Abstract
Background
Respiratory syncytial virus (RSV) infection has been identified to serve as the primary cause of acute lower respiratory infectious diseases in children under the age of one and a significant risk factor for the emergence and development of pediatric recurrent wheezing and asthma, though the exact mechanism is still unknown.
Methods and results
In this study, we discuss the key routes that lead to recurrent wheezing and bronchial asthma following RSV infection. It is interesting to note that following the coronavirus disease 2019 (COVID-19) epidemic, the prevalence of RSV changes significantly. This presents us with a rare opportunity to better understand the associated mechanism for RSV infection, its effects on the respiratory system, and the immunological response to RSV following the COVID-19 epidemic. To better understand the associated mechanisms in the occurrence and progression of pediatric asthma, we thoroughly described how the RSV infection directly destroys the physical barrier of airway epithelial tissue, promotes inflammatory responses, enhances airway hyper-responsiveness, and ultimately causes the airway remodeling. More critically, extensive discussion was also conducted regarding the potential impact of RSV infection on host pulmonary immune response.
Conclusion
In conclusion, this study offers a comprehensive perspective to better understand how the RSV infection interacts in the control of the host’s pulmonary immune system, causing recurrent wheezing and the development of asthma, and it sheds fresh light on potential avenues for pharmaceutical therapy in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In young children, respiratory syncytial virus (RSV), which is spread through respiratory droplets and close contact, mostly affects the lower respiratory tract and causes symptoms including fever, coughing, asphyxia, and shortness of breath [9]. One of the most prevalent forms of acute lower respiratory tract infections (LRTI) among children under the age of 1, particularly in infants between the ages of 2 and 6 months, is acute bronchiolitis. RSV re-infection is frequently observed because the immune defense mechanism developed as a result of prior RSV infection does not sustain long. Around 18% of pediatric inpatients under the age of 1 are hospitalized due to acute bronchiolitis triggered by RSV infection [26]. According to a cohort study, between 30 and 40% of infants hospitalized for bronchiolitis, especially the severe variety, may develop recurrent wheezing [34]. RSV is one of the pathogens that frequently give rise to viral illnesses such as severe bronchiolitis [59]. As a result of the persistently high incidence rate of RSV bronchiolitis, RSV is a significant risk factor for asthma and recurrent wheeze. Hence, a connection between RSV bronchiolitis in infancy and the development of bronchial asthma in children has been proposed [37]. The epidemiological evidence providing the association between viral bronchiolitis in childhood and subsequent recurrent wheeze or asthma was first brought up in 1959 by Wittig H and Glaser J [108]. Supported by some prospective studies, RSV bronchiolitis is currently linked to recurrent wheezing, asthma, and airway remodeling. Sigurs N. et al. [88] conducted the longest follow-up study to date with a final cut-off age of 18 years old. This compelling evidence showed that severe RSV bronchiolitis in childhood can lead to long-term allergic asthma and airway remodeling. Together, the RSV-LRTI was considered to substantially harm the physical and mental health, especially in early life and was thought to contribute to a predisposition for the development of asthma, and may even bring about the chronic pulmonary disorders in their adult life [18].
The ongoing COVID-19 pandemic, as a significant threat to public health, has caused a surge in patients infected with SARS-CoV-2 and other common respiratory tract viruses that may lead to acute and chronic respiratory illnesses. As a result, the circumstances surrounding the infection of respiratory tract viruses are quite complex, and we need to act promptly to empower ourselves with understanding and insight. Additionally, the development of several ground-breaking public health policies have reshaped our lives and had a big impact on how many other pathogenic viruses, including RSV, were originally transmitted.
Here, this study seeks to update our understanding of the role that RSV infection plays in the occurrence and progression of recurrent wheezing, asthma, and other respiratory disorders in pediatric patients, as well as the mechanism of host-RSV immune response and immune escape, particularly in the COVID-19 pandemic.
The infection with respiratory syncytial virus and its derived pulmonary pathology
Brief introduction to the structure of respiratory syncytial virus
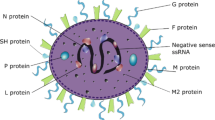
RSV, initially identified from chimpanzees in 1956, mostly affects newborns and causes catastrophic lower respiratory tract disorders [9]. RSV, as the single-stranded negative RNA envelope virus, belongs to Paramyxoviridae, Pneumoviridae, and Pneumovirus genus. The genome of RSV is composed of two non-structural proteins (NS) named NS1 and NS2, the nucleoprotein (N), phosphoprotein (P), matrix protein (M), small hydrophobic protein (SH), attachment glycoprotein (G), fusion protein (F), M2, and large protein (L). RSV has two subtypes, A and B, and these strains typically co-proliferate even if one of them predominates [39].
Epidemiological evidence showing lower respiratory tract infection with RSV could cause recurrent wheezing and even asthma in pediatric patients
RSV is a major contributor to pediatric lower respiratory tract infections worldwide, according to epidemiological statistics. RSV can result in 33.1 million acute LRTI cases, 3.2 million hospital admissions, and 118,000 under-5-year-old fatalities annually (mostly in underdeveloped nations). Since it is so widespread, nearly every kid is at risk for LRTI following initial RSV infection in infancy [92]. The most frequent RSV-related LRTI illness is bronchiolitis, which also accounts for the majority of hospitalizations in children under the age of 2 and baby deaths under the age of 1 [29]. Additionally, RSV-related LRTI is closely linked to the development and occurrence of other chronic respiratory diseases. For instance, in patients with RSV-related LRTI in early childhood (under 3 years old), the incidence of recurrent wheezing ranges from 4 to 47%, while the incidence of asthma ranges from 8 to 76% [21]. The majority of investigations consistently came to the conclusion that RSV-related LRTI may increase the prevalence of asthma in children [15]. Additionally, RSV-related LRTI in infancy has been linked to lower pulmonary function and increased airway reactivity in kids of school age [15, 21].
SARS-CoV-2 sharing single-stranded RNA nature with RSV could cause the resultant respiratory disorders
SARS-CoV-2, which gives rise to COVID-19, is a positive-sense, single-stranded RNA virus [109], as explained in our most recent study [43], and it has a number of crucial parts, including the membrane (M), nucleocapsid (N), envelope (E), and spike (S) proteins [51]. Since both RSV and SARS-CoV-2 are single-stranded RNA envelope viruses, although RSV is a negative-sense RNA virus, they still share substantial structural similarities. Additionally, SARS-CoV-2 infection may result in serious respiratory conditions. According to reports, SARS-CoV-2 infection can cause acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) by triggering an initial host immune response, intense inflammation, and occasionally even a catastrophic cytokine storm [101].
Importantly, the children population was previously widely recognized with a lower incidence and milder course of COVID-19, possibly due to less inflammatory response, while the monitor of the blood cytokine levels in special pro-inflammatory disorders such as the Kawasaki disease and multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 was recommended (B. M. [55]. In contrast, the studies in pediatric patient population with underlying primary immunodeficiency also demonstrated the favorable outcome among immunosuppressed pediatric patients rather than other comorbidities (B. M. [54], which indicated that a weaker inflammatory response might play a protective role in this condition. Hence, in the parts that followed, RSV and SARS-CoV-2 infection were discussed side by side. The major routes to trigger pediatric asthma following RSV infection were discussed, and its effects on immunological response in the respiratory system were especially emphasized.
The major routes of respiratory syncytial virus to trigger pediatric asthma
RSV infection is linked to the majority of acute asthma attacks; it is also thought to be an independent risk factor for recurrent asthma attacks and exacerbations, which can worsen asthma through promoting an inflammatory response, airway hyper-responsiveness, and airway remodeling [76, 87]. The aforementioned factors are now thought to be the primary means that an RSV infection causes recurrent wheezing and bronchial asthma. The physical barrier of the airway epithelial tissue may be immediately destroyed by RSV invasion, which may also encourage inflammatory and hyper-responsive airway responses. As a result, we went into each of these pathogenic episodes individually in this review study.
