Abstract
Most soda ash originates from the Solvay process that involves the aqueous reaction of NaCl, CO2, and NH3 rendering, after five steps, sodium carbonate as well as solid and liquid wastes. In the final stage, the NH4Cl originated in the first step reacts with Ca(OH)2 (lime milk), recovering most of the NH3, recycled in the process. In this work, we propose the use of steel slag, a low cost and abundant waste material of siderurgy, as source of CaO in the Solvay process. In our approach, the lime milk is partially replaced by steel slag in the NH3 recycling, recovering up to 40 wt% of the total ammonia. It can decrease the amount of CaCO3 and fossil fuels required and consequently decrease the amount of wastes originated in the procedure. In addition, we found that a simple thermal treatment of the mother liquor, immediately before the reaction with Ca(OH)2, could recover about 17–26 wt% of the NH3, reducing reagent demands and consequently effluent amounts. Another advantage is the lesser Ca(II) detected in the waste waters and the fewer caustic sludge produced in this new process. Despite it is a preliminary work, at the laboratory scale, our procedure suggests a synergic connection of the steel and soda ash productions that might reduce energy and costs, as well as the carbon dioxide emissions related to both the Na2CO3 and steel industries.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1861, Ernest Solvay patented a continuous process for the production of sodium carbonate (soda ash), replacing Leblanc’s method. After more than 150 years, the so-called “Solvay Process” is still known for its economic feasibility and careful integration of chemical and physical operations. The starting materials, salt brine, NaCl(aq), and limestone, CaCO3, are readily available at low cost, and most of the ammonia utilized in the process can be recovered. All together, these factors make the overall methodology very attractive. Sodium carbonate, a major commodity, is an essential raw product for many industrial applications, for example, in the production of glass, detergents, and papers (Steinhauser 2008). More than three quarters of the Na2CO3 expend nowadays are produced by this ammonia-based soda process. The remaining is supplied by natural resources, for example, from mines of Trona—sodium sesquicarbonate, Na2CO3·NaHCO3·2H2O in US, among other occurring minerals.
Reactions from I to V represent the chemical transformations occurring in each step of the Solvay process. Enthalpy and Gibbs free energy at standard conditions provided for each reaction were estimated utilizing HSC Chemistry 6.1 software.
The first step comprises the chemical reaction of NaCl(aq), CO2(g), and NH3(g), resulting in NaHCO3(s), NH4Cl(aq), unprocessed reagents, and other products. After vacuum filtration, which separates the solid sodium hydrogen carbonate from the mother liquor, the crude NaHCO3 is calcined, at temperatures ranging between 160 and 230 °C, producing soda ash (Na2CO3), water, and carbon dioxide (step II). The carbon dioxide required in the process is produced by limestone calcination at 950–1100 °C, which is converted into quicklime (CaO) and CO2, represented by step III. The carbon dioxide produced by reactions II and III is utilized in reaction I. In step IV, calcium oxide is transformed into a milky suspension, Ca(OH)2, which is utilized in the ammonia recovery process. The required ammonia is reclaimed in step V by distilling the mother liquor in the presence of lime milk (Kasikowski et al. 2004; Gao et al. 2007; Steinhauser 2008). When properly designed and operated, a Solvay plant can reclaim almost all the required ammonia and consumes only small amounts to replace losses. The major inputs are salt, limestone, and thermal energy that alone represents ca. of 30 % of the Solvay costs (Trypuc and Lyjak 2001; Trypuc and Druzynski 2007; Trypuc and Bialowicz 2011).
Conventional production of soda ash is accompanied by large amounts of undesirable liquid and solid wastes (Trypuc and Lyjak 2001). The amount of liquid waste after step V can be 10 times greater than that of the produced soda ash (Trypuc and Bialowicz 2011). The liquid effluent comprises CaCl2, unreacted NaCl, small amounts of CaCO3, and Ca(OH)2, and it is usually discharged in rivers, lakes, or in the sea. The caustic sludge, consisting mainly of CaCO3, SiO2, CaO, Mg(OH)2, and CaSO4, is normally used as fertilizer, abandoned in inactive salt mines, or disposed in water reservoirs (Gao et al. 2007; Steinhauser 2008). The necessary effluent control and residues disposal make Solvay process less competitive than natural soda ash from Trona (sodium sesquicarbonate, Na2CO3·NaHCO3·2H2O). American natural soda ash has a sale price two times lower than that from the Solvay production (Kasikowski et al. 2004).
