Abstract
Conventional Solvay process, which utilizes CO2 to synthesize sodium carbonate, has been well-known for more than a century, but the only purpose of this process is to produce soda ash. Annually, significant amounts of CO2 are converted into soda ash using Solvay process; however, this conventional process does not sustain CO2 and waste cycle. Increasing the awareness of global warming and climate change linking to CO2 emission has pressured this industry to move for a more eco-friendly process that should exploit its potential for CO2 utilization and sequestration and thus contribute to both CO2 emission and waste management. In conventional Solvay process, to produce one mole of Na2CO3, at least one mole of CO2 might be emitted to atmosphere due to the use of CaO. Therefore, a number of studies have been done to explore novel processes or to modify the Solvay process in order for it to engage more in CO2 mitigation. This chapter introduces the most up-to-date modified Solvay process and novel pathways to produce soda ash and baking soda in the consideration of waste and CO2 utilization. Simultaneous waste and CO2 utilization offers a great opportunity for shifting to a green production, not only soda ash industry but others where their exhausted CO2, alkaline solid wastes, or reject brine can be utilized. However, there are challenges which require further research and technological development initiatives for the idea to be industrially implemented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Soda ash
- CO2 utilization
- CO2 emission
- Reject brine
- Slag
- Fly ash
- Layered double hydroxide
- Hydrotalcite
- Solvay process
9.1 Potential Contribution of Inorganic Waste in CO2 Emission Mitigation
9.1.1 Carbon Utilization with Alkaline Solid Waste
Industrial wastes such as slag or fly ash may have composition similar to cement; therefore, they have great potential to use as active additives to replace a certain fraction of cement in concrete [1,2,3]. Recent study indicated that the strength of cement mortar can be significantly enhanced by adding carbonated slag or fly ash [4, 5]. These wastes contain significant amount of CaO and MgO which has been extensively investigated for CO2 sequestration [6]. The most effective CO2 sequestration for those wastes is gas–liquid method in which slag is dispersed in water and then CO2 gas is introduced into the slurry. Divalent ions such as Ca2+ leached from slag react with dissolved CO2 according to Eqs. 9.1–9.3 to form calcium carbonate [7,8,9]. The CaCO3 reacts with Ca3Al2O6 (C3A) phase in concrete as shown in Eq. 9.4 that results in the formation of calcium carboaluminate hydrate (C3A · CaCO3 · 11H, where H stands for H2O) and (C3A · 0.5CaCO3 · 0.5Ca(OH)2 · 11.5H). The formation of calcium carboaluminate hydrate could contribute to enhance the mechanical strength of concrete [4]. With an estimated 400 million tonnes of iron and steel slag (for every tonne of steel produced, two tonnes of CO2 emission, 400 kg of blast and basic oxygen furnace slag, and 200 kg of electric arc furnace slag are generated) [10], 750 million tonnes of fly ash from coal-fired power plant [11], and huge quantity of alkaline waste from other industries’ discharges annually, this alternative could contribute to reduce a great amount of CO2 emission.
Global phosphate industry, mainly fertilizer producers, discharges approximately over 200 million tonnes of phosphogypsum annually (4.5–5.5 tonnes for one tonne of P2O5 produced), and about 85% of this is disposed without any treatment [12, 13]. This waste is dumped in large stockpile causing serious environmental impact. Phosphogypsum has high content of calcium sulfate, about 24–34 wt% as CaO and 48–58 wt% as SO3. This make it high potential for CO2 utilization and sequestration [14,15,16,17,18,19,20]. However, not only sequestration, this solid waste could be converted to marketable (NH4)2SO4 fertilizer [21]. CO2 utilization and (NH4)2SO4 fertilizer production is based on the conventional Merseburg process in which CaSO4 reacts with (NH4)2CO3 to form CaCO3 and (NH4)2SO4 as Eq. 9.5 [22, 23].
Conventionally, (NH4)2CO3 that previously produced by bubbling CO2 into ammonium solution is added into the slurry of gypsum or phosphogypsum. Recent studies reveal that bubbling CO2 or flue gas directly into ammonium-saturated phosphogypsum solution helps reduce reaction process from two steps to one step which could result in saving expense and lower production cost [8, 24, 25]. This modified Merseburg process is chemically described in Eq. 9.6. Accordingly, for every tonne of (NH4)2SO4 produced, 0.9 tonnes of CaCO3 is generated which can permanently sequester 0.4 tonnes of CO2, and 1.6 tonnes of waste gypsum is consumed [25]. With over 200 million tonnes of phosphogypsum from phosphate industry together with significant amount gypsum from flue gas desulfurization process in power plant [24], its reveals the high potential of gypsum waste and CO2 utilization to produce marketable products such as CaCO3 and (NH4)2SO4.
9.1.2 CO2 Utilization with Aqueous Inorganic Waste
Beside the solid alkaline wastes that contain mainly Ca2+ and Mg2+ with the large capacity of CO2 sequestration, there are also aqueous solution wastes that have high concentration of Na+, Mg2+, and Ca2+ such as reject brine from desalination plant [26, 27], saline brine from CO2 storage sites [28] or textile industry [29]. High demand on clean water due to the world population growth and scarcity of freshwater has driven the increase in the deployment of desalination plants worldwide. Desalination has become an important source of drinking water production with the capacity of estimated >86 million m3/day from >18,000 plants in 2015 [28]. Over 15,000 desalination plants were installed in 2009 in which reverse osmosis technology accounted for 80% and global market for reverse osmosis alone is predicted to reach $8.1 billion by 2018 [30,31,32]. Desalination is the most viable way that can produce enough clean water to meet the high demand. However, this method faces some issues that are high energy consumption and reject brine management and disposal. According to Dawoud and Mulla, every 1 m3 clean water produced results in 2 m3 of reject brine [33]. Advanced technologies may enhance the water recovery and reduce the reject flow, but the reject volume still remains very large. Reject brine disposal is a costly process which accounts for 3–33% of the total cost of desalination depending on the selection of disposal method [34]. The major constituents in reject brine comprise of calcium, magnesium, sodium cations, sulfate and chloride anions, and sometime antiscalants. Their concentrations are dependent on the water sources, desalination technologies, and water recovery ratio or concentration factor [27, 34]. Seawater desalination plant recovery is typically limited to 40–65%, and its total dissolved solid concentration usually ranges from 65,000 to 85,000 mg/l [27]. Reject brine disposal either inland or into the sea may result in negative environmental impact due to locally increasing in temperature and salinity; hence, a proper management and treatment procedure is required for the disposal [31, 35]. A number of methods such as solar evaporation, membrane distillation, electrodialysis, forward osmosis, zero liquid discharge, salt recovery have been proposed to reduce and eliminate the brine disposal in which the methods that combine brine treatment with the recovery of valuable component have drawn significant interest [31, 32, 35,36,37]. Recently, several studies have considered the recovery of salt and minerals from rejected brine in the context of CO2 capture and utilization [38,39,40,41,42]. Simultaneous CO2 and brine utilization is based on the reaction between CO2 and major inorganic cations prevailed in the rejected brine. Generally, CO2 utilization with brine can be divided into two methods: direct precipitation of earth alkaline cations of Ca2+ and Mg2+ by CO2 gas to form CaCO3 and MgCO3 [43,44,45,46] and indirect precipitation of Na+ to form sodium bicarbonate [41]. In the former method, less soluble carbonates removed from water can be value-added products; nevertheless, with the low Ca2+ and Mg2+ concentrations of several hundred to several thousand ppm, this method is potentially used for brine pre-treatment other than the recovery of CaCO3 for marketing [45], whereas, the latter method makes up a core technology for the industrial production of soda ash (Na2CO3) [47]. Soda ash is an important raw material used in many industries such as glass, chemicals, soaps and detergents, metallurgy, water treatment, pulp, and paper. Producing soda ash from rejected brine and CO2 is an opportunity for at the same time utilizing two waste streams in one product. By producing soda ash, CO2 can be sequestered in the way that generates the added value for CO2 captured. In the following section, the CO2 utilization in the most updated sodium bicarbonate (NaHCO3) and sodium carbonate production process will be introduced; especially, its relevance to CO2 mitigation strategy and brine and solid waste treatment will be considered.