RSV invasion could directly destruct the physical barrier of airway epithelial tissue
The primary target cells for RSV invasion and the host’s initial line of defense against the infection are airway epithelial cells. The airway epithelium is frequently reported to be impaired in various pulmonary illnesses, such as asthma [105]. RSV infection could cause necrosis and exfoliation of airway epithelial cells, infiltration of peripheral lymphocytes, hyperplasia of glands, increased secretion of mucus, or even severe airway obstruction leading to emphysema, atelectasis, etc. The main symptoms of RSV infection are wheezing and dyspnea, which are mostly caused by airway blockage [25].
The surface mucus layer and the apical junction complex between individual airway epithelial cells form a barrier defense against the invasion of harmful bacteria. Tight connections and sticky junctions, which make up the apical junction complex of the epithelium, are crucial for controlling the permeability of the barrier and preserving cell polarization [25]. RSV infection may cause damage to the airway barrier in asthmatic airway epithelial cells, according to research by Kast J et al. on the influence of RSV infection on the control of tight junctions [44]. According to studies conducted by Masaki T. et al. and Tsutsumi H. et al., RSV infection could upregulate the tight junction-related proteins claudin-4 and occluding and ZO-1, raise the cell polarity, thus promote virus budding in human nasal epithelial cells [63, 98].
Mucins, which are significant elements of the mucus layer lining the airways, include MUC5AC, MUC1, MUC5B, and others [8]. The innate immune defense is strengthened by the released mucins’ interactions with other mucus elements like immunoglobin A (IgA) and lectins [6]. It was noted that RSV infection resulted in the loss of MUC5AC’s negative regulation because its target miRNA, hsa-miR-34b/c, was downregulated [19]. According to other research, RSV infection significantly increased the secretion of transforming growth factor (TGF) and extracellular matrix protein, and TGF- triggered epithelial-mesenchymal transition (EMT), which produced extracellular matrix by secreting collagen and fibronectin and increased the expression of MMPs to support airway remodeling [114]. The homeostasis of epithelial growth and repair is supported by the expression of various mediators, such as matrix metalloproteinase (MMP), cytokines, growth factors, and others by airway epithelial cells themselves [106]. These mediators also play a significant role in the secretion of mucin and the control of tight junctions between epithelial cells [8, 73, 83, 95]. The repair of the bronchial epithelium was found to be delayed by RSV, similar to many other respiratory viruses. The activity of the matrix degradative enzymes MMP 2 and MMP 9 may rise in response to RSV infection, interfering with the normal repair process and increasing the synthesis of the fibroblast growth factor (FGF) and epidermal growth factor (EGF) [48, 106].
RSV infection could promote airway inflammatory responses
In addition to being the weakened defense after virus infection, airway epithelial cells play a significant role in the beginning of inflammation. RSV infection primarily affects airway epithelial cells (AECs) at the bronchial, bronchiolar, and alveolar levels [71]. The RSV infection invades the airway epithelial cells, damages the airway mucosal barrier, and causes airway inflammation by secreting a number of cytokines, chemokines, and inflammatory mediators. It also contributes to the development of bronchial asthma and acute attacks.
Everard M et al. [20] discovered neutrophil and eosinophil infiltration in the airway in the nasal secretion of children infected with RSV, while RSV infection could drive the airway epithelial cells to secrete massive cytokines [75], such as interferon (IFN)-γ, interleukin (IL)-4, IL-5, IL-10, IL-12, and IL-13, chemokines such as CCL2, CCL3, and CCL4, CXC chemokine ligand (CXCL) 8, CXCL10, and CXCL11, and inflammatory mediators such as cysteine leukotrienes (CysLTs), which in turn causes multiple inflammatory cells to infiltrate and generate inflammatory response, leading to the condition of local airway inflammation, and eventually participating in the occurrence and acute attack of bronchial asthma. RSV infects AECs, recruits and activates neutrophils and NK cells via production of tumor necrosis factor (TNF)-α, CXCL8 (also named as IL-8), and inducible T cell chemokine (ITAC/CXCL11), promotes secretion of chemokines including macrophage inflammatory protein (MIP) 1α/CCL3, MIP1β/CCL4, MIP2, interferon-induced protein 10 (IP-10)/CXCL10, and monocyte chemokine (MCP) 1/CCL2, and induce the recruitment of monocytes and memory T cells (Z. [32, 69]. CysLTs, the inflammatory mediators with increased secretion caused by RSV infection, were also implicated in asthma. Hsu C et al. [40] discovered the significantly increased CysLTs in bronchoalveolar lavage fluid (BALF) and lung tissues, together with the RSV-triggered pulmonary inflammation in human RSV (A2 strain) infected 6–8 weeks old Balb/c mice. Han J et al. [30] observed the effective therapeutic effect of the CysLTs receptor antagonist, Montelukast, on airway inflammation triggered by RSV infection. Han Z et al. [32] found that concentration of CXCL4 within primary differentiated airway cells is positively correlated with the clinical severity of RSV infection. Increased CXCL4 can bind to heparan sulfate (HS), the main receptor of RSV, to block RSV attachment to host cells and alleviate pulmonary inflammation. Furthermore, Zeng S et al. [113] reported that IL-33 receptor (suppression of tumorigenicity 2, ST2) signaling is crucial in RSV-infected mouse lungs. Th2 and Th17 cytokine levels were also increased, which act on lung stromal cells to recruit more neutrophils to infected local sites [46].
RSV infection could exacerbate airway hyper-responsiveness
After inhaling small amounts of irritants or allergens, some individuals may experience abnormal and excessive contractions in their airways, leading to airway stenosis and a significant increase in airway resistance. This condition is known as airway hyper-responsiveness (AHR). While the exact mechanism of AHR is not yet fully understood, it is widely accepted that airway infection and inflammation play an important role.
It was discovered that neutrophils, which are activated by lipopolysaccharide (LPS) during airway remodeling, play a pivotal role in the augmented response to acetylcholine in the airway, leading to increased airway AHR [80]. Besides, Zhou N et al. [116] established a LPS (major component of Gram-negative bacteria, natural ligand of toll-like receptor 4, TLR4) plus RSV infection animal model in Balb/c mice. The results demonstrated that Gram-negative bacteria–derived LPS could switch the dominant AHR mediator from IFN-γ (the Th1 type cytokine) to extracellular degradative MMP 9 in RSV-infected mice, which exacerbates the pathological AHR and airway remodeling. These pathways present new targets for drug development in the future.
It was discovered that RSV infection could affect AHR through neurological regulation. RSV was observed to induce excess production of neuropeptide substance P by upregulating the mRNA expression of neurokinin-1 (NK-1, the receptor of substance P in the airway), leading to increased AHR phenotypes including bronchoconstriction and excessive secretion of mucus [93]. These clues suggest that balancing neuropeptide production in the airway may help alleviate AHR caused by RSV infection, providing insight into potential clinical therapy.
Various inflammatory cells, mediators, and cytokines also contribute to AHR. Skewed T helper (Th) cell profiling has long been emphasized in AHR. Compared with its major counterpart Th1 cell type, the Th2 cells mainly secrete interleukins such as IL-4, IL-5, IL-10, and IL-13, along with the IL-9 contributing neutrophils. They contribute to AHR by inducing the production of airway immunoglobulin E (IgE) and eosinophil infiltration, increasing inflammatory mediators and cells, promoting mucus secretion, affecting ciliated cell differentiation and cilia movement, triggering smooth muscle cell contraction, and depressing the reactivity of the β adrenergic receptor in airway smooth muscle cells [117]. Saravia J et al. [79] discovered increased lung type 2 innate lymphoid cells (ILC2) and IL-33 (another Th2 cytokine) [33, 52], along with accumulated eosinophils, resulting in AHR in lung homogenate in acute RSV-infected neonatal mice.