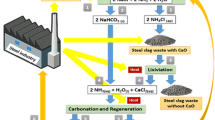
In view of these drawbacks, we have studied possible modifications in the Solvay method, aiming to improve efficiency by reducing energy inputs and diminishing the amount of wastes. Consequently, this work has been focused on the use of industrial wastes as sources of chemicals required in the ammonia-based soda process. Among various possible materials, we have chosen the steel slag, a low cost residue of the iron metallurgy, with high Ca(II) and Mg(II) oxides concentration and easily found in the siderurgy areas (Chiang et al. 2011; Eloneva et al. 2008). In general lines, we propose two modifications in the Solvay process, Fig. 1, as follows:
-
(a)
Partial replacement of lime in the regeneration of ammonia, step V, using steel slag milk.
-
(b)
A thermal treatment of the mother liquor, prior to step V, reclaiming part of the NH3 used in step I.
Therefore, we believe these results can serve as starting point for a cleaner production of NaHCO3, through a synergic eco-industrial network connecting the steel and soda ash productions (Alnouri et al. 2014; Jing et al. 2013; Murillo-Alvarado et al. 2013; Gutiérrez-Arriaga et al. 2015; Li et al. 2015). Thus, resources might be shared (raw materials, water, energy, infrastructure, and informations), minimizing wastes, environmental impact, and enhancing economic performance.
Materials and methods
Material and reagents
The industry that has donated the steel slag will remain unidentified. All chemical reactions were performed with deionized water. The chemicals NaCl, NH4Cl, NH4OH, EDTA, HCl, NaOH, and KBr were purchased from Merck Company and used as received. Methyl orange and Eriochrome black T were purchased from Sigma-Aldrich Company, and the cylinder of CO2 was purchased from Linde.
Chemical procedures and equipment
The mother liquor comprises a complex mixture of ions: Na+, NH4 +, HCO3 −, CO3 2−, and Cl−, dominated by the amount of NH4 + and Cl−. In order to study possible modifications in the Solvay process, several reactions between steel slag and NH4Cl solutions have been examined regarding the influence of reaction time, temperature, and ammonium chloride concentration. In addition, we have evaluated how reduced concentrations of HCO3 − in the mother liquor can improve the amount of the recovered NH3.
The steel slag reaction with ammonium chloride solution
Chemical reactions of steel slag with NH4Cl have been carried out at 85 °C for 45 min with aqueous ammonium chloride solutions (0.1 and 1 mol L−1), in a closed system, varying the steel slag/NH4Cl solution ratio. The produced NH3 was monitored by titration with HCl using methyl orange as indicator. The amount of Ca(II) and Mg(II) in solution was determined by titration with EDTA and using Eriochrome black T as indicator. The presence of other cations leached from the steel slag was measured by atomic absorption spectroscopy in the Varian atomic absorption spectrometer (AA 240 FS) under the following conditions: wavelength 248.3 nm, slit 02, and acetylene gas flame. The steel slag was chemically characterized before and after leaching with ammonium chloride by X-ray diffraction, infrared spectroscopy, and thermal analysis. XRD patterns were collected with a Siemens D5000 instrument using a Ni-filtered Cu Kα radiation (λ = 1.5418 Å) and a graphite monochromator in the diffracted beam. A scan rate of 1o/min was applied to record a pattern in the 2θ range of 5°–70°. Silicon was used as internal standard. The XRD lines were encountered in JCPDS (Joint Committee on Powder Diffraction Standard). Infrared spectra were obtained from a Perkin-Elmer Spectrum RX FT-IR spectrometer, using KBr disks in the 4000–400 cm−1 region with 64 scans and 4 cm−1 of spectral resolution. Thermogravimetry (TG) and Differential Thermal Analysis (DTA) were carried out by DTG-60 Shimadzu instrument at temperatures ranging from ambient to 900 °C with a heating rate of 10 °C min−1 in argon, with a flux of 30 mL min−1. The TA program 60 was used to determine the temperatures and heat involved in thermal events. The chemical composition of the steel slag was determined using an Energy Dispersive X-ray Fluorescence Spectrometer Shimadzu EDX-720. The following operating conditions were selected: voltage of 15 kV tube (Na–Sc) and 50 kV (Ti-U) with current in the tube 188 and 37 μA, respectively; collimator 10 mm; real time integration of 300 s; dead time of the detector 40 and 39 %, in vacuum and detector Si(Li) cooled with liquid nitrogen. The analytical method used is called the fundamental parameters method, and it was implemented within the equipment’s software.