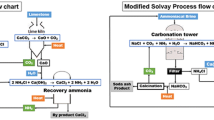
9.1.3 Conventional Solvay Process and Its Potential for CO2 Utilization
Solvay process developed in 1861 by Ernest Solvay is the most commercially accepted process for soda ash production due to its low investment and maintenance costs compared to others. This process produces soda ash from brine (NaCl) and limestone (CaCO3) with the overall reaction given in Eq. 9.7 [48]. In fact, it is more complicated and goes through many different steps that are schematically described in Fig. 9.1. Brine after purification is ammoniated and carbonated by passing the CO2 gas (Eq. 9.8). The carbonation leads to the precipitation of bicarbonate (NaHCO3) that is subsequently filtered and calcined to produce soda ash (Eq. 9.9). Filtrate mainly consisting of NH4Cl and residual NaCl is mixed with CaO in the distillation process to regenerate NH3 that is returned to ammoniation process (Eq. 9.10). CaO reacts with NH4Cl and NaCl in the filtrate to form CaCl2 as a major chemical species in distiller waste (Eqs. 9.10 and 9.11). Limestone is burned to supply CaO for NH3 regeneration and CO2 for carbonation process (Eq. 9.12). The conventional Solvay process faces a significant problem of waste management [48,49,50]. To ease its impact on the environment, the waste utilization is usually added into the traditional Solvay process in which CaCl2 can be converted to CaCO3, calcium phosphate, magnesium phosphate, or gypsum that are commercializable by-products [50,51,52,53,54,55]. The Solvay process itself does not substantially contribute to reduce CO2 emission due to the burning fossil fuel for CaO and CO2. This process consumes a significant amount of CO2 because of the high demand on soda ash and therefore has high potential for CO2 utilization and sequestration, but it can become reality only when the whole process is revised with the replacement of CaO and CO2 sources. These chemicals are abundant in many industrial wastes such as fly ash, slag, and flue gas. Thus, the exploration of those wastes as major sources for CaO and CO2 instead of burning CaCO3 should be considered. This strategy does not only reduce CO2 emission but also mitigate other wastes to be discharged to the environment. In the following parts of this chapter, the modified Solvay method and related methods to produce NaHCO3 and Na2CO3 in the consideration of waste and CO2 utilization will be introduced.
9.2 Modified Solvay Process and Solid Waste Utilization
9.2.1 Solid Waste Utilization in a Modified Solvay Process
Looking at the Solvay process as shown in Fig. 9.1, we can find a number of precursors or process steps where the traditional sources can be partially or even completely replaced by wastes. First of all, let us analyze the CO2 cycle in the process; every mole of Na2CO3 produced requires one mole of CO2 from burned CaCO3 fed into system. This process looks like a carbon neutral one emission because CO2 in a close loop, however, there is a hidden event, i.e., CaCO3 burning process that combusts fossil fuel and generate significant amount of CO2 [56]. The emission from fossil fuel combustion in lime production may vary with the type of kiln and fuel, but according to Shan et al., an average of 56.55 million tonnes CO2 emitted for the production of 207.5 million tonnes lime in China in 2012 [56]. This means that every tonne of CaO produced generates 0.27 tonnes CO2 that are not used in soda ash production. Steinhauser indicated that the production of one tonne of soda ash emits from 0.2 to 0.4 tonnes of CO2 [48]. This makes the traditional Solvay process not attractive in the term of CO2 emission. CO2 is however very abundant in flue gas from power plant or industry that burns fossil fuel as energy source. Flue gas from coal-fired power plant, for example, contains approximately 15 vol.% of CO2 that can be a viable CO2 supply for soda ash industry. The issue now lies in the CaO source because burning CaCO3 does not only provide CO2 but also provide CaO. Stop burning CaCO3 means the alternatives for CaO source must be taken into account. Fortunately, as discussed in earlier section, a variety of alkaline solid wastes such as ash and slag which have high content of CaO are available from coal-fired power plants and other industries that burn coal for energy as well. Depending on industry, technology, and type of coal, chemical composition and alkaline contents of solid wastes can be varied. In the industrial process, e.g., steelmaking illustrated in Fig. 9.2, slag can be collected from different process steps with different quantities and qualities. Table 9.1 shows the chemical composition of major alkaline wastes that are available from the literatures [6, 7, 57, 58]. The slag collected from blast furnace (BF slag) contains about 38–42 wt% CaO, while that collected from basic oxygen furnace (BOF slag) has 46.3–55.9 wt% CaO. As can be seen in this table, there are a number of wastes that have high CaO content with maximum above 50 wt% such as municipal solid waste (MW) incinerator bottom ash (53 wt%), fly ash class C (54.8 wt%), blended hydraulic slag cement (56.9 wt%), air pollution control residue (60 wt%), argon oxygen decarburization (AOD) slag from steel industry (60.7 wt%), and paper sludge incineration ash (69 wt%). These two waste streams, flue gas and alkaline solid waste, could be perfect alternatives for CO2 and CaO sources and positive contribution to CO2 emission and solid waste mitigation.

Reprinted with permission from [59], copyright 2008 American Chemical Society
Flowchart of a typical integrated steelmaking process.
In the Solvay process, thermal energy consumption accounts for 30% of production cost and considerable fraction of it comes from firing CaCO3 for CaO [63]. The use of solid waste instead of CaO does not only reduce CO2 emission but also directly contributes to lower the cost of product thanks to the reduction in energy consumption and low cost of solid waste. In order to improve the input parameters such as energy and material cost and diminish the amount of waste in conventional process, Carvalho Pinto et al. proposed a modified Solvay process as shown in Fig. 9.3 where lime used in the regeneration of ammonia is partially replaced by steel slag milk [63]. The steel slag having 40.1 wt% CaO was utilized in the laboratory investigation. The effect of various conditions such as temperature, time, NH4Cl concentration, and slag/NH4Cl ratio on the extraction of Ca2+ ions from slag was investigated. The maximum Ca2+ extraction efficiency reaches 5 wt% at the optimum condition of NH4Cl 1.5 mol/L at 90 °C for 60 min with a solid-to-liquid ratio of 2 wt%. The Ca2+ leaching is however significantly affected by the NaHCO3 concentration remained in the filtrate from the Solvay process after solid NaHCO3 is separated. For a better Ca2+ leaching, it requires a thermal treatment to decompose NaHCO3 prior to the addition of alkaline waste [54, 63]. By thermal treatment, the Ca2+ concentration is improved from 192 to 1520 mg/L, which is close to leaching efficiency of pure NH4Cl (1840 mg/L). NH3 recovery also varies with reaction conditions and is possible to reach a maximum of 40 wt%. The study at bench scale indicated that the maximum NH3 recovery of 40 wt% can be reached only when multi-recovery stages is applied.

Reprinted with permission from [63], copyright 2015 Springer Nature publisher
Conventional Solvay process and its modified one by partial replacement of CaO with steel slag.
It is easily understandable that the success of alkaline solid wastes as alternatives to CaO depends on the amount of Ca2+ ions that can be extracted as active components. The extraction is controlled by several factors including the type of solid waste, CaO content of waste, the nature of calcium in solid waste, extracting solution, and temperature [7, 59,60,61,62, 65, 68,69,70,71, 73]. CaO content in solid waste is a primary parameter to evaluate the potential of a waste utilization. The CaO content greatly varies with the type of solid waste. As shown in Table 9.1, CaO contents in different solid wastes are variable, the higher CaO wastes are usually expected to have more amount of Ca2+ extracted, but it also depends on which type of crystalline phase existing in the wastes. In a research to extract Ca2+ from steel slag containing 40.1 and 30.5 wt% CaO using NH4Cl solution and the filtrate of the Solvay process conducted by de Carvalho Pinto et al., the maximum Ca2+ extraction reached only 22 wt%, much lower compared to many others as shown in Table 9.2 [54, 63]. Even though only 22 wt% calcium was extracted, 84.3 wt% of the NH3 inlet can be recovered, demonstrating a great potential of solid waste use in a modified Solvay process. Therefore, the key to the success of this process now lies on calcium extraction method. In the following section, the essential parameters for effective calcium extraction using the NH4Cl solution or the filtrate from the Solvay process will be intensively discussed based on the available literatures.