RSV infection could eventually cause airway remodeling
Airway remodeling is a prominent feature of pediatric bronchial asthma, and RSV infection has been found to induce overexpression of vascular endothelial growth factor (VEGF), transforming growth factor β1 (TGF-β1), and insulin-like growth factor 1 (IGF-1) as biomarkers of airway remodeling [103]. The role of VEGF in regulating airway repair and remodeling has been experimentally validated. For example, Lee C et al. found that VEGF induces an asthma-like phenotype characterized by inflammation, parenchymal and vascular remodeling, edema, mucus metaplasia, myofibroblast proliferation, and airway hyper-responsiveness in lung-specific VEGF165 transgenic mice [49]. TGF-β1, a fibrogenic cytokine, can promote fibroblast transdifferentiation into myofibroblasts, myofibroblast proliferation, and airway smooth muscle cell migration toward the epithelial layer, thereby facilitating the process of tissue remodeling [42]. IGF-1 can increase the content of collagen and elastin, the major components of pulmonary extracellular matrix, and increase smooth muscle thickness, thus participating in the repair of lung tissue injury and airway remodeling (Y. Y. [31]. It is therefore understandable that the induced expression of these three remodeling factors following RSV infection in vivo could result in lasting tissue remodeling and physiological consequences.
New situation of RSV infection prevalence and RSV-related LRTI post-COVID-19 era (Fig. 1)
One illustration depicts the current state of the prevalence of RSV infection in the post-COVID-19 period. RSV-LRTI, respiratory syncytial virus–lower respiratory tract infection; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Created with BioRender.com
During the past few years of the COVID-19 pandemic, the prevalence of RSV infections has sharply decreased. This can be explained by the fact that RSV and SARS-CoV-2 share the same mode of aerosol transmission, which made it easier to understand how the restrictive measures of public health implemented worldwide had a significant impact on the prevalence of RSV [17]. For example, in Belgium, it was reported that the number of registered RSV cases significantly decreased (> 99%) compared to 2019 [100]. Additionally, RSV, being a seasonal virus, typically peaks between January and February in Poland and other countries in the northern hemisphere [13]. However, during the COVID-19 pandemic, the epidemiological profile of RSV throughout the world underwent a drastic change, and the annual RSV epidemic seemed to be absent in both the winter and spring of 2020–2021 [14, 90, 99]. This conclusion was supported by the data from Stamm P et al. [90], who carried out point-of-care test (POCT)-PCR detecting RSV and SARS-CoV-2 in pediatric emergency rooms during December 1st, 2018, to March 31th, 2021, and compared the RSV incidence before and after COVID-19 epidemic. Moreover, Manti S et al. [60] analyzed the epidemiological data of pediatric RSV-LRTI from hospitalized children below 2 years old from October 2018 to December 2021. In summary, it is widely believed that the RSV-LRTI epidemic has been suppressed thanks to COVID-19-related public health measures and changes in population-level social behavior patterns.
To further compare the disease state differences between hospitalized pediatric patients with COVID-19 and RSV, Fedorczak A et al. [22] studied children aged 36 months admitted from September 2021 to March 2022 (52 cases with COVID-19 vs. 43 cases with RSV). They compared the clinical manifestations, treatments, and length of hospital stays between the two groups. The RSV group displayed the higher incidence of major clinical manifestation including cough, rhinitis, short of breath, moist rales, and wheezing, while the COVID-19 group displayed higher incidence of fever and seizure. Furthermore, the RSV patients required more bronchodilator or oxygen therapy, as well as longer hospital stay. These results suggest that infants infected with RSV may display more severe clinical manifestations than those infected with COVID-19, as the RSV-infected patients required longer hospital stays and more subtle treatment. However, it is important to note that these findings were unexpected, and it is possible that the lower number of RSV cases during the COVID-19 epidemic could have contributed to a “survivor bias” in the data.
Considering the similar single-stranded RNA nature of RSV and SAR-CoV-2 viruses, it is reasonable to speculate whether the prevalence and severity of respiratory infection caused by RSV might be affected by SARS-CoV-2 infection. Concerns have been raised about the potential co-infection of RSV and SARS-CoV-2 contributing to more severe disease conditions and higher mortality rates. To investigate this possibility, Swets M and colleagues analyzed the clinical outcomes of 6965 hospitalized adults in England between February 6th, 2020, and December 8th, 2021, among whom 220 individuals were co-infected with RSV and SARS-CoV-2. Surprisingly, no significant difference was found in disease severity, as indicated by the need for medical support, such as invasive mechanical ventilation, between those with co-infections and those with single SARS-CoV-2 infection [91].
The mechanism of host-RSV immune response and immune escape
Innate immune response following RSV infection
During RSV invasion, the innate immune response is quickly activated as the first line of defense against foreign pathogens, which trigger various types of pattern recognition receptors (PRRs), including TLRs and retinoid inducible gene-1 like receptors (RLRs), to stimulate the production of type I IFNs and recruitment of immune cells for defense action. Type I IFNs play antiviral roles by inhibiting protein synthesis and increasing pro-apoptosis factors, which safeguard the host by preventing the spread of infection by halting the release of newly replicated progeny virus. However, excessive production of type I interferon can also generate pro-inflammatory mediators. Therefore, the type I IFN response in respiratory mucosa is linked to recurrent wheezing in the first 2 years of life in infants with acute RSV infection [37].
Among various TLRs, the TLR3 and TLR4 are expected to play a role during RSV infection based on the ligands they sense. During the RSV infection, TLR3 can sense the dsRNA intermediates during RSV replication, while TLR4 recognizes the RSV-F protein. According to Groskreutz D et al. [27], the expression of both TLR3 mRNA and protein, as well as its membrane distribution, increases in A549 cell lines and human bronchial epithelial cells infected with RSV. Both TLR3 and TLR4 could initiate the expression of type I IFN including IFN-α and IFN-β through transcriptional regulation via myeloid differentiation factor 88 (MyD88)–independent mediation of IFN regulatory factor 3 (IRF3) [110]. The significant secretion of IFN-α and IFN-β provides local antiviral benefits, effectively restraining virus replication at the infected site [104]. Interferon type I demonstrates antiviral activity by inhibiting protein synthesis and promoting apoptosis factors; however, excessive expression of type I interferon can also generate pro-inflammatory mediators [97]. Compared with rhinovirus infection, pediatric patients with RSV bronchiolitis exhibit greater expression of IFN-I and IFN-III, and increased IFN-I expression in the nasal cavity of RSV-infected individuals correlates with disease severity [68].
The TLR3/TRIM56/GRP78/TRIF/IFN signaling plays crucial role in the antiviral defense and consequential immunological response potentiated by the sensing of extracellular dsRNA (Y. [86, 107]. RSV infection was found to induce the inflammatory cytokines and chemokines such as CCL5 production directly through TLR3 signaling pathways, independent on the interferon (IFN) receptor signaling in the human epithelial cells [78]. Previous studies have also emphasized the critical role of TLR4 in RSV-induced innate immune responses, further validated in TLR4 knockout mice [36]. TLR4 specifically recognizes RSV-F protein, activating nuclear factor-kappa B (NF-κB) signaling and the secretion of inflammatory-related molecules [47]. NF-κB activation via nuclear translocation leads to transcription of downstream pro-inflammatory factors and cytokines [28]. TLR4 senses RSV-F protein to induce human neutrophils to facilitate the formation of the extracellular traps of neutrophils (NETs) to capture RSV, and the NETs will form dense mucus emboli distributed all over the small airways and lungs, leading to airway obstruction and wheezing [24]. RSV SH protein, which is involved in the pathogenesis of human asthma, can activate TLR4 to promote the release of IL-1β and IL-18, thereby mediating inflammatory responses [62].
RLR, consisting of retinoid inducible gene 1 (RIG1) and MDA5, serves as a critical cytoplasmic receptor for intracellular viral RNA. In children infected with RSV, RLR expressed by airway epithelial cells, dendritic cells (DCs), macrophages, and lymphocytes, facilitates the activation of the transcription factor NF-κB and regulates the expression of inflammatory cytokines and type I interferon. RIG1 detects viral RNA products and subsequently interacts with the mitochondrial protein mitochondrial antiviral signaling protein (MAVS). This leads to the phosphorylation of the transcription factor IRF3 by IRF3 kinase and the dimerization and translocation of activated IRF3 to induce the transcription activation of IFN-β [102]. It was reported that the relative gene expression level of melanoma differentiation–associated protein 5 (MDA5) and RIG1 in nasopharyngeal washes from RSV-infected infants was significantly higher than that in RSV free infants. Additionally, RIG1 mRNA expression level is positively correlated with RSV viral load [81].