The influence of temperature, time of reaction, steel slag/NH4Cl solution ratio, and NH4Cl solution concentration in the chemical reaction between steel slag and NH4Cl
The influence of temperature in the reaction was evaluated by treating 2 g of steel slag with 100 mL of a 0.3 mol L−1 ammonium chloride solution (2 wt% steel slag/NH4Cl solution ratio). The stirring speed was kept at 300 rpm during the 30 min reaction conducted in temperatures ranging from 33 to 85 °C. The time of reaction varied from 0 to 90 min, with a fixed temperature of 85 °C. Steel slag/NH4Cl mixtures, in the range of 2–50 wt%, were stirred during 45 min at T = 85 °C, with fixed amount of ammonium chloride (0.3 mol L−1). In another experiment, the concentration of NH4Cl solution varied from 0.1 to 5 mol L−1 using T = 85 °C, t = 45 min, and 2 wt% steel slag/NH4Cl solution ratio. Such parameter ranges were chosen based on the operating conditions of a normal Solvay process and the typical composition of the mother liquor (Trypuc and Bialowicz 2011). The leaching efficiency was evaluated by the amount of Ca(II) found in solution. The extraction of Ca(II) from the steel slag was calculated using the expression
where C is the Ca(II) concentration (g L−1) in the NH4Cl solution after reaction with the steel slag; V is the volume of the NH4Cl solution (L), and M is the amount of steel slag (g).
Ammonia recovery with steel slag in a simulated Solvay process at laboratory scale
Ammoniated brine was prepared by adding 26 g of NaCl (100 mL of deionised water) and 8.3 % ammonia (Shreve 1945). An immediate carbonation was observed by injecting pure CO2 at room temperature, leading to NaHCO3 precipitation. The CO2 injection proceeded until pH = 7.5. Then NaHCO3 was filtered, and the resulting solution (mother liquor) was boiled, for 30 min, to evaluate the effect of HCO3 − ions in the thermal decomposition. The concentrations of ammonium chloride and hydrogen carbonate in the mother liquor solution during the thermal treatment were studied by automatic potentiometric titration with HCl and NaOH. The remaining mother liquor was treated with steel slag (2 wt% steel slag/NH4Cl solution ratio) at 85 °C, during 45 min, under constant stirring (300 rpm). The resulting amount of NH3 was monitored by titration with HCl using methyl orange as indicator. The amount of Ca(II) and Mg(II) was evaluated using EDTA titration. The presence of other cations was monitored by atomic absorption spectroscopy.
Results and discussion
The efficiency of the Ca(II) leaching in the steel slag with NH4Cl and the NH3 recover
Before using steel slag in the Solvay process, we have performed the reaction of this waste with pure NH4Cl. It was carried out in order to determine the best amount of steel slag, the most favorable concentration of NH4Cl, the temperature of the reaction, and the influence of pH. Results are shown in Table 1. Granulometry was not monitored since the size of the used powder was roughly constant (<50 µm). The reaction between steel slag and NH4Cl occurs in more than one step (Kodama et al. 2008):
In view of the low kps of calcium hydroxide, 7.9 × 10−6, at the equilibrium, the amount of dissolved Ca(OH)2 shall be very small, Eq. (7).