9.2.2 Calcium Extraction by Extracting Agent
The number of studies has been conducted to extract the calcium from solid waste for a purpose of CO2 sequestration and calcium carbonate production [6, 7, 58, 61, 65, 67, 70, 73, 74]. The calcium extraction efficiency is used as a major parameter to evaluate the effectiveness of an extraction system. The extraction efficiency is defined as the percentage of Ca extracted by extracting solution to the total calcium in the solid waste used in the test as expressed in Eq. 9.13. Pure water has very poor calcium extraction because it can dissolve only part of free CaO in solid waste. In a research conducted by Said et al. [61], only 0.81 wt% of calcium in steel-converted slag was extracted by pure water. However, in another research, He et al. can extract more than 10 wt% of calcium from fly ash using pure water even though the fly ash contains less CaO, 30.47 wt% (Table 9.2) [62]. The difference in calcium extraction efficiency is probably due to the different contents of free calcium oxide or the waste microstructure that prevents the migration of Ca2+ into water phase. The calcium extraction efficiency offered by water is usually far below the requirement for an application, and thus, it needs a more effective method. Various extracting agents such as acids (HCl, HNO3, CH3COOH, and CH3CH2COOH) [59, 74,75,76] and ammonium salts (NH4Cl, NH4NO3, CH3COONH4, and (NH4)2SO4) [59,60,61,62, 71] have been used to improve the Ca2+ extraction. Recent works have indicated that the addition of these extracting agents helps enhance the Ca2+ extraction significantly thanks to their possibility to selectively dissolve some calcium compounds in solid waste, e.g., the dissolution of calcium silicate and calcium ferrite phases given in Eqs. 9.14 and 9.15 [65, 66, 68, 70, 72].
where mCa is the mass of Ca extracted by the extracting solution (g), mt is the mass of solid waste used (g), CCaO is the CaO content in solid waste (wt%), MCa is molecular weight of calcium (g/mol), and MCaO is molecular weight of CaO (g/mol).
The calcium extraction from fly ash by water and ammonium salts at different temperatures was reported by He et al. [62] (Fig. 9.4). It is evident that the calcium extraction improves significantly by adding those extracting agents. Temperature also contributes to the improvement, but it should be controlled in a moderate range. The higher temperatures do not significantly improve the extraction but incur the water vaporization that causes the loss of solvent, difficult process control, and energy intensiveness. In another research, Lee et al. showed the effect of ammonium salts on two types of slag, granulated slag and air-cooled slag [71]. They again confirm the extraction efficiency caused by the type of both extracting solvent and solid waste. NH4NO3, NH4Cl, and CH3COONH4 have a comparable extraction efficiency, approximately 49–52 wt% for granulated slag and 11–17 wt% for air-cooled slag (Fig. 9.5). (NH4)2SO4 has worse extraction among four ammonium salts due to the formation of less soluble CaSO4 on the outer layer of slag granules that prevents the dissolution of Ca2+ into liquid phase. Using 2 mol/L of NH4Cl, NH4NO3, and CH3COONH4, Said et al. can extract from 45 to 54% calcium from BOF slag that has the particle size of 74–125 µm. Similar results were observed by Sanni et al. in which acids that form soluble salts with calcium such as CH3COOH, HNO3, and CH3CH2COOH possess the highest extraction efficiency (85–100%) followed by ammonium salts (NH4NO3, NH4Cl, and CH3COONH4) with the efficiency ranging from 50 to 80%, while (NH4)2SO4, (NH4)2HPO4, and NH4H2PO4 showed a poor extraction [67]. For cement waste, 1 mol/L solution of NH4NO3 and CH3COONH4 has the extraction efficiency of ~69% that is better than NH4Cl and (NH4)SO4 with only 32 and 4%, respectively [60]. Generally, ammonium salts of NH4Cl, NH4NO3, and CH3COONH4 were recognized as the best solution for extracting calcium from solid waste thanks to their high extraction efficiency and NH3 recyclability.

Reprinted with permission from [62], copyright 2013 American Chemical Society
Influence of temperature and the type of extraction solution on Ca2+ extraction efficiency (ammonium salt concentration: 1 mol/L, solid-to-liquid ratio: 50 g/L, mixing rate: 500 rpm, time: 60 min).

Reprinted with permission from [71], copyright 2016 Elsevier
Calcium extraction efficiency of (a) granulated slag, and b air-cooled slag when different ammonium salts are used. Solvent concentration (mol/L), reaction temperature (°C), reaction time (min), and solid-to-liquid ratio are 2 mol/L, 30 °C, 60 min, and 10 for the condition 9.1 and 1 mol/L, 30 °C, 30 min, and 10 for the condition 9.2, respectively. Further detail on the condition 9.1 and 9.2 can be found in Table 9.2. Abbreviations for the solvents: AN: NH4NO3, AC: NH4Cl, AA: CH3COONH4, and AS: (NH4)2SO4.
Variety of agents have proved to be effective for calcium extraction, however, to apply in the Solvay process, and only NH4Cl is a suitable candidate because it is the main component in the filtrate that will react with CaO for NH3 recovery. In a typical Solvay process, NH4Cl concentration in the filtrate is around 3.6–4.9 mol/L, but it could not directly apply for calcium extraction because of the presence of HCO3+ in the filtrate that reacts with Ca2+ to form insoluble CaCO3 [54, 55, 63]. The CaCO3 may form the less soluble carbonated shell around slag particles that prevents the dissolution of Ca2+ ions [7, 9, 58]. Thermal treatment can decompose the HCO3+ to release CO2, but also remove NH4+ as NH3 in the gas phase; this causes the decrease in the NH4Cl concentration. The reduction in NH4Cl concentration may influence the extraction capacity of the filtrate. Recent researches have indicated that NH4Cl concentration plays a very important role in the calcium extraction [60,61,62,63, 65, 67, 70, 72]. Table 9.2 shows the calcium extraction from various solid wastes using NH4Cl in the literatures. In this table, numerous parameters including NH4Cl concentration, solid-to-liquid ratio, particle size, temperature, and extraction time and their influence on the extraction efficiency, calcium selectivity, and calcium concentration in leachate are introduced. A good agreement among published works is that the extraction efficiency improved with the increase in NH4Cl concentration; however, its concentration above 1 mol/L would be preferable. A rapid increase in the extraction efficiency was observed at the NH4Cl ranging from 0 to 1 mol/L. Above 1 mol/L, the increase is not significant (Fig. 9.6a). The decrease in NH4+ concentration due to thermal treatment of the filtrate has been verified by de Carvalho Pinto et al. [54, 63]. By boiling the filtrate containing NH4Cl 4.2 mol/L for 30 min, they can recover 16.7% NH3 which causes the NH4Cl concentration decrease to 3.5 mol/L [63]. In another study, the reduction on the NH4Cl concentration from 3.6 to 2.8 mol/L was found by boiling the filtrate [54]. These suggest that a fraction of NH3 can be recovered by thermal treatment, but it does not influence on the following extraction activity because the NH4Cl concentration is remained in preferable range. By boiling the filtrate for 30 min prior to the addition of slag, de Carvalho Pinto et al. can enhance the calcium extracted into filtrate solution from 192 to 1520 mg/L [63] confirming the effectiveness of the thermal treatment.

Reprinted with permission from [63], Springer Nature publisher
Effect of NH4Cl concentration (a) and solid-to-liquid ratio (b) on the calcium extraction efficiency.