Exposure by other respiratory viruses like influenza (the similar negative-sense RNA virus like RSV) was found to upregulate the expression of various antiviral genes (including RIG1, TLR3, and MDA5, the above-mentioned important players in viral defense) in mouse bone marrow–derived eosinophils [50]. However, other findings suggest some non-structural protein components of RSV might play sophisticated “pro-viral” functions by fine-tuning the IFN response following viral infection [64]. Whether RSV infection could boost the innate immune response in its infected and recruited various types of immune cells or not is still called for further investigation.
Furthermore, previous research has revealed that NF-κB is a crucial factor in the activation of NLRP3 inflammasomes during RSV infection. These two factors are positively correlated with each other. As we comprehend, during virus infection and lung injury, the excessive activation of inflammasomes causes the generation of a large number of inflammatory factors in the airway, leading to exacerbation of the existing lung injury (C. [85]. Though the latency of RSV infection is relatively short, it is still recommended to consider both the acute infection and the latent reactivation phase when interpreting RSV infection–induced intersection of innate immune pathways and the interactive immune response [16].
Adaptive immune response following RSV infection
During an asthmatic attack, the imbalance between Th1/Th2 [84] and Th17/Treg [74] pathways is considered a significant mechanism, which also exists in patients infected with RSV. The G protein is crucial to Th2 cytokine bias in RSV-induced pulmonary disorders, promoting the production of IL-4 and IL-5 by Th2 and inhibiting the production of IFN-γ by Th1 [10]. Following RSV infection, the suppressed Th2 population becomes out of control as secreted IFN-γ decreases and highly expressed IL-4 induces recurrent wheezing and even asthma attacks [41].
The interaction between RSV-G protein and host CX3CR1 is a crucial event during the adaptive immune response of airway epithelial inflammation and infection. The G protein can bind with chemokine receptor CX3CR1 to induce a strong cytokine/chemokine response, including the production of IL-6, IL-8, MIP-1α, and MCP-1, which act on the host-RSV response [12].
IL-8 is produced by nasal mucosa epithelial cells, HBECs, and recruited neutrophils [94, 112]. Besides chemotaxis of neutrophils, IL-8 can induce T lymphocytes to migrate to inflammatory sites by itself [58], and also make the recruited neutrophils release chemotactic substances and mediate the migration of T lymphocytes [66]. Previous studies [1] have verified that RSV infection can cause excessive release of IL-8 in airway epithelium, mainly involved in inflammatory diseases dominated by neutrophil infiltration. This evidence indicates that RSV infection in bronchial epithelial cells induces IL-8 release, which may contribute to susceptibility to RSV infection in asthma.
Interactive immune response following RSV infection
In various TLRs as important player in innate immune system, double-stranded RNA (dsRNA) selectively activates TLR3 and aids in the production of thymic stromal lymphopoietin (TSLP) in epithelial cells [45]. During the inflammatory process, RSV generates numerous dsRNA intermediates during its replication within epithelial cells, stimulating TSLP secretion. Activation of TLR3 by RNA ligands significantly increases TSLP mRNA expression (by 66.8 times) [65], which plays a crucial role in pulmonary inflammation (Y. J. [57] in BALF from both the asthmatic wild-type mice and lung-specific TSLP transgenic mice, causing notable pulmonary pathologies such as airway inflammation, goblet cell hyperplasia, and airway hyper-responsiveness. Likewise, TSLP receptor–deficient mice demonstrate a tendency toward Th1 responses, failing to produce the pulmonary inflammatory response triggered by allergens [3, 115]. TSLP also accumulates in airways of asthmatic patients [111]. As TSLP is secreted by airway epithelium, it induces the regulation of dendritic cells in differentiating CD4+ Th0 cells into Th2 subtype, consequently leading to the release of IL-4, IL-5, IL-13, and TNF-α, rather than IL-10 and IFN-γ (Y. J. [56]. Besides, the mechanisms such as the killer-cell immunoglobulin-like receptors–HLA interactions indicated that during viral infection, the innate receptors could also modulate adaptive T cell responses and contribute to interactive immune response [67].
Immune escape of RSV against human host system
The RSV-G protein binds to adhesion molecules on host cells to initiate virus attachment. The protein’s variable region leads to diverse antigens, resulting in repeated infections in a single individual. The conserved region contains a CX3C-like cysteine domain, which can compete with CX3C chemokine receptors and dampen immune response mediated by immune cells [96].
RSV SH protein was discovered to hinder the signaling cascade of TNF-α triggered NF-κB activation [23]. The non-structural proteins NS1/NS2 synergistically inhibit the mRNA and protein expression of IFN-α and IFN-β, thus blocking their downstream signaling pathways in macrophages. Spann et al. additionally found that the RSV NS1 and NS2 proteins could obstruct interferon production through the MyD88 independent pathway mediated by TLR3 and TLR4 by interfering with the activation of IRF3, thereby outlasting the potential immune clearance from host cells [89]. NS2 could also bind with RIG1 and inhibit its interaction with MAVS [53]. Besides, NS1 directly binds with the promoter of IFN-β, and sabotages its binding with calmodulin binding peptide (CBP), eventually affecting the accessibility of IFN-β promoter to transcriptional factor and cofactors such as IRF3 [77]. In vitro experiments also demonstrate that NS1 and NS2 enhance proteasome-mediated degradation by forming a ubiquitin ligase complex, thereby diminishing the level of signal transducer and activator of transcription 2 (STAT2) and its related signaling [35].
RSV-infected dendritic cells (DCs) exhibit compromised assembly of the immunological synapse, possibly regulated by the N protein of RSV. Expression of N protein in DCs and lung epithelial cells is associated with the decreased antigen determinant of major histocompatibility complex (MHC) on cell surface [11]. It was found that human leukocyte antigen (HLA, MHC of human beings)–DR expression is suppressed on monocytes, and the reduced HLA-DR expression is correlated with greater disease severity in infected infants [2].
Potential mechanisms underlying host-virus immune response and immune escape in both COVID-19 and RSV-LRTI (Fig. 2)
Similar pathways mediated in host-virus immune response and immunological escape following COVID-19 and RSV infection. COVID-19, coronavirus disease 2019; dsRNA, double-stranded RNA; HLA, human leukocyte antigen; RSV, respiratory syncytial virus; ssRNA, single-stranded RNA; TLR, toll-like receptor. Created with BioRender.com
After acute viral infection, pathogen-associated molecular patterns (PAMPs) from invading foreign pathogens and damage-associated molecular patterns (DAMPs) from injured host cells are sensed by PRRs such as TLRs and NLRs. This activates the NF-kB signaling and NLRP3 inflammasomes [72]. As we mentioned earlier, TLRs play a major role in RSV defense, and are also found to be activated in COVID-19 [61]. However, TLRs and their downstream signaling can be double-edged swords and dysregulation can lead to excessive production of pro-inflammatory mediators, resulting in severe respiratory complications.
The activation of NLRP3 inflammasomes plays a significant role in the human innate immune response against viral attacks [7]. It has been observed that such activation of NLRP3 inflammasomes is associated with an excessive immune response and a cytokine storm following SARS-CoV-2 infection [38]. Likewise, this hyperactive immune response can lead to intense inflammation and sometimes even a destructive cytokine storm, resulting in ALI and ARDS in COVID-19 [70]. As previously mentioned, the production of a massive amount of pro-inflammatory cytokine resulting from TLRs mediated over activation of NLRP3 inflammasome has also been identified as a major player in pulmonary inflammation and tissue injury resulting from RSV infection. These clues indicate that both RSV and COVID-19 can cause severe disease phenotype through the underlying activation of NLRP3 inflammasomes.