The effect of temperature
The steel slag and NH4Cl chemical reaction are strongly influenced by changes in the temperature, Fig. 2a; however, the best one has been set at 90 °C, since more heat might imply in pressurized reaction vessels in order to avoid losses of water to the vapor phase, therefore raising costs. The extraction of Ca(II) from the steel slag to the solution increases the consumption of calcium oxide according to the reaction mechanism between the steel slag and NH4Cl, Eqs. (6) (7), and (8), contributing to the conversion of ammonium into NH3.
The effect of NH4Cl concentration
The concentration of ammonium chloride solutions is another parameter that influences the leaching efficiency of Ca(II) and the NH3 recover. The results display in Fig. 2b have been obtained by studying how the amount of leached Ca(II) is affected by the action of different solutions of NH4Cl in 2 wt% steel slag/NH4Cl solution ratio. Despite the leaching of Ca(II) have been performed by using solutions of NH4Cl with concentrations varying from 0.1 to 5.0 mol L−1, concentrations greater than 1.5 mol L−1 have not improved the amount of Ca(II) in the leachate. As shown in Table 1, for a concentration of 0.1 mol L−1 (NH4Cl), the amount of Ca(II) raises from 336 to 752 mg L−1 as the steel slag ratio increases from 2 to 50 wt%. In this situation, the amount of NH3 ranges from 0.027 to 0.040 mol L−1, which corresponds to a recovery ratio between 27 and 40 wt%. For a concentration of 1 mol L−1 (NH4Cl), the amount of Ca(II) in the solution is 5520 mg L−1 and that of generated NH3 is 0.307 mol L−1, corresponding to a recovery ratio of 30.7 wt%. Therefore, it is possible to conclude that the reaction between steel slag and ammonium chloride is favored by NH4Cl solutions up to 1 mol L−1.
The effect of the time of reaction
The extraction of Ca(II) from the steel slag does not increase greatly after 60 min of reaction, reaching a maximum efficiency of 5 wt%, Fig. 2c. Thus, it is possible to say that after 60 min, the reaction reaches the equilibrium, Eqs. (6), (7), and (8), and no further conversion of ammonium into ammonia is detected beyond this time.
The effect of steel slag/NH4Cl solution ratio
This study was performed in order to investigate how different amounts of steel slag affect the process. We have observed that increasing amounts of steel slag, 2, 5, 10, 30, and 50 wt%, added to NH4Cl solutions (0.3 mol L−1) increase not only pH, due to Ca(OH)2, but also the amount of Ca(II) in solution and NH3, Table 1. However, this tendency stabilizes even after the addition of generous amounts of steel slag, since no significant changes have been detected in pH, in the Ca(II) concentration or in the amount of NH3. It is expected that the amount of Ca(II) in the solution for 30 wt% steel slag ratio would be three times the concentration for 10 wt% ratio. Nonetheless, this behavior was not observed in the experiments. As the steel slag ratio increases, its reactivity with the NH4Cl solution decreases, diminishing the amount of leachate, Fig. 2d. Consequently, the ammonia formed in the reaction remains between 27 and 40 wt%, as shown in Table 1. Three reasons may be associated to that (a) the increasing amount of water contributes to the Ca(II) solubilization and ammonium conversion, Eqs. (6), (7), and (8); (b) the higher buffer effect of the NH4 +/NH3 mixture is reached at pH = 9.25. In this pH, NH4 + and NH3 concentrations are the same, and the inter-conversion between this chemical species, Eq. (9), prevents further Ca(II) extraction; (c) we have observed a drastic change in the fluidity of the mixture as more steel slag is added to the same NH4Cl solution, preventing proper mixing and consequently decreasing Ca(II) extraction. Despite this apparent drawback, an efficiency of NH3 recovery between 27 and 40 wt% is still a good result, in view of a possible use to replace lime in step III of the Solvay process.