9.2.3 Type of Solid Waste
Types of solid waste and the crystalline phases existing in solid waste are the most influent factors that decide the success of extraction using NH4Cl. CaO content and its compounds in waste vary with the waste sources and directly affect the extraction efficiency. The calcium extraction from different wastes under various conditions is provided in Table 9.2. In general, BOF slag has a high extraction efficiency. Most of BOF slag samples show the extraction efficiency above 50 wt%, while only few samples of desulphurization lag and BF slag attain 50 wt% calcium extraction. Investigating on the calcium extraction from different slags, Sanni et al. recognized that free lime in BOF and desulphurization slags contributes to high extraction with about 70 and 50%, respectively, while all calcium in BF and ladle slags exists in silicate form that explains for a poor extraction with a efficiency below 15% [67]. According to Lee et al., air-cooled slag contains higher CaO content (32.9 wt%) compared to granular slag (22.5 wt%) but the extraction efficiency is much lower, 11–17 wt% compared to 49–52 wt%, respectively [71]. The differences are attributed to the crystallinity of these two slags caused by collecting methods. Granular slag is generated by rapid cooling using water produced about 50% amorphous phase and 50% crystalline phases including mainly wustite (15.23%), Ca2Fe2O5 (13.32%), beta-Ca2SiO4 (13.10%), and magnetite (10.96%), whereas air-cooled slag is obtained by slow cooling under an ambient atmosphere containing mostly crystalline phases, which account for the poor extraction from this slag. To demonstrate the high extraction contributed by free CaO in solid waste, the comparison between MgO + CaO mixture and fly ashes was made by Hosseini et al. [69]. Two fly ashes collected from electrostatic precipitator of coal power plant that are Hazelwood fly ash (32.4 wt% CaO) containing mainly CaSO4, CaO, calcium ferrite (CaFe2O4), and silicate and Yallourn fly ash (9.4 wt% CaO) containing mostly magnesia ferrite, quartz, and amorphous CaO were tested. As shown in Fig. 9.7, above 80 wt% of calcium in MgO + CaO mixture can be extracted, while only 32 and 37 wt% of calcium was leached from Hazelwood and Yallourn fly ash in the first cycle using NH4Cl, respectively.

Reprinted with permission of [69], copyright 2014 American Chemical Society
Calcium extraction efficiency for five leaching-carbonation cycles at optimum conditions (80 °C and 30 min) using MgO + CaO mixture, Hazelwood and Yallourn fly ash.
9.2.4 Particle Size and Microwave Treatment
Particle size distribution is another crucial characteristic that influences on the calcium extraction. Data on the effect of size on the calcium extraction can be found in Table 9.2. Investigating the calcium leaching from various slags with size <150 and 150–500 µm by Hall et al. indicated an impressive increase in extraction efficiency by size reduction with a maximum 50% higher in small size samples [68]. By reducing the size of slag from 250–500 to 0–125 µm, Said et al. can improve almost 50% of the extraction efficiency of a steel converter slag which contains the majority of CaSiO4, Ca2Fe2O5, Fe0.925O, and Fe3O4 without free CaO (Fig. 9.8) [61]. Further extraction improvement can be executed by the application of microwave [72], ball milling, and heat treatment [70]. Tong et al. found that the calcium leaching can be improved about 10% by the combination of NH4Cl extracting agent with microwave treatment on electric arc furnace slag [72]. The research indicated that Ca2SiO3, Ca2SiO4, and Ca3SiO5 can be dissolved by NH4Cl solution generating leaching Ca2+ and SiO2. Lee et al. showed a calcium extraction improvement by combining NH4Cl extracting agent with ball milling [70]. The improvement is attributed to the particle size reduction that decreased from <10 to 1–5 µm by ball milling.

Reprinted with permission from [61], copyright 2012 Elsevier
Effect of particle size on calcium extraction from steel converter slag in NH4Cl solution 1 mol/L (solid-to-liquid ratio: 20 g/l).
9.2.5 Solid-to-Liquid Ratio
Solid-to-liquid ratio (g/L) for extraction is another key parameter. Most of studies agree that lower solid-to-liquid ratio offers better extraction efficiency (Table 9.2) [61,62,63, 65]. The relation was clarified by de Carvalho Pinto et al. as shown in Fig. 9.6b; the extraction efficiency drastically declines as the slag concentration increases [63]. This is in congruence with the observation by Said et al. where the calcium extraction from BOF slag by ammonium salts reached a maximum (73%) at the lowest solid concentration (5 g/L) and the lowest extraction (6%) by the highest solid concentration (100 g/L) [61]. The analogous effect was also observed when calcium was extracted from fly ash [62]. The reason for this reduction in the extraction efficiency is most likely due to the high viscosity causing the low mobility of solution that prevents proper mixing and consequently reducing calcium extraction. It is also believed that the higher buffer effect of the NH4+/NH3 couple is attained at pH = 9.25. At this pH, NH4+ and NH3 concentrations are equal and their interconversion occurs that prevents the further Ca2+ extraction [63]. In contrast to the extraction efficiency, the Ca+ concentration in the liquid increased a long with the increase in solid-to-liquid ratio; this relation is illustrated by He et al. in Fig. 9.9. In a practical application, an optimum solid-to-liquid ratio must be determined to balance the extraction efficiency, Ca2+ concentration, and water consumption for efficient production.

Reprinted with permission from [62], copyright 2013 American Chemical Society
Effect of solid-to-liquid ratio on calcium extraction efficiency (a) and Ca2+ concentration in leachate (b) at 25 °C for 60 min with the NH4Cl concentration of 1 mol/L and mixing rate 500 rpm.
9.2.6 Extraction Selectivity
Since solid waste has a diverse chemical composition, the extraction may simultaneously leach various metal ions into solution. Selectivity of a metal is a fraction of its concentration to total metal concentration in the leachate. A good solid waste should generate high selectivity for Ca over others. Impurity leached into the filtrate does not impact on the quality of resulting NaHCO3 or Na2CO3 product, but it may affect the NH3 recovery efficiency and relate to the strategy for later waste management. High selectivity of unreactive species toward NH4Cl or metal ions that form stable complexes with NH3 will result in low NH3 recovery. In general, ammonium salt solution shows a good selectivity for calcium against undesired species (Table 9.2) [54, 60, 65, 68, 70]. Relatively small quantity of undesired metals, e.g., Mn, Fe, Ni, Al, Si are detected in the leachate. A study by Jo et al. on the calcium extraction from cement waste using ammonium salts showed that most of ammonium salts have a good selectivity for calcium, which is greater at higher salt concentration, reached above 98% at the salt concentration of 1.0 mol/L (Fig. 9.10) [60]. Other studies on steel slag by Hall et al. and Lee et al. show that the calcium extraction can attain more than 95% selectivity [68, 70]. Sun et al. found only 75.7% Ca selectivity when extracting it with NH4Cl 2 mol/L from oxy furnace steel slag; however, total selectivity for two alkaline metals of Ca and Mg reached 96% [64]. Since magnesium could contribute well for NH3 recovery in the Solvay process, the high selectivity for these two elements is acceptable.

Reprinted with permission from [60], copyright 2014 Elsevier
Selectivity of major metal ions in different ammonium salts at the concentration of a 0.5 mol/L and b 1.0 mol/L.
9.3 Modified Solvay Process with Amines and Reject Brine Utilization
Finding a way to prevent or mitigate sea disposal of concentrated brine wastes has huge environmental benefits. The idea to combine CO2 utilization with waste brine treatment derives from the unique opportunity provided by the availability of vast amounts of brine wastes from desalination plant. Due to its ability to treat two environmental wastes, CO2 utilization with brine is a promising technology for solving the problem of desalination brine wastes [36]. Over the years, different ideas have been proposed to accomplish this kind of process, most of which are based on the Solvay process first developed by Ernst Solvay in 1861 [77]. Recently, methods involving the use of CaO and layered double hydroxides have also been shown to give promising results with regard to this process. The goal of the following sections is to provide a more detailed description of these processes and their performances with respect CO2 utilization and reject brine treatment.
The conventional Solvay process involves reacting CO2 with ammonia/brine solution to produce NaHCO3 and ammonium chloride (Fig. 9.1). The NaHCO3 is precipitated and separated, while the ammonium chloride solution (filtrate) is reacted with lime to produce ammonia, which is recycled, and CaCl2 as a waste product. While this process scheme can be applied directly for CO2 utilization and brine treatment as demonstrated by El-Naas [78, 79], it is not very appropriate for the processes where sodium removal is the main goal due to its low sodium conversion. This, in addition to the volatility of ammonia at the temperatures at which CO2 capture processes operate, poses a problem for the application of Solvay process for CO2 utilization and brine treatment [80, 81]. Also, Solvay process applies CaO for the regeneration of ammonia. This is counterproductive for the CO2 capture process due to the fact the production of CaO is CO2 intensive. For every two moles of CO2 from power plants captured, one mole of CO2 from calcination of limestone is released [82].
Modifications of the Solvay process for CO2 capture and utilization processes have been mostly focused on the replacement of volatile ammonia with alcohol amines which are stable at the temperature range of CO2 capture processes. Alcohol amines also tend to give better performance results (Na conversion/removal) than ammonia with dilute CO2 streams such as flues gas as shown in Table 9.3.