HLAs as the MHC of human origin play a critical role in the diversity of host immune response and are therefore closely involved in the immune escape process. Recent research has revealed that suppressed HLA-DR expression in monocytes can lead to increased disease severity in infants under 2 years old with RSV and adults aged 20–43 years with COVID-19 [2, 4]. Furthermore, the immune-suppressive molecule HLA-G has been found to be induced in lung alveolar epithelial cells in COVID-19 patients aged 36–96 years [82], which is believed to promote immune escape and subsequent disease progression [5]. However, it remains to be investigated whether HLA-G is also implicated in RSV infection. Although the age stratification of investigated population is not consistently focused on the infants, these findings still suggest important clues about the similar HLA interference from RSV and SARS-CoV-2 might help explain their potentially similar pattern of recurrent infection, and more importantly to understand how both viruses might promote aggressive and severe tissue injuries due to compromised immune surveillance.
Conclusion
The evidence presented indicates that RSV infection in infants and young children can lead to RSV bronchitis with recurrent asthmatic symptoms and may be closely related to the later development of bronchial asthma. Current understanding suggests that RSV infection can exacerbate asthma development by damaging airway epithelial tissue, promoting inflammation, increasing airway hyper-responsiveness, and ultimately causing airway remodeling.
Given the similar single-stranded RNA nature of SARS-CoV-2 and RSV, we extensively discussed the potential for SARS-CoV-2 to cause similar respiratory disorders. Dysregulation of key TLR signaling, overactive NLRP3 inflammasomes, and compromised monocyte HLA-DR were found to be strongly implicated in both RSV and SARS-CoV-2 infections. These similar underlying mechanisms may explain the recurring infections and the viruses’ ability to promote aggressive and severe tissue injury due to compromised immune surveillance.
The incidence of RSV was significantly reduced and the infection appeared to be competitively suppressed due to the COVID-19 pandemic. This, combined with public health measures and changes in population-level social behavior patterns, may result in a significant decrease or even elimination of other respiratory viruses, including RSV. Moreover, this could offer innovative ways to prevent respiratory infections, emphasizing the crucial role of preventive public health interventions. In infants, a single RSV infection was associated with more severe clinical manifestations during a prolonged hospital stay. However, the severity of co-infection between RSV and SARS-CoV-2 did not exhibit a significant difference compared to single infections.
Discussion and perspectives
Although previous studies have fed us with lots of information about the strong implication of RSV in pediatric recurrent wheezing and asthma, there are still remaining controversial issues. The systematic in vivo experimental evidence is still in short to conclude whether RSV infection is an independent causative agent of pediatric bronchial asthma, and more detailed molecular mechanism underlying RSV triggered development of asthma. Hopefully, our review could shed some light on how to prevent and cure RSV infection in early life, and more importantly, how to block the triggered recurrent wheezing and asthma after one-time RSV infection.
Despite previous studies providing significant data on the correlation between RSV and recurrent wheezing and asthma in children, there are still some controversial issues to be addressed. The current in vivo experimental evidence is insufficient to confirm whether RSV infection is an independent cause of pediatric bronchial asthma, and the molecular mechanism behind RSV-induced asthma development requires further elucidation. This review aims to provide insights into the prevention and treatment of early-life RSV infection and, crucially, how to prevent recurrent wheezing and asthma triggered by one-time RSV infection. In this study, there is an unexpected finding that infants with single RSV infection experience more severe pulmonary disorders than those with single SARS-CoV-2 infection. However, it is unclear if this conclusion is due to survivor bias and requires further investigation. There is a slight concern that some relevant reports focused on COVID-19 in adults rather than infants, who are the population most susceptible to RSV-LRTI. Hopefully, more studies can be conducted in children to provide us with better insights and more convincing evidence.
Data availability
None original data included in this review work.
References
Abu-Harb M, Bell F, Finn A, Rao WH, Nixon L, Shale D, Everard ML (1999) IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J 14(1):139–143. https://doi.org/10.1034/j.1399-3003.1999.14a23.x
Ahout IM, Jans J, Haroutiounian L, Simonetti ER, van der Gaast-de Jongh C, Diavatopoulos DA, ..., Ferwerda G (2016) Reduced expression of HLA-DR on monocytes during severe respiratory syncytial virus infections. Pediatr Infect Dis J 35(3):e89–96. https://doi.org/10.1097/inf.0000000000001007
Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ (2005) A role for TSLP in the development of inflammation in an asthma model. J Exp Med 202(6):829–839. https://doi.org/10.1084/jem.20050199
Awasthi NP, Mishra S, Tiwari V, Agarwal J, Das PK, Jain P, Husain N (2023) Monocyte HLADR and immune dysregulation index as biomarkers for COVID-19 severity and mortality. Indian J Clin Biochem 38(2):204–211. https://doi.org/10.1007/s12291-022-01087-z
Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, ..., Akdis CA (2020) Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 75(7):1564–1581. https://doi.org/10.1111/all.14364
Baños-Lara Mdel R, Piao B, Guerrero-Plata A (2015) Differential mucin expression by respiratory syncytial virus and human metapneumovirus infection in human epithelial cells. Mediators Inflamm 2015:347292. https://doi.org/10.1155/2015/347292
Batiha GE, Al-Gareeb AI, Rotimi D, Adeyemi OS, Al-Kuraishy HM (2022) Common NLRP3 inflammasome inhibitors and Covid-19: divide and conquer. Sci Afr 18:e01407. https://doi.org/10.1016/j.sciaf.2022.e01407
Bonser LR, Erle DJ (2017) Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med 6(12):112. https://doi.org/10.3390/jcm6120112
Borchers AT, Chang C, Gershwin ME, Gershwin LJ (2013) Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol 45(3):331–379. https://doi.org/10.1007/s12016-013-8368-9
Boyoglu-Barnum S, Chirkova T, Todd SO, Barnum TR, Gaston KA, Jorquera P, ..., Anderson LJ (2014) Prophylaxis with a respiratory syncytial virus (RSV) anti-G protein monoclonal antibody shifts the adaptive immune response to RSV rA2-line19F infection from Th2 to Th1 in BALB/c mice. J Virol 88(18):10569–10583. https://doi.org/10.1128/jvi.01503-14
Céspedes PF, Bueno SM, Ramírez BA, Gomez RS, Riquelme SA, Palavecino CE, ..., Kalergis AM (2014) Surface expression of the hRSV nucleoprotein impairs immunological synapse formation with T cells. Proc Natl Acad Sci U S A 111(31):E3214–3223. https://doi.org/10.1073/pnas.1400760111
Chirkova T, Lin S, Oomens AGP, Gaston KA, Boyoglu-Barnum S, Meng J, ..., Anderson LJ (2015) CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J Gen Virol 96(9):2543–2556. https://doi.org/10.1099/vir.0.000218
Chrobak E, Machura E, Wrzask M, Krakowczyk H, Mielczarek M (2011) RSV infection course in infants and young children during hospitalization. Przegl Lek 68(1):63–67
Coma E, Vila,J, Méndez-Boo L, Antón A, Mora N, Fina F, ..., Medina M (2022) Respiratory syncytial virus infections in young children presenting to primary care in catalonia during the COVID-19 pandemic. J Pediatric Infect Dis Soc 11(2):69–72. https://doi.org/10.1093/jpids/piab121