The maximum steel slag leaching efficiency is reached at [NH4Cl] = 1.5 mol L−1, T = 90 °C, t = 60 min, and steel slag/NH4Cl solution ratio of 2 wt%. In this conditions, the maximum Ca(II) extraction reaches 5 wt% of the steel slag total mass, Fig. 2b, d, which is much smaller than the total amount of Ca(II) in the steel slag (28.5 wt%; calculated by % CaO × Ca molar mass/CaO molar mass), Table 2.
Composition of the mother liquor (bench scale)
The carbonation of ammoniated brine, at bench scale, resulted in the formation of a solid product with a yield of 65 %, compared to the weight of NaCl. The remaining hydrogen carbonate concentration was 0.7 mol L−1 as measured by titration with HCl.
After the addition of HCl and a gentle heating, to remove HCO3 − and CO2, the concentration of NH4 + in the mother liquor was found at 4.2 mol L−1 by titration with NaOH. Therefore, a small loss of ammonia might have happened since we expected an ammonium concentration of 4.9 mol L−1.
Thermal treatment of the mother liquor of a Solvay process at bench scale
We have simulated a Solvay process at laboratory scale in order to study the generation of the mother liquor. Despite the several possible crossing reactions, our study reveals an inverse dependence of the ammonia recovery efficiency and the remaining amounts of HCO3 − in solution. A reflux of the mother liquor for 30 min, preceding the addition of lime milk, can lead to the thermal decomposition of HCO3 − into CO3 2−, CO2, and H2O (Vogel 1981), reducing the concentration of HCO3 − from 0.7 to 0.05 mol L−1. We believe that the formed carbonate can react further with NH4 + yielding hydrogen carbonate and NH3:
According to Eq. (10), the HCO3 − can be thermally removed until no further CO3 2−, CO2, or HCO3 − is left. Hence, the concentration of NH4 + in the mother liquor decreased from 4.2 to 3.5 mol L−1. Thus, 16.7 wt% of ammonia could be recovered just by heating the mother liquor prior the reaction with lime milk. According to literature data regarding the mother liquor composition under typical operating conditions (Trypuc and Bialowicz 2011), the regeneration of NH3 through mother liquor boiling in a real Solvay process could vary from 17 to 26 wt%, depending on how rich the mother liquor is in terms of hydrogen carbonate anion. In view of the reaction stoichiometry, 56 kg of lime, coming from 100 kg of limestone, is required to reclaim 34 kg of NH3. An efficient control of this proposed heating cycle can decrease the amount of lime from 17 to 26 wt%, and therefore less Ca(II) will be found in the wastewaters. Reduced amounts of HCO3 − before the addition of lime milk can also decrease the quantity of caustic sludge (CaCO3) (Gao et al. 2007), produced according to Eq. (11).
Approximately, 35 kg of caustic sludge is avoided in the mother liquor for each cubic meter of solution. The necessary heat might come from water vapor easily found in Solvay plants.
Reaction of steel slag with the mother liquor of the Solvay process at bench scale
After setting the effects of temperature, time, concentration of NH4Cl, and steel slag ratio, we have studied the use of this waste in the bench-scaled Solvay process. After step I, the solid NaHCO3 was filtered, and the resultant mother liquor was used in this procedure. As discussed previously in this paper, the concentration of NH4Cl was found at 4.2 mol L−1. We have observed that the direct reaction of steel slag with this mother liquor is ineffective, Table 3 (i). The presence of hydrogen carbonate somehow decreases the efficiency of the leaching reaction, and small amounts of Ca(II) are detected in solution, 192 mg L−1, Table 3. However, after thermal treatment, Table 3 (ii), which decomposes the HCO3 − ions, the Ca(II) concentration in solution reaches 1520 mg L−1, which is close to 1840 mg L−1, Table 3 (iii), obtained using pure ammonium chloride. It was possible to recover 2 wt% of the total ammonia from the mother liquor when 2 wt% of steel slag is utilized in the reaction, Table 3. Although this ammonia recovery efficiency is quite low, the amount of steel slag cannot be continuously increased, because its reactivity with ammonium chloride is not favoured with steel slag ratios above 25 wt%, Fig. 2d, or in NH4Cl concentrations greater than 1.5 mol L−1, Fig. 2b. Therefore, we believe several ammonia recovery stages would be required, in which fresh steel slag would be fed in each stage. In this manner, it would be possible to reach the maximum ammonia recovery efficiency with steel slag, (40 %), as shown in Table 1.
