fuel to the present fuel can save Rs. 40,000 million per year (Gopal et al. 2014). According to the India’s biofuel policy (Aradhey 2014), the target is to blend petroleum diesel with 20% biodiesel by the end of 12th Five-Year Plan (2017). Till date only 5% blending target has been attained. The target of Government of India for a mandatory 20% blending was made in accordance with the availability of the feedstock.
Some gaps were identified in the previous studies:
-
No estimation was made in order to ensure the amount of used frying oil that is disposed off as waste.
-
Analysis of results on basis of gas chromatography was not implemented.
-
Blending of biodiesel and simultaneous testing of blends for determining exhaust emissions was not carried out.
This was the first comprehensive study in India which encompassed the estimation of amount of waste cooking oil generated on daily basis by small and big eating houses in the vicinity of the institute and its characterization. Optimization and standardization of the biodiesel production process, i.e., transesterification process was accomplished by simple factorial method (user friendly), so as to obtain highest yield. Biodiesel so produced was further characterized for its important fuel properties, and analysis of the results on basis of gas chromatography was also done. The exhaust emission characteristics of the biodiesel blends at varying brake loads were determined on diesel engine as biodiesel mixed with conventional diesel in some proportions can be used to run any existing conventional compression ignition engine and does not require any engine modifications (Sunthitikawinsakul and Sangatith 2012).
Materials and methods
In order to estimate the quantity of cooking oil being discarded as a waste in accordance with the consumption of the oil on daily basis, four different eating houses were selected randomly in a nearby location. The information so collected was:
-
Type of oil used for cooking purpose.
-
Amount of oil used per day.
-
Amount of oil leftover after usage per day.
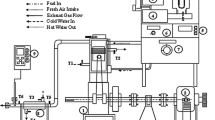
The waste cooking oil sample collected from different eating houses was mixed, and this mixture was the sample used for study. The optimization of the transesterification process was carried out by standardizing three different parameters that affect the level of ester recovery, i.e., varying the molar ratio (methanol:oil) from 4:1 to 7:1, catalyst concentration, i.e., KOH (%) from 0.5 to 2% and reaction temperature from 30 to 75 °C. Sets of graphs were drawn that represented the relationship between percent yield of the biodiesel corresponding to the molar ratio and catalyst concentration at different reaction temperatures. The result was analyzed in accordance with the maximum yield of ester obtained by optimization. The biodiesel production process was carried out in certain steps that can be explained in Fig. 1 that follows.
The oil was heated at around 60 °C and then filtered using the muslin cloth. The unwanted particles, if not removed, might hinder the further process for biodiesel production. Therefore, filtration of the sample was replicated thrice in order to obtain the sample which was clear enough and therefore free from any other contaminants.
The oil sample was treated with KOH–methanol mixture by taking the results of the standardization process into consideration. The flask was shaken constantly for few seconds so as to ensure uniform mixing of the components throughout. The flasks were kept in the water bath shaker that was preset at desired temperature. The speed of the shaker was regulated accordingly. The process was carried out for the desired time period.
After removing the flask from the water bath shaker, the mixture was allowed to settle for 4 h in order to ensure complete separation of the ester layer from the glycerol layer. Boiling water was used to wash the contents of the separating funnel in order to remove the traces of the contaminants such as unreacted catalyst and methanol. The washing process was carried out 3–4 times, and after each washing, the sample was allowed to settle for 15–20 min.
After washing, in order to remove the traces of water present in the sample the solution was heated at boiling temperature of water so that the water can be driven out from the solution. Therefore, after heating a clear solution was obtained, and this was the biodiesel.
The physical and chemical parameters of the waste cooking oil sample were determined to analyze its potential as a feedstock for biodiesel production. The prepared biodiesel was characterized in order to ensure its compliance with the ASTM D6751 standard biodiesel specifications for 7 parameters, i.e., acid value ASTM (D664), kinematic viscosity at 40 °C ASTM (D445), cloud point ASTM (D2500), pour point ASTM (D97), free fatty acid ASTM (D5555), gross heat of combustion ASTM (D2015) and carbon residue ASTM (D4530).