Coutts J, Fullarton J, Morris C, Grubb E, Buchan S, Rodgers-Gray B, Thwaites R (2020) Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr Pulmonol 55(5):1104–1110. https://doi.org/10.1002/ppul.24676
Cuddy SR, Cliffe AR (2023) The intersection of innate immune pathways with the latent herpes simplex virus genome. J Virol 97(5):e0135222. https://doi.org/10.1128/jvi.01352-22
Di Mattia G, Nenna R, Mancino E, Rizzo V, Pierangeli A, Villani A, Midulla F (2021) During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol 56(10):3106–3109. https://doi.org/10.1002/ppul.25582
Driscoll AJ, Arshad SH, Bont L, Brunwasser SM, Cherian T, Englund JA, ..., Feikin DR (2020) Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization-sponsored meeting. Vaccine 38(11):2435–2448. https://doi.org/10.1016/j.vaccine.2020.01.020
Du X, Yang Y, Xiao G, Yang M, Yuan L, Qin L, ..., Liu C (2020) Respiratory syncytial virus infection-induced mucus secretion by down-regulation of miR-34b/c-5p expression in airway epithelial cells. J Cell Mol Med 24(21):12694–12705. https://doi.org/10.1111/jcmm.15845
Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, ..., Milner AD (1994) Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 71(5):428–432. https://doi.org/10.1136/adc.71.5.428
Fauroux B, Simões EAF, Checchia PA, Paes B, Figueras-Aloy J, Manzoni P, ..., Carbonell-Estrany X (2017) The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther 6(2):173–197. https://doi.org/10.1007/s40121-017-0151-4
Fedorczak A, Zielińska N, Nosek-Wasilewska P, Mikołajczyk K, Lisiak J, Zeman K, Tkaczyk M (2022) Comparison of COVID-19 and RSV infection courses in infants and children under 36 months hospitalized in paediatric department in fall and winter season 2021/2022. J Clin Med 11(23):7088. https://doi.org/10.3390/jcm11237088
Fuentes S, Tran KC, Luthra P, Teng MN, He B (2007) Function of the respiratory syncytial virus small hydrophobic protein. J Virol 81(15):8361–8366. https://doi.org/10.1128/jvi.02717-06
Funchal GA, Jaeger N, Czepielewski RS, Machado MS, Muraro SP, Stein RT, ..., Porto BN (2015) Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One 10(4):e0124082. https://doi.org/10.1371/journal.pone.0124082
Ganesan S, Comstock AT, Sajjan US (2013) Barrier function of airway tract epithelium. Tissue Barriers 1(4):e24997. https://doi.org/10.4161/tisb.24997
Garcia-Garcia ML, Calvo Rey C, Del Rosal Rabes T (2016) Pediatric asthma and viral infection. Arch Bronconeumol 52(5):269–273. https://doi.org/10.1016/j.arbres.2015.11.008
Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW (2006) Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol 176(3):1733–1740. https://doi.org/10.4049/jimmunol.176.3.1733
Haeberle HA, Takizawa R, Casola A, Brasier AR, Dieterich HJ, Van Rooijen N, ..., Garofalo RP (2002) Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J Infect Dis 186(9):1199–1206. https://doi.org/10.1086/344644
Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, ..., Szilagyi P (2009) The burden of respiratory syncytial virus infection in young children. N Engl J Med 360(6):588–598. https://doi.org/10.1056/NEJMoa0804877
Han J, Jia Y, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, Gelfand EW (2010) Montelukast during primary infection prevents airway hyperresponsiveness and inflammation after reinfection with respiratory syncytial virus. Am J Respir Crit Care Med 182(4):455–463. https://doi.org/10.1164/rccm.200912-1811OC
Han YY, Yan Q, Chen W, Forno E, Celedón JC (2021) Serum insulin-like growth factor-1, asthma, and lung function among British adults. Ann Allergy Asthma Immunol 126(3):284-291.e282. https://doi.org/10.1016/j.anai.2020.12.005
Han Z, Rao J, Xie Z, Wang C, Xu B, Qian S, ..., Wang J (2020) Chemokine (C-X-C Motif) ligand 4 is a restrictor of respiratory syncytial virus infection and an indicator of clinical severity. Am J Respir Crit Care Med 202(5):717–729. https://doi.org/10.1164/rccm.201908-1567OC
Hardman C, Ogg G (2016) Interleukin-33, friend and foe in type-2 immune responses. Curr Opin Immunol 42:16–24. https://doi.org/10.1016/j.coi.2016.05.004
Hasegawa K, Mansbach JM, Bochkov YA, Gern JE, Piedra PA, Bauer CS, ..., Camargo CA Jr (2019) Association of rhinovirus C bronchiolitis and immunoglobulin E sensitization during infancy with development of recurrent wheeze. JAMA Pediatr 173(6):544–552. https://doi.org/10.1001/jamapediatrics.2019.0384
Hastie ML, Headlam MJ, Patel NB, Bukreyev AA, Buchholz UJ, Dave KA, ..., Gorman JJ (2012) The human respiratory syncytial virus nonstructural protein 1 regulates type I and type II interferon pathways. Mol Cell Proteomics 11(5):108–127. https://doi.org/10.1074/mcp.M111.015909
Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA (2001) Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol 75(22):10730–10737. https://doi.org/10.1128/jvi.75.22.10730-10737.2001
Heinonen S, Rodriguez-Fernandez R, Diaz A, Oliva Rodriguez-Pastor S, Ramilo O, Mejias A (2019) Infant immune response to respiratory viral infections. Immunol Allergy Clin North Am 39(3):361–376. https://doi.org/10.1016/j.iac.2019.03.005
Heneka MT (2017) Inflammasome activation and innate immunity in Alzheimer’s disease. Brain Pathol 27(2):220–222. https://doi.org/10.1111/bpa.12483
Hon KL, Leung AKC, Wong AHC, Dudi A, Leung KKY (2023) Respiratory syncytial virus is the most common causative agent of viral bronchiolitis in young children: an updated review. Curr Pediatr Rev 19(2):139–149. https://doi.org/10.2174/1573396318666220810161945
Hsu CH, Hu CM, Lu KH, Yang SF, Tsai CH, Ko CL, ..., Lue KH (2012) Effect of selective cysteinyl leukotriene receptor antagonists on airway inflammation and matrix metalloproteinase expression in a mouse asthma model. Pediatr Neonatol 53(4):235–244. https://doi.org/10.1016/j.pedneo.2012.06.004
Hu X, Li X, Hu C, Qin L, He R, Luo L, ..., Feng J (2017) Respiratory syncytial virus exacerbates OVA-mediated asthma in mice through C5a-C5aR regulating CD4(+)T cells immune responses. Sci Rep 7(1):15207. https://doi.org/10.1038/s41598-017-15471-w
Ito I, Fixman ED, Asai K, Yoshida M, Gounni AS, Martin JG, Hamid Q (2009) Platelet-derived growth factor and transforming growth factor-beta modulate the expression of matrix metalloproteinases and migratory function of human airway smooth muscle cells. Clin Exp Allergy 39(9):1370–1380. https://doi.org/10.1111/j.1365-2222.2009.03293.x
Jiang C, Jiang K, Li X, Zhang N, Zhu W, Meng L, ..., Lu S (2023) Evaluation of immunoprotection against coronavirus disease 2019: Novel variants, vaccine inoculation, and complications. J Pharm Anal 13(1):1–10. https://doi.org/10.1016/j.jpha.2022.10.003
Kast JI, McFarlane AJ, Głobińska A, Sokolowska M, Wawrzyniak P, Sanak M, ..., Wanke K (2017) Respiratory syncytial virus infection influences tight junction integrity. Clin Exp Immunol 190(3):351–359. https://doi.org/10.1111/cei.13042
Kato A, Favoreto S Jr, Avila PC, Schleimer RP (2007) TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 179(2):1080–1087. https://doi.org/10.4049/jimmunol.179.2.1080
Khader SA, Gaffen SL, Kolls JK (2009) Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2(5):403–411. https://doi.org/10.1038/mi.2009.100
Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, ..., Finberg RW (2000) Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 1(5):398–401. https://doi.org/10.1038/80833
Lechapt-Zalcman E, Prulière-Escabasse V, Advenier D, Galiacy S, Charrière-Bertrand C, Coste A, ..., Escudier E (2006) Transforming growth factor-beta1 increases airway wound repair via MMP-2 upregulation: a new pathway for epithelial wound repair? Am J Physiol Lung Cell Mol Physiol 290(6):L1277–1282. https://doi.org/10.1152/ajplung.00149.2005
Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, ..., Elias JA (2004) Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 10(10):1095–1103. https://doi.org/10.1038/nm1105