A possible drawback of this process relates to the presence of undesired species, Mn(II), Cr(III), Fe(III), Ni(II), and Al(III) and their related silicates and aluminosilicates. However, we have observed that NH4Cl present in the mother liquor reacts with the steel slag with certain selectivity (Kodama et al. 2008), since little amounts of these cations were found in solution, Table 4.
Chemical characterization of steel slag at bench scale
Steel slag was chemically characterized before, (a), and after, (b), the reaction with the mother liquor at bench scale, Fig. 3. The X-ray diffraction patterns revealed little chemical difference on going from (a) to (b). Both samples comprised wollastonite (CaSiO3) (W) and gehlenite (Ca2Al2SiO7) (G), and the main crystalline phases of CaO were not detected. Perhaps, it is present as an amorphous phase.
The NH4Cl present in the mother liquor could not chemically react with dead-burnt CaO or with calcium silicate or aluminosilicate. Therefore, we believe that the mother liquor targets only the amorphous Ca(II) or Mg(II) in the form of oxide or hydroxide (Bonenfant et al. 2008). This would explain the poor Ca(II) extraction from the steel slag, reaching a maximum of 5 wt%. As shown by the X-ray diffraction, most of the Ca(II) present in the steel slag belongs to the crystalline silicate structure.
Infrared spectroscopy and thermal analysis experiments showed no difference in the steel slag before and after the reaction with the thermally treated mother liquor, which confirms that no significant changes occurred in the steel slag chemical structure, Figs. 4 and 5.
Simulation of the chemical equilibrium between steel slag and NH4Cl
Chemical equilibrium compositions for the system steel slag/NH4Cl solution were calculated utilizing the software HSC Chemistry 6.1. This program is able to evaluate chemical equilibrium compositions based on Gibbs free energy minimization. In such simulations, the temperature was fixed at 85 °C, and pressure of 1 bar was utilized. The NH4Cl solution concentration varied from 0.1 to 5 mol L−1 in step sizes of 0.1 mol L−1 in each simulation. Several simulations were performed, varying the steel slag/NH4Cl solution ratio from 2 to 50 wt%. The steel slag composition utilized in the simulations is shown in Table 2, Fig. 6.
When the steel slag ratio is 2 wt%, ions present in the steel slag other than Ca(II) are solubilized in the solution, Fig. 6. Although leaching is favored, the amount of steel slag is small, and the conversion of ammonium into NH3 rapidly reaches a maximum and does not increase further. The conversion ratio at this point is 7.3 wt%, and virtually all CaO is extracted from the steel slag. When the steel slag/NH4Cl ratio increases to 50 wt%, not all CaO is extracted from the solid phase, but the ammonia recovery reaches 80 wt%. These theoretical calculations comply with experimental results; however, theoretical results show higher recovery efficiencies since losses, buffering, and mixing effects are not taken into account. In addition, most of the Ca(II) in the steel slag is not in the form of oxide but in the form of silicates, decreasing the ammonium conversion. According to the experiments, conversions between 27 and 40 wt% are reachable.
Energy consumption
The overall Solvay process is
The production of each ton of NaHCO3 requires 1.54 tons of limestone, considering an efficiency of 69 % (Trypuc and Bialowicz 2011). Modern lime kilns requires 3.5 GJ fuel ton−1 CaO (Halmann and Steinfeld 2003) or 3 GJ for the calcination of 1.54 tons of CaCO3. In many plants, this amount of calcium carbonate comes from the burning of 172 kg of coal (Halmann and Steinfeld 2003). Consequently, the use of steel slag partially replacing lime milk might reduce the fuel consumed in step III of the Solvay process, settled at 12 US$ for each ton of Na2CO3 (Infomine 2015).