Huang et al. used a sterically hindered alcohol amine and methyl aminoethanol (MAE), in the place of ammonia, and reported a CO2 capacity of 0.9 mol/mol MAE and a NaHCO3 yield of 14.3 g NaHCO3/mole MAE (~17.14 g/kg solution) at MAE and NaCl concentration of 1.2 and 3.4 M, respectively [82]. In similar vein, Dindi et al. investigated the use of different classes of alcohol amines as replacement for ammonia and found that secondary or sterically hindered amines such as 2-amino, 2-propanol-amine gave the best performance [83, 86]. The reaction of amines with CO2 could follow either a carbamate or the bicarbonate route depending on the class of the amine. Primary and secondary amines react with CO2 to form carbamates following the zwitterion mechanism Eq. 9.16, while tertiary and sterically hindered amines react with CO2 to form bicarbonates according to Eq. 9.17 [87].
Primary or secondary amines react with CO2 to form carbamate (Eq. 9.16) accordingly, two molecules of amine are needed to capture one mole of CO2. For this reason, the CO2 absorption capacity of such amines cannot exceed 0.5 mol CO2 per mole of amine theoretically. Meanwhile, the tertiary or sterically hindered amines which follow the bicarbonate pathway have the potential of reaching a capacity of 1 mol CO2 per mol amine as shown in Eq. 9.17, although they may be prevented from reaching this capacity due to kinetic limitations of the bicarbonate pathway. Sometimes, the capacity of primary or secondary amines exceeds the theoretical limit due to the carbamate hydrolysis (Eq. 9.18) which releases an amine to react with more CO2, thereby increasing the CO2 absorption capacity beyond the theoretical limit. This effect is even more pronounced in the presence of brine.
The CO2 absorption capacity of amines increases as a result of precipitation of NaHCO3. Na in the brine reacts with \( {\text{HCO}}_{3}^{ - } \) generated in Eq. 9.17 (tertiary amines) and Eq. 9.18 (primary/secondary amines), thereby shifting the equilibrium to the right and causing more CO2 to be absorbed and more NaHCO3 to be precipitated in Eqs. 9.19 and 9.20 [88]. The process continues until the solution pH reduces below 9 in which case NaHCO3 cannot be precipitated and equilibrium is reached or until all the amine is consumed to form an amine chloride. This explains why amines give better CO2 absorption results in the presence of NaCl [82, 83].
Although amines give better results during the carbonation stage, a major drawback of the use of amines in the Solvay process is the difficulty of regenerating the free amine from the amine chloride by-product produced during the carbonation step. Although Huang proposed a method for regenerating ammonia from NH4Cl using activated carbon as a means of avoiding the use of CaO for ammonia regeneration, no method was proposed for regenerating the MAE chloride [82]. The activated carbon was used to remove HCl from a solution of NH4Cl with the liberation of ammonia according to Eq. 9.21.
The HCl-loaded activated carbon was then regenerated with water and used repeatedly to produce about 0.0012 mol HCl per cycle. In addition, the ultra-high lime with aluminum (UHLA) method was proposed by Dindi et al. to recover AMP from the AMP-HCl formed during the carbonation step. However, the efficiency of this process was limited by increasing amine concentration and by the presence CO2 in the solution [83]. No other method for regenerating amines from amine chlorides has been reported in the literature. Hence, this constitutes a major obstacle in the application of the amine-based Solvay process for brine treatment and CO2 capture and utilization
Recently, El-Naas proposed a process in which the CaO was used to replace NH3 in the Solvay process. The CaO is dispersed in brine to increase its pH and then applied for CO2 capture. The higher pH facilitates absorption of CO2 into the brine and subsequently the precipitation of NaHCO3. The system was effective for CO2 capture and Na removal with an optimum temperature of 10 °C and CaO concentration of 20 g/L. Beyond this concentration, Na removal was reduced due to competitive formation of CaCO3. The modified process had a maximum CO2 capture capacity of 1.44 mol/L and sodium removal efficiency of 35%, while the conventional process had a maximum CO2 capacity of 1.14 mol/L and a 29% sodium removal efficiency. The modified process is also potentially less costly than the conventional process due to the elimination of energy-intensive ammonia regeneration, fewer unit operations, and lower costs of CaO relative to the cost of ammonia [89].
9.4 Layered Double Hydroxide Process
Due to the shortcomings of the modified Solvay processes for the simultaneous treatment of desalination brines and CO2 utilization, a new, layered double hydroxide (LDH)-based process has been recently proposed which has the potential to perform better than modified Solvay processes [90]. LDHs, also known as hydrotalcite, are clay materials consisting of alternating layers of mixed metal hydroxides and anion-bearing interlayers, similar to structure of the hydrotalcite mineral as shown in Fig. 9.11 [46]. The mixed metal hydroxides layer are made up of positively charged di or trivalent cations, while the anion layer contains negatively charged balancing anions which combine to give the formula \( \left[ {{\text{M}}_{{1{-}x}}^{2 + } {\text{M}}_{x}^{3 + } \left( {\text{OH}} \right)_{2} } \right]_{x} + \left( {{\text{A}}^{-} } \right){\text{ x}}/{\text{n}} \cdot {\text{mH}}_{ 2} {\text{O}} \). M2+ represents divalent cations like Mg, Fe, Co, Cu, Ni, or Zn, M3+ represents trivalent cations like Al, Cr, Ga, Mn or Fe, and A− is the interlayer anion such as \( {\text{CO}}_{3}^{ - 2} \), \( {\text{HCO}}_{3}^{ - } \), \( {\text{SO}}_{4}^{ - 2} \), Cl−, or OH−. The value of x is equal to the molar ratio of M2+/(M2+ + M3+) and is generally in the range 0.2–0.33.

Reprinted with permission from [90], copyright 2018 Elsevier
Hydrotalcite structure.
Carbonate LDHs, in which a \( {\text{CO}}_{3}^{2 - } \) ion exists within the layers, are the most common LDH forms due to the strong affinity of the LDHs for the \( {\text{CO}}_{3}^{2 - } \) ion. While LDHs can exchange their interlayer ions with other ions present in a solution, this is not always possible depending on the relative affinity of the anion to the LDH. However, when calcined, they release the interlayer anion and OH groups to produce mixed metal oxides, which can reconvert to the original LDH structure when being dispersed in an aqueous solution of the appropriate anion, making them excellent materials for the sorption anions. This property of LDHs has been harnessed for the removal of different kinds of contaminants from wastewater like iodide [91], silicate [92], chlorides [93], heavy metals [94], and rare earth elements [95].
In the case of reject brine treatment, the objective is to reduce chloride and sodium concentrations to levels that are safe for disposal. LDHs can be used to remove chloride from brine as shown in several studies by Kameda and co-workers [93, 96, 97]. Given that the removal of chlorides from brines by Mg–Al–O also results in the increase in OH− ions in the solution leading to a rise in solution pH, this increased alkalinity can then be employed for CO2 absorption and precipitation of NaHCO3 which ultimately leads to sodium removal from the brine. In this way, CO2 capture/utilization and reject brine treatment are combined in one single process as further described in the following section.
9.4.1 Process Description
The process consists of three major stages which are the chloride removal, carbonation, and regeneration stages as shown in Fig. 9.12. In the chloride removal stage, Mg–Al mixed metal oxide, derived from Mg–Al hydrotalcite, is dispersed in the brine to remove the chloride ions via an ion exchange process. Chloride moves from the liquid phase to the solid phase, while the solution is enriched in OH− ions due to the solvation of the mixed metal oxide. When the slurry is separated, the brine solution becomes more alkaline while mixed metal oxide is transformed to a chloride-form hydrotalcite, Cl−–HT, according to Eq. 9.22. The resulting alkaline brine solution, a mixture of NaOH and NaCl, is then sent to the carbonation stage for the precipitation of NaHCO3. Depending on the initial concentration of the brine and the extent of chloride removal, a concentration step may be required to bring this solution to a concentration high enough to precipitate NaHCO3.
In the carbonation stage, the CO2 captured from flue gas reacts with the alkaline solution to precipitate NaHCO3, removing the Na+ from the brine in the process as described in Eqs. 9.23–9.28. The liquid effluent from this stage has reduced salinity and can therefore be disposed as treated brine, while the NaHCO3 can be dried and packaged for sale. To ensure efficiency of the process, the spent Cl–HT must be reused. This is done thermally in the regeneration stage.