LeMessurier KS, Rooney R, Ghoneim HE, Liu B, Li K, Smallwood HS, Samarasinghe AE (2020) Influenza A virus directly modulates mouse eosinophil responses. J Leukoc Biol 108(1):151–168. https://doi.org/10.1002/jlb.4ma0320-343r
Li X, Geng M, Peng Y, Meng L, Lu S (2020) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10(2):102–108. https://doi.org/10.1016/j.jpha.2020.03.001
Liew FY, Girard JP, Turnquist HR (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16(11):676–689. https://doi.org/10.1038/nri.2016.95
Ling Z, Tran KC, Teng MN (2009) Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol 83(8):3734–3742. https://doi.org/10.1128/jvi.02434-08
Liu BM, Hill HR (2020) Role of host immune and inflammatory responses in COVID-19 cases with underlying primary immunodeficiency: a review. J Interferon Cytokine Res 40(12):549–554. https://doi.org/10.1089/jir.2020.0210
Liu BM, Martins TB, Peterson LK, Hill HR (2021) Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: a review. Cytokine 142:155478. https://doi.org/10.1016/j.cyto.2021.155478
Liu YJ (2006) Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med 203(2):269–273. https://doi.org/10.1084/jem.20051745
Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, ..., Ziegler SF (2007) TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol 25:193–219. https://doi.org/10.1146/annurev.immunol.25.022106.141718
Mackay CR (2001) Chemokines: immunology’s high impact factors. Nat Immunol 2(2):95–101. https://doi.org/10.1038/84298
Mansbach JM, Geller RJ, Hasegawa K, Piedra PA, Avadhanula V, Gern JE, ..., Camargo CA (2021) Detection of respiratory syncytial virus or rhinovirus weeks after hospitalization for bronchiolitis and the risk of recurrent wheezing. J Infect Dis 223(2):268–277. https://doi.org/10.1093/infdis/jiaa348
Manti S, Giallongo A, Parisi GF, Papale M, Presti S, Lo Bianco M, ..., Leonardi S (2022). Impact of COVID-19 pandemic and lockdown on the epidemiology of RSV-mediated bronchiolitis: experience from our centre. Children (Basel) 9(11). https://doi.org/10.3390/children9111723
Mantovani S, Oliviero B, Varchetta S, Renieri A, Mondelli MU (2023) TLRs: innate immune sentries against SARS-CoV-2 infection. Int J Mol Sci 24(9):8065. https://doi.org/10.3390/ijms24098065
Marzec J, Cho HY, High M, McCaw ZR, Polack F, Kleeberger SR (2019) Toll-like receptor 4-mediated respiratory syncytial virus disease and lung transcriptomics in differentially susceptible inbred mouse strains. Physiol Genomics 51(12):630–643. https://doi.org/10.1152/physiolgenomics.00101.2019
Masaki T, Kojima T, Okabayashi T, Ogasawara N, Ohkuni T, Obata K, ..., Sawada N (2011) A nuclear factor-κB signaling pathway via protein kinase C δ regulates replication of respiratory syncytial virus in polarized normal human nasal epithelial cells. Mol Biol Cell 22(13):2144–2156. https://doi.org/10.1091/mbc.E10-11-0875
Mesev EV, LeDesma RA, Ploss A (2019) Decoding type I and III interferon signalling during viral infection. Nat Microbiol 4(6):914–924. https://doi.org/10.1038/s41564-019-0421-x
Meyer EH, DeKruyff RH, Umetsu DT (2008) T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med 59:281–292. https://doi.org/10.1146/annurev.med.59.061506.154139
Milewski Ł, Dziunycz P, Barcz E, Radomski D, Roszkowski PI, Korczak-Kowalska G, ..., Malejczyk J (2011) Increased levels of human neutrophil peptides 1, 2, and 3 in peritoneal fluid of patients with endometriosis: association with neutrophils, T cells and IL-8. J Reprod Immunol 91(1–2):64–70. https://doi.org/10.1016/j.jri.2011.05.008
Mora-Bitria L, Asquith B (2023) Innate receptors modulating adaptive T cell responses: KIR-HLA interactions and T cell-mediated control of chronic viral infections. Immunogenetics 75(3):269–282. https://doi.org/10.1007/s00251-023-01293-w
Nenna R, Ferrara M, Nicolai A, Pierangeli A, Scagnolari C, Papoff P, ..., Midulla F (2015) Viral load in infants hospitalized for respiratory syncytial virus bronchiolitis correlates with recurrent wheezing at thirty-six-month follow-up. Pediatr Infect Dis J 34(10):1131–1132. https://doi.org/10.1097/inf.0000000000000825
Nuriev R, Johansson C (2019) Chemokine regulation of inflammation during respiratory syncytial virus infection. F1000Res 8:F1000 Faculty Rev-1837. https://doi.org/10.12688/f1000research.20061.1
Onder G, Rezza G, Brusaferro S (2020) Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323(18):1775–1776. https://doi.org/10.1001/jama.2020.4683
Openshaw PJM, Chiu C, Culley FJ, Johansson C (2017) Protective and harmful immunity to RSV infection. Annu Rev Immunol 35:501–532. https://doi.org/10.1146/annurev-immunol-051116-052206
Paroli AF, Gonzalez PV, Díaz-Luján C, Onofrio LI, Arocena A, Cano RC, ..., Gea S (2018) NLRP3 inflammasome and caspase-1/11 pathway orchestrate different outcomes in the host protection against Trypanosoma cruzi acute infection. Front Immunol 9:913. https://doi.org/10.3389/fimmu.2018.00913
Petecchia L, Sabatini F, Usai C, Caci E, Varesio L, Rossi GA (2012) Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab Invest 92(8):1140–1148. https://doi.org/10.1038/labinvest.2012.67
Pukelsheim K, Stoeger T, Kutschke D, Ganguly K, Wjst M (2010) Cytokine profiles in asthma families depend on age and phenotype. PLoS One 5(12):e14299. https://doi.org/10.1371/journal.pone.0014299
Qin L, Hu CP, Feng JT, Xia Q (2011) Activation of lymphocytes induced by bronchial epithelial cells with prolonged RSV infection. PLoS One 6(12):e27113. https://doi.org/10.1371/journal.pone.0027113
Quinn LA, Shields MD, Sinha I, Groves HE (2020) Respiratory syncytial virus prophylaxis for prevention of recurrent childhood wheeze and asthma: a systematic review. Syst Rev 9(1):269. https://doi.org/10.1186/s13643-020-01527-y
Ren J, Liu T, Pang L, Li K, Garofalo RP, Casola A, Bao X (2011) A novel mechanism for the inhibition of interferon regulatory factor-3-dependent gene expression by human respiratory syncytial virus NS1 protein. J Gen Virol 92(Pt 9):2153–2159. https://doi.org/10.1099/vir.0.032987-0
Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW (2005) Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol 79(6):3350–3357. https://doi.org/10.1128/jvi.79.6.3350-3357.2005
Saravia J, You D, Shrestha B, Jaligama S, Siefker D, Lee GI, ..., Cormier SA (2015) Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog 11(10):e1005217. https://doi.org/10.1371/journal.ppat.1005217
Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA (2002) Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol 283(5):L952-962. https://doi.org/10.1152/ajplung.00420.2001
Scagnolari C, Midulla F, Pierangeli A, Moretti C, Bonci E, Berardi R, ..., Antonelli G (2009) Gene expression of nucleic acid-sensing pattern recognition receptors in children hospitalized for respiratory syncytial virus-associated acute bronchiolitis. Clin Vaccine Immunol 16(6):816–823. https://doi.org/10.1128/cvi.00445-08
Seliger B, Jasinski-Bergner S, Massa C, Mueller A, Biehl K, Yang B, ..., Bauer M (2022) Induction of pulmonary HLA-G expression by SARS-CoV-2 infection. Cell Mol Life Sci 79(11):582. https://doi.org/10.1007/s00018-022-04592-9
Seo GS, Jiang WY, Park PH, Sohn DH, Cheon JH, Lee SH (2014) Hirsutenone reduces deterioration of tight junction proteins through EGFR/Akt and ERK1/2 pathway both converging to HO-1 induction. Biochem Pharmacol 90(2):115–125. https://doi.org/10.1016/j.bcp.2014.05.006
Shamji MH, Durham SR (2017) Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol 140(6):1485–1498. https://doi.org/10.1016/j.jaci.2017.10.010
Shen C, Zhang Z, Xie T, Ji J, Xu J, Lin L, ..., Zhao X (2019) Rhein suppresses lung inflammatory injury induced by human respiratory syncytial virus through inhibiting NLRP3 inflammasome activation via NF-κB pathway in mice. Front Pharmacol 10:1600. https://doi.org/10.3389/fphar.2019.01600