Environmental impacts
The modified Solvay process suggested in this paper can diminish the corresponding carbon emissions. The partial replacement of lime milk reduces the importance of step III of the normal Solvay. In this step, limestone is calcined to produce CaO and CO2. The former reagent is depleted in the next step, and CO2 returns to step I. Not only step II and III contributes to CO2 emissions of Solvay process, but also the burning of fossil fuel, to provide the energy required in the calcination. The proposed approach can also reduce CO2 emissions and limestone transportation costs. The amount of emitted CO2 ranges from 0.9 × 107 to 1.5 × 107 ton for each ton of CaCl2, disposed or not (Forster 2012). We estimate reductions between 8 and 20 % in CO2 emissions, which could imply in an insufficient amount of carbon dioxide required for the carbonation step. A process simulation is being developed in order to better quantify this issue and investigate the impacts of such changes in this approach. As suggested in this paper, if this new Solvay plant is built near a siderurgy, the latter can provide the necessary carbon dioxide in the carbonation step, heat, and the steel slag, therefore reducing its net CO2 emissions and raw materials consumption. This synergic approach can have positive consequences for cleaner industrial productions, Fig. 7. The steel production is one of the major CO2 emitting industrial activity. The main carbon dioxide source is the blast furnace, whose exhaust gases contain between 14 and 33 % of CO2 (IEA 2013). In addition, for every ton of steel, 150 kg of steel slag is produced, as well as a considerable amount of heat (Zhang et al. 2013). The steel slag is produced at temperatures above 1000 °C; however, this waste heat is usually not recovered as in other cases.
A possible disadvantage of our proposal concerns the amount of the generated residue, which cannot be ignored (0.5 kg L−1 of mother liquor). However, steel slag is already a residue in the steel industry, which is normally commercialized as asphalt concrete aggregate, granular base, or used in construction applications (FHWA 2015). Perhaps, the huge amount wastewater is the real concern. Nevertheless, as in most industries before disposal, it might be recycled in order to recover water and remove heavy metal cations.
Since it is a preliminary work, we have not provided a balance of costs and energy input. Such calculations might be more appropriate in a pilot plant, where the effects of our approach in the overall process can be looked more closely. For example, (a) the reduction of the amount of Ca(II) in the solid and liquid wastes; (b) the reduction of the amount of lime and lime milk; (c) the effect of an increased time of the NH3 regeneration; and (d) the costs of crushing, grinding, and with the transport of the steel slag to the Solvay plant.
Conclusions
We have proposed a modified Solvay process in which lime can be partially replaced by steel slag, in the NH3 recovery step of the Solvay process. It was found that it is possible to recover 2 wt% of the total ammonia when the steel slag/NH4Cl solution ratio is 2 wt%, and hydrogen carbonate ions are not present. Increasing the amount of steel slag and dividing the leaching process in several steps, it would be possible to achieve 40 wt% efficiency of the ammonia recovery. Energy and raw material costs could be reduced, because of the partial elimination of the calcination of limestone, which is the most energy demanding reaction. However, an accurate energy balance must be carefully produced considering other possible variables.
We have observed that a simple thermal treatment of the mother liquor, before the reaction with milk of lime, can decompose HCO3 − into H2O and CO3 2−. The carbonate in solution reacts with NH4 + recovering approximately 17–26 wt% of the NH3 required in the process depending on the HCO3 − concentration in the mother liquor. The proposed modified Solvay can reduce the CO2 emissions, associated to step III. The amount of lime can decrease from 17 to 26 wt%, as well as the amount of caustic sludge (CaCO3) in the step of the NH3 recovery. Moreover, the amount of Ca(II) in the wastewaters is also reduced (17–26 wt%) in comparison to the normal Solvay process. Finally, we conclude the synergic integration of steel industry and Solvay plant, and it can produce positive results concerning costs and environment. Perhaps, it is the correct approach when sustainability is the focus of the production.