Chloride removal step:
Carbonation step:
At high pH,
At low pH when OH− has been depleted by reaction 9.24,
During the regeneration, the Cl–HT is calcined at above 450 °C to produce the original Mg–Al mixed metal oxide (Mg–Al–O) by releasing Cl, OH, and CO2 present in the Cl–HT structure. The mixed metal oxide is then returned to the chloride removal stage and the process loop continues, while the released chloride, on the other hand, provides opportunity for the production of Cl-based products which brings additional value to the process. Overall, the process results in a more extensive treatment of the brine because of the removal of both Na and Cl ions while creating more potential for revenue through the sale of CO2- and Cl−-based chemical products.
9.4.2 Process Evaluation and Performance
A detailed assessment of the proposed process has been presented in [90]. The process was evaluated based on its ability to accomplish the two process objectives of brine treatment and CO2 utilization. The performance indices for the evaluation of brine treatment were expressed as overall chloride and sodium removal efficiencies, and for CO2 capture and utilization, as CO2 absorption capacity and yield of NaHCO3, respectively. Firstly, hydrotalcite and mixed metal oxide were synthesized; thereafter, cyclic tests were carried out to determine the performance indices. Conversion between hydrotalcite and the mixed metal oxide in a process cycle can be demonstrated by XRD investigation (Fig. 9.13).

Reprinted with permission from [90], copyright 2018 Elsevier
XRD spectra and of the synthesized CO3–HT and Mg–Al–O.
The Mg–Al–O was found to be effective for the removal chloride from the brine across the six cycles tested as shown in Fig. 9.14. Chloride removal efficiencies varied from 90 to 58%, from the first cycle to the sixth cycle, with an average of 70% across six cycles. The extent of sodium removal from the brine, due to precipitation of NaHCO3, varied from 26 to 17% from the first cycle to the sixth cycle, with an average of 20% across six cycles, while an average NaHCO3 yield of 44 g/kg carbonated solution was also obtained over six cycles of the process. The characterization of the produced NaHCO3 confirmed that a pure NaHCO3 product can be obtained from the process as presented in Fig. 9.15. Based on these results, the proposed process was found to be feasible for the simultaneous treatment of the reject brine and CO2 utilization.

Reprinted with permission from [90], copyright 2018 Elsevier
Initial and final concentrations of Cl− (a) and Na+ (b) in brine.

Reprinted with permission from [90], copyright 2018 Elsevier
SEM images at different magnifications (a, b), XRD spectra (c), and EDS spectra (d) of precipitated NaHCO3.
The proposed process concept is an important contribution to research efforts on brine treatment and CO2 utilization. It promises to be more effective than earlier processes because it is able to remove both Na and Cl ions from brine, whereas other proposed processes remove only Na ions. Also, in many regions where reject brines are generated in large volumes, desalination plants are usually integrated with power plants; hence, there will be little logistical constraint to the implementation of this process and the potential cost savings from the combined treatment of two industrial wastes as well as the revenue from the sale of two chemical products might justify the investment in this technology. Nonetheless, a detailed techno-economic study is required to determine the economic viability of the proposed process concept.
9.5 Conclusion
Current progress has been demonstrated promising outcomes from solid waste, reject brine, and CO2 utilization for greener soda ash production. No doubt that the waste and CO2 utilization in an integrated process has huge potential on the mitigation of CO2 emission and waste management. Instead of burning CaCO3 for CO2 and CaO in the conventional Solvay process, those agents can be extracted from the waste streams of power plant or steel plant in a modified Solvay process. The process can further integrate with reject brine treatment from desalination plant to create process that completely consumes wastes as raw materials. This process is suitable to combine with other industries such as power plant or steel plant so that it can at the same exploit both waste material and waste heat for a marketable product production. The revenue received may be partially offset the cost of waste treatment and CO2 capture that will encourage the effort toward waste and CO2 emission mitigation.
The calcium extraction from solid waste is influenced by multiple parameters including CaO content and its crystalline phase, particle size distribution, extracting agent concentration (NH4Cl), and solid-to-liquid ratio. CaO content, particularly free CaO, and the size of solid waste has the most decisive role for an efficient extraction. While the NH4Cl concentration in the filtrate of the Solvay process is usually in the range of optimum concentration for extraction (>1 mol/L), the solid-to-liquid ratio becomes a very important parameter for balancing Ca2+ concentration in the filtrate and extraction efficiency. Recent initiative reveals that steel slag can partly replace CaO in the Solvay process to recover 40% NH3 [63]; however, it could be significantly improved with a more suitable solid waste (high CaO content with low crystallinity and small particle size).
The modified Solvay process by replacing NH3 with alcohol amine may render high soda ash recovery, but it faces the critical problem of amine recyclability. The layered double hydroxide process using Al2O3–MgO mixture to adsorb Cl− ions generating NaOH solution that can capture CO2 for soda ash production is novel and promising route, but it is still in the research stage.
To accelerate the waste and CO2 utilization in the soda ash production, several challenges must be solved in future research, i.e., (1) to recognize the waste sources that are most suitable for NH4Cl extraction and NH3 recovery, (2) to find calcium extraction assistant method to effectively boost the extraction, (3) to revise the extractor, e.g., using multiple stage or combine mixing with microwave, (4) to look for an efficient way to recycle alcohol amine in modified Solvay process, (5) to prevent the formation of spinel phase during calcination of the layered double hydroxide for the long recyclability of Al2O3–MgO system, and finally, an economic evaluation for the proposed process is necessary.
Abbreviations
- C3A:
-
Ca3Al2O6
- H:
-
H2O
- AN:
-
NH4NO3
- AC:
-
NH4Cl
- AA:
-
CH3COONH4
- AS:
-
(NH4)2SO4
- MAE:
-
Methyl aminoethanol
- LDH:
-
Al layered double hydroxide
- HT:
-
Hydrotalcite-like materials
- Cl–HT:
-
Chloride-form hydrotalcite
- AOD:
-
Argon oxygen decarburization
- BOF:
-
Basic oxygen furnace
- BF:
-
Blast furnace
- EAF:
-
Electric arc furnace
- MW:
-
Municipal solid waste
- XRD:
-
X-ray diffraction
- EDS:
-
Energy dispersive X-ray spectroscopy
References
Salman M, Cizer Ö, Pontikes Y, Santos RM, Snellings R, Vandewalle L, Blanpain B, Van Balen K (2014) Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2 sequestering construction material. Chem Eng J 246:39–52
Pan S-Y, Chen Y-H, Chen C-D, Shen A-L, Lin M, Chiang P-C (2015) High-gravity carbonation process for enhancing CO2 fixation and utilization exemplified by the steelmaking industry. Environ Sci Technol 49:12380–12387
Chen K-W, Pan S-Y, Chen C-T, Chen Y-H, Chiang P-C (2016) High-gravity carbonation of basic oxygen furnace slag for CO2 fixation and utilization in blended cement. J Clean Prod 124:350–360
Pan S-Y, Chung T-C, Ho C-C, Hou C-J, Chen Y-H, Chiang P-C (2017) CO2 mineralization and utilization using steel slag for establishing a waste-to-resource supply chain. Sci. Rep. 7:17227