Shen Y, Li NL, Wang J, Liu B, Lester S, Li K (2012) TRIM56 is an essential component of the TLR3 antiviral signaling pathway. J Biol Chem 287(43):36404–36413. https://doi.org/10.1074/jbc.M112.397075
Shi T, Ooi Y, Zaw EM, Utjesanovic N, Campbell H, Cunningham S, ..., Nair H (2020) Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis 222(Suppl 7):S628-s633. https://doi.org/10.1093/infdis/jiz311
Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM (2010) Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 65(12):1045–1052. https://doi.org/10.1136/thx.2009.121582
Spann KM, Tran KC, Collins PL (2005) Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol 79(9):5353–5362. https://doi.org/10.1128/jvi.79.9.5353-5362.2005
Stamm P, Sagoschen I, Weise K, Plachter B, Münzel T, Gori T, Vosseler M (2021) Influenza and RSV incidence during COVID-19 pandemic-an observational study from in-hospital point-of-care testing. Med Microbiol Immunol 210(5–6):277–282. https://doi.org/10.1007/s00430-021-00720-7
Swets MC, Russell CD, Harrison EM, Docherty AB, Lone N, Girvan M, ..., Baillie JK (2022) SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 399(10334):1463–1464. https://doi.org/10.1016/s0140-6736(22)00383-x
Tabor DE, Fernandes F, Langedijk AC, Wilkins D, Lebbink RJ, Tovchigrechkob A, ..., Abram ME (2020) Global molecular epidemiology of respiratory syncytial virus from the 2017–2018 INFORM-RSV Study. J Clin Microbiol 59(1). https://doi.org/10.1128/jcm.01828-20
Tan YR, Yang T, Liu SP, Xiang Y, Qu F, Liu HJ, Qin XQ (2008) Pulmonary peptidergic innervation remodeling and development of airway hyperresponsiveness induced by RSV persistent infection. Peptides 29(1):47–56. https://doi.org/10.1016/j.peptides.2007.10.020
Tang H, Sun Y, Shi Z, Huang H, Fang Z, Chen J, ..., Li B (2013) YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-κB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol 190(1):438–446. https://doi.org/10.4049/jimmunol.1201827
Terakado M, Gon Y, Sekiyama A, Takeshita I, Kozu Y, Matsumoto K, ..., Hashimoto S (2011) The Rac1/JNK pathway is critical for EGFR-dependent barrier formation in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300(1):L56–63. https://doi.org/10.1152/ajplung.00159.2010
Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ (2001) CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2(8):732–738. https://doi.org/10.1038/90675
Troy NM, Bosco A (2016) Respiratory viral infections and host responses; insights from genomics. Respir Res 17(1):156. https://doi.org/10.1186/s12931-016-0474-9
Tsutsumi H, Kojima T, Hirakawa S, Masaki T, Okabayashi T, Yokota S, ..., Sawada N (2011) Respiratory syncytial virus infection and the tight junctions of nasal epithelial cells. Adv Otorhinolaryngol 72:153–156. https://doi.org/10.1159/000324777
Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N (2021) Resurgence of Respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg Infect Dis 27(11):2969–2970. https://doi.org/10.3201/eid2711.211565
Van Brusselen D, De Troeyer K, Ter Haar E, Vander Auwera A, Poschet K, Van Nuijs S, ..., Vanden Driessche K (2021) Bronchiolitis in COVID-19 times: a nearly absent disease? Eur J Pediatr 180(6):1969–1973. https://doi.org/10.1007/s00431-021-03968-6
Vaninov N (2020) In the eye of the COVID-19 cytokine storm. Nat Rev Immunol 20(5):277. https://doi.org/10.1038/s41577-020-0305-6
Vareille M, Kieninger E, Edwards MR, Regamey N (2011) The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev 24(1):210–229. https://doi.org/10.1128/cmr.00014-10
Varricchi G, Ferri S, Pepys J, Poto R, Spadaro G, Nappi E, ..., Canonica WG (2022) Biologics and airway remodeling in severe asthma. Allergy 77(12):3538–3552. https://doi.org/10.1111/all.15473
Vercammen E, Staal J, Beyaert R (2008) Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev 21(1):13–25. https://doi.org/10.1128/cmr.00022-07
Villenave R, Shields MD, Power UF (2013) Respiratory syncytial virus interaction with human airway epithelium. Trends Microbiol 21(5):238–244. https://doi.org/10.1016/j.tim.2013.02.004
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92(8):827–839. https://doi.org/10.1161/01.Res.0000070112.80711.3d
Wei D, Li NL, Zeng Y, Liu B, Kumthip K, Wang TT, ..., Li K (2016) The molecular chaperone GRP78 contributes to toll-like receptor 3-mediated innate immune response to hepatitis C virus in hepatocytes. J Biol Chem 291(23):12294–12309. https://doi.org/10.1074/jbc.M115.711598
Wittig HJ, Glaser J (1959) The relationship between bronchiolitis and childhood asthma; a follow-up study of 100 cases of bronchiolitis. J Allergy 30(1):19–23. https://doi.org/10.1016/0021-8707(59)90054-1
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, ..., Zhang YZ (2020) A new coronavirus associated with human respiratory disease in China. Nature 579(7798):265–269. https://doi.org/10.1038/s41586-020-2008-3
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, ..., Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science, 301(5633):640–643. https://doi.org/10.1126/science.1087262
Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, ..., Corrigan C (2005) Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 174(12):8183–8190. https://doi.org/10.4049/jimmunol.174.12.8183
Yoon BN, Choi NG, Lee HS, Cho KS, Roh HJ (2010) Induction of interleukin-8 from nasal epithelial cells during bacterial infection: the role of IL-8 for neutrophil recruitment in chronic rhinosinusitis. Mediators Inflamm 2010:813610. https://doi.org/10.1155/2010/813610
Zeng S, Wu J, Liu J, Qi F, Liu B (2015) IL-33 receptor (ST2) signalling is important for regulation of Th2-mediated airway inflammation in a murine model of acute respiratory syncytial virus infection. Scand J Immunol 81(6):494–501. https://doi.org/10.1111/sji.12284
Zhao Y, Jamaluddin M, Zhang Y, Sun H, Ivanciuc T, Garofalo RP, Brasier AR (2017) Systematic analysis of cell-type differences in the epithelial secretome reveals insights into the pathogenesis of respiratory syncytial virus-induced lower respiratory tract infections. J Immunol 198(8):3345–3364. https://doi.org/10.4049/jimmunol.1601291
Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, ..., Ziegler SF (2005) Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6(10):1047–1053. https://doi.org/10.1038/ni1247
Zhou N, Li W, Ren L, Xie X, Liu E (2017) An interaction of LPS and RSV infection in augmenting the AHR and airway inflammation in mice. Inflammation 40(5):1643–1653. https://doi.org/10.1007/s10753-017-0604-7
Zhu Y, Chen L, Huang Z, Alkan S, Bunting KD, Wen R, ..., Huang H (2004) Cutting edge: IL-5 primes Th2 cytokine-producing capacity in eosinophils through a STAT5-dependent mechanism. J Immunol 173(5):2918–2922. https://doi.org/10.4049/jimmunol.173.5.2918
Funding
We are grateful for the financial support from National Natural Science Foundation of China (Grant Nos.: 81974014 and 82211530115), the Natural Science Foundation of Shaanxi Province (Project No.: 2021JQ-024), Xi’an Talent Program (Project No.: XAYC200023), Xi’an Health Commission (COVID-19 special project), and research funds of Xi’an Children’s Hospital (Project No.: 2022A01).
Author information
Authors and Affiliations
Contributions
P. S.: validation, visualization, writing—original draft preparation; C. J.: validation, visualization, writing—original draft preparation, funding acquisition, reviewing and editing; Y. Z.: conceptualization, project administration, supervision, conceptualization, funding acquisition, writing—reviewing and Editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, P., Jiang, C. & Zhang, Y. The implication of infection with respiratory syncytial virus in pediatric recurrent wheezing and asthma: knowledge expanded post-COVID-19 era. Eur J Clin Microbiol Infect Dis 43, 403–416 (2024). https://doi.org/10.1007/s10096-023-04744-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04744-0