Gas chromatography was carried out using NUCON 5700 gas chromatograph in order to determine the constituents of fatty acids in biodiesel. Biodiesel comprises of esters of saturated acids and unsaturated esters. Thus, fatty acid profile was deduced by using gas chromatographic analysis. In the present study, capillary column and F I Detector were used. About 10 microliter of sample was injected to the injector inlet by an additional pressure regulated stream of gas. Results were obtained on integrator in the form of a plot as shown.
Five different biodiesel blends were prepared by mixing petroleum diesel with biodiesel in the desired ratios at room temperature as shown in Table 1.
The experiment was carried out on a variable compression ratio diesel engine. Table 2 signifies the engine specifications. The emission parameters were analyzed by operating the engine at different biodiesel blends at varying brake loads and diverting the exhausts to the gas analyzer. NUCON-500 model-type gas analyzer was used to obtain the exhaust emission characteristics.
Results and discussion
The data shown in Table 3 represent that all eating joints (small or big) use considerable amount of edible oil for frying on daily basis. These eating houses also generate considerable amount of waste cooking oil on daily basis (29–46%) which cannot be used anymore. This oil ultimately finds its way in drainage. Thus, a waste management option can be put forward to effectively utilize the oil to convert it to biodiesel. The production cost of biodiesel is majorly dedicated to the feedstock that is used for the process. Therefore, using waste cooking oil as a feedstock for producing biodiesel is both economically and environmentally viable.
Three different parameters, i.e., reaction temperature, catalyst concentration and molar ratio of alcohol–oil were optimized, and the effects of these parameters on the ester recovery were studied. Following graphs were obtained that represented the catalyst concentration versus yield data and molar ratio versus yield data at four different temperatures, i.e., 30, 45, 60 and 75 °C (Figs. 2, 3).
At 30 °C, maximum yield of 62% was obtained at catalyst concentration of 1.5% and at the molar ratio of 6:1 (Figs. 4, 5).
At 45 °C, maximum yield of 66.7% was obtained at catalyst concentration 1% and molar ratio 6:1 (Figs. 6, 7).
At 60 °C, maximum yield of 93.7% was obtained at catalyst concentration of 1% and molar ratio 5:1 (Fig. 8).
At 75 °C, maximum yield of 72% was obtained at catalyst concentration of 1% and molar ratio 6:1.
The most favorable optimum condition that resulted in the maximum yield of 93.7% was at 60 °C; catalyst concentration 1% and molar ratio 5:1. Hence, the effect of various parameters on methyl ester recovery can be discussed as follows:
Methanol-to-oil ratio greatly influences the yield of biodiesel. Transesterification is carried out with and an additional quantity of alcohol although the requirements amount to the stoichiometric ratio of 3:1. This is done in order to shift the chemical equilibrium of the reaction to the product side. Cetinkaya and Karaosmanoglu (2004) reported that transesterification is not efficient below the ratio of 5:1. In Figs. 3 and 5, an increase in molar ratio up to 6:1 resulted in an increase in the ester content. Increasing the ratio beyond that resulted in a slight decrement in the ester yield. On the other hand, Fig. 7 showed a declination when the ratio was increased beyond 5:1. A maximum yield of 93.7% was observed at this ratio. Figure 9 represents a decreasing ester yield when the molar ratio was increased beyond 6:1. This decrement in the yield of ester beyond a specified level could be because of the excess alcohol that might have hindered the process at the particular temperature. Thus, the complete separation of ester from the glycerol could not take place result of which a foamy phase had developed (Freedman et al. 1984).
The catalyst was tested for the concentrations lying in the range of 0.5–2%, taking into consideration the data from the review of the literature. All the four figures showed that maximum amount of ester were recovered at the catalyst concentration of 1%. While increasing the concentration of the catalyst from 0.5 till 1%, the yield of the ester increased. This is because on increasing the amount of catalyst the catalyst surface increases which in turn requires more amount of methanol to react resulting in an increase in the rate of reaction. Thus, the product, i.e., biodiesel yield increased. On further increasing the concentration of the catalyst, the yield was reduced considerably. This reduction could be attributed to the soap formation which might have taken place during the washing process which is the consequence of the saturation of the catalyst pores. As a result, the ester layer could not have been separated properly due to the formation of emulsions of the soap with water (Saqib et al. 2012).