Pei S-L, Pan S-Y, Gao X, Fang Y-K, Chiang P-C (2018) Efficacy of carbonated petroleum coke fly ash as supplementary cementitious materials in cement mortars. J Clean Prod 180:689–697
Sanna A, Dri M, Hall MR, Maroto-Valer M (2012) Waste materials for carbon capture and storage by mineralisation (CCSM)—a UK perspective. Appl Energy 99:545–554
Sanna A (2015) Reduction of CO2 emissions through waste materials recycling by mineral carbonation. Handb Clean Energy Syst
Xie H, Yue H, Zhu J, Liang B, Li C, Wang Y, Xie L, Zhou X (2015) Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals. Engineering 1:150–157
Ukwattage NL, Ranjith PG, Li X (2017) Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement 97:15–22
W.S. Association (2016) Steel industry by-products, vol 2
Blissett RS, Rowson NA (2012) A review of the multi-component utilisation of coal fly ash. Fuel 97:1–23
Tayibi H, Choura M, López FA, Alguacil FJ, López-Delgado A (2009) Environmental impact and management of phosphogypsum. J Environ Manage 90:2377–2386
Saadaoui E, Ghazel N, Ben Romdhane C, Massoudi N (2017) Phosphogypsum: potential uses and problems—a review. Int J Environ Stud 74:558–567
Hammas I, Horchani-Naifer K, Férid M (2013) Solubility study and valorization of phosphogypsum salt solution. Int J Miner Process 123:87–93
Ennaciri Y, Bettach M, Cherrat A, Zegzouti A (2015) Conversion of phosphogypsum to sodium sulfate and calcium carbonate in aqueous solution. J Mater Environ Sci 7:1925
Mattila HP, Zevenhoven R (2015) Mineral carbonation of phosphogypsum waste for production of useful carbonate and sulfate salts. Front Energy Res 3
Zhao H, Li H, Bao W, Wang C, Li S, Lin W (2015) Experimental study of enhanced phosphogypsum carbonation with ammonia under increased CO2 pressure. J CO2 Utilization 11:10–19
Romero-Hermida I, Santos A, Pérez-López R, García-Tenorio R, Esquivias L, Morales-Flórez V (2017) New method for carbon dioxide mineralization based on phosphogypsum and aluminium-rich industrial wastes resulting in valuable carbonated by-products. J CO2 Utilization 18:15–22
Cárdenas-Escudero C, Morales-Flórez V, Pérez-López R, Santos A, Esquivias L (2011) Procedure to use phosphogypsum industrial waste for mineral CO2 sequestration. J Hazard Mater 196:431–435
Azdarpour A, Asadullah M, Junin R, Manan M, Hamidi H, Mohammadian E (2014) Direct carbonation of red gypsum to produce solid carbonates. Fuel Process Technol 126:429–434
Bao W, Zhao H, Li H, Li S, Lin W (2017) Process simulation of mineral carbonation of phosphogypsum with ammonia under increased CO2 pressure. J CO2 Utilization 17:125–136
Kandil A-HT, Cheira MF, Gado HS, Soliman MH, Akl HM (2017) Ammonium sulfate preparation from phosphogypsum waste. J Radiat Res Appl Sci 10:24–33
Abbas KK (2011) Study on the production of ammonium sulfate fertilizer from phosphogypsum. Eng Technol J 29:814
Chou M-IM, Bruinius JA, Benig V, Chou S-FJ, Carty RH (2005) Producing ammonium sulfate from flue gas desulfurization by-products. Energy Sour 27:1061–1071
Msila X, Billing DG, Barnard W (2016) Capture and storage of CO2 into waste phosphogypsum: the modified Merseburg process. Clean Technol Environ Policy 18:2709–2715
Ahmed M, Shayya WH, Hoey D, Mahendran A, Morris R, Al-Handaly J (2000) Use of evaporation ponds for brine disposal in desalination plants. Desalination 130:155–168
Voutchkov N (2011) Overview of seawater concentrate disposal alternatives. Desalination 273:205–219
Kaplan R, Mamrosh D, Salih HH, Dastgheib SA (2017) Assessment of desalination technologies for treatment of a highly saline brine from a potential CO2 storage site. Desalination 404:87–101
Krishnaveni V, Palanivelu K (2013) Recovery of sodium bicarbonate from textile dye bath effluent using carbon dioxide gas. Ind Eng Chem Res 52:16922–16928
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P (2009) Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res 43:2317–2348
Morillo J, Usero J, Rosado D, El Bakouri H, Riaza A, Bernaola F-J (2014) Comparative study of brine management technologies for desalination plants. Desalination 336:32–49
Joo SH, Tansel B (2015) Novel technologies for reverse osmosis concentrate treatment: a review. J Environ Manage 150:322–335
Dawoud MA (2012) Environmental impacts of seawater desalination: Arabian Gulf case study
Ahmed M, Shayya WH, Hoey D, Al-Handaly J (2001) Brine disposal from reverse osmosis desalination plants in Oman and the United Arab Emirates. Desalination 133:135–147
Pérez-González A, Urtiaga AM, Ibáñez R, Ortiz I (2012) State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res 46:267–283
Giwa A, Dufour V, Al Marzooqi F, Al Kaabi M, Hasan SW (2017) Brine management methods: recent innovations and current status. Desalination 407:1–23
Ahmad N, Baddour RE (2014) A review of sources, effects, disposal methods, and regulations of brine into marine environments. Ocean Coast Manag 87:1–7
Dziedzic D, Gross KB, Gorski RA, Johnson JT (2006) Feasibility study of using brine for carbon dioxide capture and storage from fixed sources. J Air Waste Manag Assoc 56:1631–1641
Bang J-H, Yoo Y, Lee S-W, Song K, Chae S (2017) CO2 mineralization using brine discharged from a seawater desalination plant. Minerals 7:207
Breunig HM, Birkholzer JT, Borgia A, Oldenburg CM, Price PN, McKone TE (2013) Regional evaluation of brine management for geologic carbon sequestration. Int J Greenhouse Gas Control 14:39–48
El-Naas MH, Al-Marzouqi AH, Chaalal O (2010) A combined approach for the management of desalination reject brine and capture of CO2. Desalination 251:70–74
Vito CD, Mignardi S, Ferrini V, Martin RF (2011) Reject brines from desalination as possible sources for environmental technologies. In: Ning RY (ed) Expanding issues in desalination
Druckenmiller ML, Maroto-Valer MM (2005) Carbon sequestration using brine of adjusted pH to form mineral carbonates. Fuel Process Technol 86:1599–1614
Soong Y, Fauth DL, Howard BH, Jones JR, Harrison DK, Goodman AL, Gray ML, Frommell EA (2006) CO2 sequestration with brine solution and fly ashes. Energy Convers Manag 47:1676–1685
Zhao Y, Zhang Y, Liu J, Gao J, Ji Z, Guo X, Liu J, Yuan J (2017) Trash to treasure: seawater pretreatment by CO2 mineral carbonation using brine pretreatment waste of soda ash plant as alkali source. Desalination 407:85–92
Wang W, Hu M, Zheng Y, Wang P, Ma C (2011) CO2 Fixation in Ca2+/Mg2+ rich aqueous solutions through enhanced carbonate precipitation. Ind Eng Chem Res 50:8333–8339
Wagialla KM, Al-Mutaz IS, El-Dahshan ME (1992) The manufacture of soda ash in the Arabian Gulf. Int J Prod Econ 27:145–153
Steinhauser G (2008) Cleaner production in the Solvay process: general strategies and recent developments. J Clean Prod 16:833–841
Jadeja RN, Tewari A (2009) Effect of soda ash industry effluent on agarophytes, alginophytes and carrageenophyte of west coast of India. J Hazard Mater 162:498–502
Kasikowski T, Buczkowski R, Cichosz M, Lemanowska E (2007) Combined distiller waste utilisation and combustion gases desulphurisation method: the case study of soda-ash industry. Resour Conserv Recycl 51:665–690
Kasikowski T, Buczkowski R, Cichosz M (2008) Utilisation of synthetic soda-ash industry by-products. Int J Prod Econ 112:971–984
Kasikowski T, Buczkowski R, Dejewska B, Peszyńska-Białczyk K, Lemanowska E, Igliński B (2004) Utilization of distiller waste from ammonia-soda processing. J Clean Prod 12:759–769
Kasikowski T, Buczkowski R, Lemanowska E (2004) Cleaner production in the ammonia–soda industry: an ecological and economic study. J Environ Manage 73:339–356
de Carvalho Pinto PC, da Silva TR, Linhares FM, de Andrade FV, de Oliveira Carvalho MM, de Lima GM (2016) A integrated route for CO2 capture in the steel industry and its conversion into CaCO3 using fundamentals of Solvay process. Clean Technol Environ Policy 18:1123–1139