References
Alnouri SY, Linke P, El-Halwagi MM (2014) Water integration in industrial zones: a spatial representation with direct recycle applications. Clean Technol Environ Policy 16:1637–1659
Bonenfant D, Kharoune L, Sauve S, Hausler R, Niquette P, Mimeault M, Kharoune M (2008) CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind Eng Chem Res 47:7610–7616
Chiang PC, Pan SY, Chen YH, Chen CH, Chang EE (2011) Performance evaluation for carbonation of steel-making slags in a slurry reactor. J Hazard Mater 186:558–564
Eloneva S, Teir S, Salminen J, Fogelholm CJ, Zevenhoven R (2008) Steel converter slag as a raw material for precipitation of pure calcium carbonate. Ind Eng Chem Res 47:7104–7111
FHWA (2015) http://www.fhwa.dot.gov/pavement/recycling/recsslag.cfm. Accessed 28 Jan 2015
Forster M (2012) Investigations for the environmentally friendly production of Na2CO3 and HCl from exhaust CO2, NaCl and H2O. J Clean Prod 23:195–208
Gao C, Dong Y, Zhang H, Zhang J (2007) Utilization of distiller waste and residual mother liquor to prepare precipitated calcium carbonate. J Clean Prod 15:1419–1425
Gutiérrez-Arriaga CG, Abdelhady F, Bamufleh HS, Serna-González M, El-Halwagi MM, Ponce-Ortega JM (2015) Industrial waste heat recovery and cogeneration involving organic Rankine cycles. Clean Technol Environ Policy 17:767–779
Halmann M, Steinfeld A (2003) Thermoneutral coproduction of calcium oxide and syngas by combined decomposition of calcium carbonate and partial oxidation/CO2-reforming of methane. Energy Fuels 17:774–778
IEA (2013) Global action to advance carbon capture and storage: a focus on industrial applications. IEA Publications, Paris
Infomine (2015) www.infomine.com/investment/metal-prices/coal/. Accessed 28 Jan 2015
Jing Z, Liu Gd, Hang G, Lian L, Shi-huai D (2013) A theoretical basis for the relationship between the industrial pollutant generation, abatement, emission and economy. Clean Technol Environ Policy 15:707–711
Kasikowski T, Buczkowski R, Lemanowska E (2004) Cleaner production in the ammonia–soda industry: an ecological and economic study. J Environ Manag 73:339–356
Kodama S, Nishimoto T, Yamamoto N, Yogo K, Yamada K (2008) Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 33:776–784
Li W, Cui Z, Han F (2015) Methods for assessing the energy-saving efficiency of industrial symbiosis in industrial parks. Environ Sci Pollut Res 22:275–285
Murillo-Alvarado PE, Ponce-Ortega JM, El-Halwagi MM, Segovia-Hernandez JG (2013) Optimal integration of gaseous emissions from new industrial plants with the surroundings. Clean Technol Environ Policy 15:93–110
Shreve RN (1945) The chemical process industries. McGraw-Hill Book Company, São Paulo, pp 276–278
Steinhauser G (2008) Cleaner production in the Solvay Process: general strategies and recent developments. J Clean Prod 16:833–841
Trypuc M, Bialowicz K (2011) CaCO3 production using liquid waste from Solvay method. J Clean Prod 19:751–756
Trypuc M, Druzynski S (2007) Investigation of the solubility in the NaVO3–NaNO3–H2O system. Ind Eng Chem Res 46:2688–2692
Trypuc M, Lyjak G (2001) Application of NaVO3 for the utilization of the after-filtration liquor from Solvay’s process. Ind Eng Chem Res 40:2188–2191
Vogel AI (1981) Química analítica qualitativa. Mestre Jou, São Paulo, pp 330–331
Zhang H, Wang H, Zhu X, Qiu YJ, Li K, Chen R, Liao Q (2013) A review of waste heat recovery technologies towards molten slag in steel industry. Appl Energy 112:956–966
Acknowledgments
This work was supported by CNPq and FAPEMIG—Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Carvalho Pinto, P.C., de Oliveira Carvalho, M.M., Linhares, F.M. et al. A cleaner production of sodium hydrogen carbonate: partial replacement of lime by steel slag milk in the ammonia recovery step of the Solvay process. Clean Techn Environ Policy 17, 2311–2321 (2015). https://doi.org/10.1007/s10098-015-0973-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-015-0973-2