Transesterification can occur at different temperature ranges. Freedman et al. (1984) reported that the first 30–35 min of the reaction supported the temperature dependency of the biodiesel yield. The yield was 94, 85 and 80% for the temperature 60, 45 and 30 °C. High temperature enhances the rate of the reaction. On the other hand, it might also propagate the saponification phenomenon. Low temperature does not ensure a complete conversion of triglycerides into ester and glycerol. On increasing the temperature from 30 till 60 °C, the yield was enhanced. This was because the conversion of triglycerides into ester requires energy for breaking the bonds of these glycerides to form diglycerides followed by the formation of monoglycerides and finally the ester. Thus, maximum yield was obtained at 60 °C. On further increasing the temperature, the yield decreased since the saponification process could have hindered the process of conversion (Kwon and Yeom 2015).
Certain parameters were characterized, and the data corresponding to the parametric determination of the feedstock, i.e., waste cooking oil and biodiesel are tabulated in Table 4. Acid value of the biodiesel was within the permissible limit, i.e., less than 0.50 mg KOH/g. If the acid value is higher, it might lead to corrosion of engine parts (Wang et al. 2008). Kinematic viscosity of biodiesel was lower and was within the range of diesel standards as well. Higher viscosity leads to certain problems such as injection of highly viscous fuel may result in poor engine operations due to poor atomization of the fuel (Rodrigues et al. 2006). Calorific value or gross heat of combustion of biodiesel was found to be within the specified range. However, it was considerably less than the calorific values of diesel. The presence of unsaturated fatty acids esters such as methyl oleate leads to low calorific value of biodiesel (Lateef et al. 2014). Carbon residue of biodiesel was within the specified range. Diesel on the other hand has a very high value. This parameter basically highlights the coke forming ability of the fuel. In the engine system, the fuel undergoes the pyrolysis process resulting in the formation of carbon deposits. Thus, diesel has a very high carbon-to-hydrogen ratio which results in the high carbonaceous content during the burning of the fuel. Biodiesel has a low carbon-to-hydrogen ratio, and thus, it accounts for a low carbon deposit (Knothe et al. 2005). Cloud point and pour point of biodiesel were found to be low in comparison with that of diesel fuel. This phenomenon can be attributed to the presence of unsaturated fatty acid contents in the methyl ester. These acids do not crystallize rapidly at lower temperatures in comparison with that of saturated fatty acid esters. Diesel on the other hand is free from fatty acid component, so it has a low melting point. Thus, biodiesel can operate in cold conditions efficiently (Rodrigues et al. 2006).
The fatty acid esters that were analyzed by using gas chromatography in the methyl ester, i.e., biodiesel are oleic acid (C18:1), stearic acid (C18:0), palmitic acid (C16:0), linoleic acid (C18:2), linolenic acid (C18:3) as represented in Table 5. Number denotes the number of carbon and double bonds. For example, while describing the chemical composition of oleic acid as C18:1, 18 represents 18 carbons and 1 double bond.
Figure 10 shows the fatty acid composition of the biodiesel. It mostly comprised of unsaturated fatty acid components with oleic acid ester being the highest in composition followed by linoleic acid ester. The saturated portion basically comprised of palmitic acid ester being the highest followed by stearic acid ester.
The biodiesel produced has high weightage of unsaturated content; it has low oxidative stability (Mittelbach and Gangl 2001). It is hence not efficient from the long-term storage point of view as oxidation might lead to formation of certain compounds which leads to its deterioration. Unsaturated fatty acids have low melting point in comparison with saturated fatty acids. During cloud and pour point study, the prepared sample had shown low values which underlined its utility at low-temperature conditions effectively and hence can be used in colder regions (Chakarbarti and Prasad 2012). As the degree of unsaturation increases, the kinematic viscosity decreases. So biodiesel having high unsaturation has low viscosity. The biodiesel prepared in this study has low kinematic viscosity, which is favorable for injection system of the engine. The presence of unsaturated fatty acid ester imparts lubricity to biodiesel. So adding biodiesel to diesel may enhance the lubricity of the petroleum diesel in spite of adding other additives to it.