Trypuć M, Białowicz K (2011) CaCO3 production using liquid waste from Solvay method. J Clean Prod 19:751–756
Shan Y, Liu Z, Guan D (2016) CO2 emissions from China’s lime industry. Appl Energy 166:245–252
Hemalatha T, Ramaswamy A (2017) A review on fly ash characteristics—towards promoting high volume utilization in developing sustainable concrete. J Clean Prod 147:546–559
Pan S-Y, Chang EE, Chiang P-C (2012) CO2 Capture by accelerated carbonation of alkaline wastes: a review on its principles and applications. Aerosol Air Qual Res 12:770–791
Eloneva S, Teir S, Salminen J, Fogelholm C-J, Zevenhoven R (2008) Steel converter slag as a raw material for precipitation of pure calcium carbonate. Ind Eng Chem Res 47:7104–7111
Jo H, Park S-H, Jang Y-N, Chae S-C, Lee P-K, Jo HY (2014) Metal extraction and indirect mineral carbonation of waste cement material using ammonium salt solutions. Chem Eng J 254:313–323
Said A, Mattila H-P, Järvinen M, Zevenhoven R (2013) Production of precipitated calcium carbonate (PCC) from steelmaking slag for fixation of CO2. Appl Energy 112:765–771
He L, Yu D, Lv W, Wu J, Xu M (2013) A novel method for CO2 sequestration via indirect carbonation of coal fly ash. Ind Eng Chem Res 52:15138–15145
de Carvalho Pinto PC, de Oliveira Carvalho MM, Linhares FM, da Silva TR, de Lima GM (2015) A cleaner production of sodium hydrogen carbonate: partial replacement of lime by steel slag milk in the ammonia recovery step of the Solvay process. Clean Technol Environ Policy 17:2311–2321
Sun Y, Yao M-S, Zhang J-P, Yang G (2011) Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem Eng J 173:437–445
Said A, Laukkanen T, Järvinen M (2016) Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl Energy 177:602–611
Yong S, Ping ZJ, Lian Z (2016) NH4Cl selective leaching of basic oxygen furnace slag: optimization study using response surface methodology. Environ Progress Sustain Energy 35:1387–1394
Sanni E, Sebastian T, Hannu R, Justin S, Arshe S, Carl-Johan F, Ron Z (2009) Reduction of CO2 emissions from steel plants by using steelmaking slags for production of marketable calcium carbonate. Steel Res Int 80:415–421
Hall C, Large DJ, Adderley B, West HM (2014) Calcium leaching from waste steelmaking slag: significance of leachate chemistry and effects on slag grain mineralogy. Miner Eng 65:156–162
Hosseini T, Selomulya C, Haque N, Zhang L (2014) Indirect carbonation of victorian brown coal fly ash for CO2 sequestration: multiple-cycle leaching-carbonation and magnesium leaching kinetic modeling. Energy Fuels 28:6481–6493
Lee SM, Lee SH, Jeong SK, Youn MH, Nguyen DD, Chang SW, Kim SS (2017) Calcium extraction from steelmaking slag and production of precipitated calcium carbonate from calcium oxide for carbon dioxide fixation. J Ind Eng Chem 53:233–240
Lee S, Kim JW, Chae S, Bang JH, Lee SW (2016) CO2 sequestration technology through mineral carbonation: An extraction and carbonation of blast slag. J CO2 Utilization 16:336–345
Tong Z, Ma G, Zhang X, Cai Y (2017) Microwave-supported leaching of electric arc furnace (EAF) slag by ammonium Salts. Minerals 7:119
Mattila HP, Zevenhoven R (2014) Chapter ten—production of precipitated calcium carbonate from steel converter slag and other calcium-containing industrial wastes and residues. In: Aresta M, van Eldik R (eds) Advances in inorganic chemistry. Academic Press, pp 347–384
Sun Y, Parikh V, Zhang L (2012) Sequestration of carbon dioxide by indirect mineralization using victorian brown coal fly ash. J Hazard Mater 209–210:458–466
Jo H, Lee M-G, Park J, Jung K-D (2017) Preparation of high-purity nano-CaCO3 from steel slag. Energy 120:884–894
Park HK, Bae MW, Nam IH, Kim S-G (2013) Acid leaching of CaOSiO2 resources. J Ind Eng Chem 19:633–639
Solvay E (1882) Manufacture of soda by the ammonia process. USA
El Naas M (2011) Reject brine management, desalination, trends and technologies. InTech, Croatia, pp 237–252
El-Naas M, Ali A-M, Omar C (2010) A combined approach for the management of desalination reject brine and capture of CO2. Desalination 251:70–74
Joris K, Andrea R, Toon VH, Arjan VH, Wim T, Andre F (2010) The impact of CO2 capture in the power and heat sector on the emission of SO2, NOx, particulate matter, volatile organic compounds and NH3 in the European Union. Atmos Environ 44:1369–1385
Dave N, Do T, Puxty G, Rowland R, Feron PHM, Attalla MI (2009) CO2 capture by aqueous amines and aqueous ammonia—a comparison. Energy Procedia 1:949–954
Huang HP, Shi Y, Li W, Chang SG (2001) Dual alkali approaches for the capture and separation of CO2. Energy Fuels 15:263–268
Dindi A, Quang DV, Abu-Zahra MR (2015) Simultaneous carbon dioxide capture and utilization using thermal desalination reject brine. Appl Energy 154:298–308
Abdel-Aal HK, Ibrahim AA, Shalabi MA, Al-Harbi DK (1996) Chemical separation process for highly saline water 1. Parametric Exp Invest Ind Eng Chem Res 35:799–804
Abdel-Aal H, Ibrahim A, Shalabi M, Al-Harbi D (1997) Dual-purpose chemical desalination process. Desalination 113:19–25
Dindi A, Quang DV, El Hadri N, Rayer A, Abdulkadir A, Abu-Zahra MR (2014) Potential for the simultaneous capture and utilization of CO2 using desalination reject brine: amine solvent selection and evaluation. Energy Procedia 63:7947–7953
Vaidya PD, Kenig EY (2007) CO2-alkanolamine reaction kinetics: a review of recent studies. Chem Eng Technol 30:1467–1474
Conway W, Wang X, Fernandes D, Burns R, Lawrance G, Puxty G, Maeder M (2013) Toward the understanding of chemical absorption processes for post-combustion capture of carbon dioxide: electronic and steric considerations from the kinetics of reactions of CO2 (aq) with sterically hindered Amines. Environ Sci Technol 47:1163–1169
El-Naas MH, Mohammad AF, Suleiman MI, Al Musharfy M, Al-Marzouqi AH (2017) A new process for the capture of CO2 and reduction of water salinity. Desalination 411:69–75
Dindi A, Quang DV, AlNashef I, Abu-Zahra MR (2018) A process for combined CO2 utilization and treatment of desalination reject brine. Desalination 442:62–74
Theiss FL, Sear-Hall MJ, Palmer SJ, Frost RL (2012) Zinc aluminium layered double hydroxides for the removal of iodine and iodide from aqueous solutions. Desalin Water Treat 39:166–175
Nenoff TM, Sasan K, Brady PV, Krumhansl JL, Paap S, Heimer B, Howe K, Stoll Z, Stomp J (2017) Waste water for power generation via energy efficient selective silica separations. Sandia National Lab. (SNL-NM), Albuquerque, NM (United States)
Kameda T, Yabuuchi F, Yoshioka T, Uchida M, Okuwaki A (2003) New method of treating dilute mineral acids using magnesium–aluminum oxide. Water Res 37:1545–1550
Setshedi K, Ren J, Aoyi O, Onyango MS (2012) Removal of Pb (II) from aqueous solution using hydrotalcite-like nanostructured material. Int J Phys Sci 7:63–72
Douglas G, Wendling L, Pleysier R, Trefry M (2010) Hydrotalcite formation for contaminant removal from Ranger mine process water. Mine Water Environ 29:108–115
Kameda T, Yoshioka T, Hoshi T, Uchida M, Okuwaki A (2005) The removal of chloride from solutions with various cations using magnesium–aluminum oxide. Sep Purif Technol 42:25–29
Kameda T, Oba J, Yoshioka T (2017) Simultaneous removal of Cl− and SO42− from seawater using Mg–Al oxide: kinetics and equilibrium studies. Appl Water Sci 7:129–136
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Quang, D.V., Dindi, A., Abu Zahra, M.R.M. (2019). The Utilization of CO2, Alkaline Solid Waste, and Desalination Reject Brine in Soda Ash Production. In: Winter, F., Agarwal, R., Hrdlicka, J., Varjani, S. (eds) CO2 Separation, Purification and Conversion to Chemicals and Fuels. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-13-3296-8_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-3296-8_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3295-1
Online ISBN: 978-981-13-3296-8
eBook Packages: EnergyEnergy (R0)