Three emission parameters were analyzed by operating the variable compression ratio engine fueled with biodiesel blends at varying break loads. The exhaust emissions were analyzed for three different emissions—nitric oxide, nitrogen dioxide and carbon monoxide. The best result for a lower range of emissions of nitrogen di-oxide and nitric oxide during the combustion process of biodiesel is obtained on implementing the B10 blend for driving the engine. On the other hand, B50 blend accounted for a very low carbon monoxide emission. Overall it can be concluded that B10 blend can be used as a fuel to run a diesel engine effectively with minimum nitrogen dioxide, nitric oxide and carbon monoxide emissions. It is observed from Figs. 11 and 12 that nitrogen dioxide and nitric oxide emission was higher in comparison with that of mineral diesel. With an increase in blending ratio, the emission characteristics also depicted an increase in the nitrogen dioxide and nitric oxide emissions. The increase in the number of double bonds in the molecules of the unsaturated fatty acids results in high molecular weight of biodiesel. Thus, during combustion process when the fuel burns, it results in a very high temperature in the engine because of the high flame temperature. This influences the greater emissions of nitrogen dioxide. This is the basis of Zeldovich mechanism (Palash et al. 2013).
It was observed from Fig. 13 that with an increase in the blending ratio the emission of carbon monoxide was found to be low. The high temperature during the combustion of the fuel resulted in the complete burning of the fuel and thereby leading to decrease in the carbon monoxide emissions (Geong et al. 2006).
Conclusion
Using waste cooking oil for deriving energy by producing biodiesel is a feasible and attainable waste management option. Waste cooking oil has a great potential to be used as a feedstock for the waste to energy process. Optimization of transesterification resulted in a very high efficiency for the conversion of waste cooking oil to the biodiesel, i.e., 93.8% yield at 60 °C, 1% catalyst concentration and molar ratio of 5:1. The biodiesel that was produced adhered to standard biodiesel specifications for acid value, kinematic viscosity, calorific value and carbon residue which implies that it can be used as a fuel. Cloud point and pour point values of prepared biodiesel suggested its effective use in colder regions. This aspect was further confirmed by results of gas chromatography also, which also depicted its low oxidative stability, less kinematic viscosity and its lubricity. It can be concluded that B10 blend can be used as a fuel to run a variable compression ratio diesel engine with minimum NO2, NO and CO emissions.
References
Aradhey A (2014) Biofuels annual, New Delhi, India. Global agricultural information network report no. IN4055, United States Department of Agriculture (USDA) Foreign Agriculture Service
Carlinia M, Castelluccib S, Cocchia S (2014) A pilot scale study of waste vegetable oil transesterification with alkaline and acidic catalyst. Energy Procedia 45:198–206. doi:10.1016/j.egypro.2014.01.022
Cetinkaya M, Karaosmanoǧlu F (2004) Optimization of base-catalyzed transesterification reaction of used cooking oil. Energy Fuel 18:1888–1895. doi:10.1021/ef049891c
Chakarbarti PP, Prasad RBN (2012) Biodiesel production from jatrophacurcas oil. Jatropha Chall New Energy Crop 1:463–490. doi:10.1007/978-1-4614-4806-8_25
Cunha A, Feddern V, Marna C, Higarashi MM, Abreu P, Coldebella A (2013) Synthesis and characterization of ethylic biodiesel from animal fat waste. Fuel 105:228–234. doi:10.1016/j.fuel.2012.06.020
Freedman B, Pryde EH, Mounts TH (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc 61:1638–1643. doi:10.1007/BF02541649
Geong GT, Oh YT, Park DH (2006) Emission profile of rapeseed methyl ester and its blend in a diesel engine. Appl Biochem Biotechnol 129(1):165–178. doi:10.1385/ABAB:129:1:165
Gopal N, Pal A, Sharma S, Samanchi C, Satyanarayanan K, Elango T (2014) Investigation of emission and combustion characteristics of CI engine fueled with waste cooking oil methyl ester and diesel blends. Alexendria Eng J 53:281–287. doi:10.1016/j.aej.2014.02.003
Kabir I, Yacob M, Radam A (2014) Awareness, attitudes and practices regarding waste cooking oil recycling in Petaling, Malaysia. J Environ Sci Toxicol Food Technol 8(10):45–51
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83(10):823–833. doi:10.1007/s11746-006-5033-y
Knothe G, Gerpen JV, Krahl J (2005) The biodiesel handbook. AOCS Press, Urbana
Kwon MH, Yeom SH (2015) Optimization of one-step extraction and transesterification process for biodiesel production from the marine microalga Nannochloropsis sp. KMMCC 290 cultivated in a raceway pond. Biotechnol Bioprocess Eng 20(2):276–283. doi:10.1007/s12257-014-0599-y
Lateef FA, Onukwuli OD, Okoro UC, Ejikeme PM, Jere P (2014) Some physical properties and oxidative stability of biodiesel produced from oil seed crops. Korean J Chem Eng 31(5):725–731. doi:10.1007/s11814-014-0028-0
Mahesh ES, Ramanathan A, Begum MS, Narayanan A (2015) Biodiesel production from waste cooking oil using KBr impregnated CaO as catalyst. Energy Convers Manag 91:442–450. doi:10.1016/j.enconman.2014.12.031
Mata TM, Martins AA, Caetano NS (2012) Valorization of waste frying oils and animal fats for biodiesel production. Adv Biofuel Bioprod 1:671–693. doi:10.1007/978-1-4614-3348-4_28
Mittelbach M, Gangl S (2001) Long storage stability of biodiesel made from rapeseed and used frying oil. J Am Oil Chem Soc 78(6):573–577. doi:10.1007/s11746-001-0306-z
Palash SM, Kalam MA, Masjuki HH, Masum BM, Fattah IMR, Mofijur M (2013) Impacts of biodiesel combustion on NO x emissions and their reduction approaches. Renew Sustain Energy Rev 23:473–490. doi:10.1016/j.rser.2013.03.003
Reed TB, Graboski MS, Gaur S (1993) Biodiesel from waste vegetable oils. Adv Thermochem Biomass Convers. doi:10.1007/978-94-011-1336-6_119
Rodrigues JA Jr, Cardoso FP, Lachter ER, Estevao LRM, Lima E, Nascimento RSV (2006) Correlating chemical structure and physical properties of vegetable oil esters. J Am Oil Chem Soc 83(4):353–357. doi:10.1007/s11746-006-1212-0
Saqib M, Mumtaz MW, Mahmood A, Abdullah MI (2012) Optimized biodiesel production and environmental assessment of produced biodiesel. Biotechnol Bioprocess Eng 17(3):617–623. doi:10.1007/s12257-011-0569-6
Sunthitikawinsakul A, Sangatith N (2012) Study on the quantitative fatty acids correlation of fried vegetable oil for biodiesel with heating value. Procedia Eng 32:219–224. doi:10.1016/j.proeng.2012.01.1260
Valente S, Pasa VMD, Belchior CRP, Sodre JR (2011) Physical-chemical properties of WCO biodiesel and castor oil biodiesel blends. Fuel 90:1700–1702. doi:10.1016/j.fuel.2010.10.045
Wang H, Tang H, Wilson J, Salley SO, Ng KYS (2008) Total acid number determination of biodiesel and biodiesel blends. J Am Oil Chem Soc 85(11):1083–1086. doi:10.1007/s11746-008-1289-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sodhi, A.K., Tripathi, S. & Kundu, K. Biodiesel production using waste cooking oil: a waste to energy conversion strategy. Clean Techn Environ Policy 19, 1799–1807 (2017). https://doi.org/10.1007/s10098-017-1357-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-017-1357-6