Abstract
Hospitals regularly seek to upgrade their antimicrobial stewardship program (ASP). Our aim was to evaluate the impact of simplified therapeutic guidelines (STGs) compared to various established tools for ASP on the rate of optimal antibiotic therapy (OAT) and antibiotic consumption. Audits of antibiotic prescriptions were carried out over a 24-month period. Feedback information led to STGs (e.g., ≤ 15 drugs). The impact of STGs was based on the rate of OAT, defined as a diagnosis of the infectious disease in the patient’s medical records associated with the corresponding therapy indicated in the STGs or in other guidelines. STGs were compared to five other means of ASP: internal or national guidelines, audit, information regarding antibiotic consumption and bacterial resistance, and restricted access to targeted antibiotics. Antibiotic consumption was measured in defined daily doses/1000 days of hospital stay, focusing on third-generation cephalosporins (TGC) and fluoroquinolones (FQ). Twenty-six hospitals were audited from April 2017 to June 2019. A total of 1,028 antibiotic prescriptions were analyzed, including 204 (20%) after STG implementation in seven hospitals. In multivariate analysis, OAT (n = 176, 17%) was associated with STGs, AOR 2.21 [1.51–3.22], and with three tools in place, 1.75 [1.24–2.48]. The relative variations of consumption of TGC and FQ for hospitals with or without STGs were − 13.1 vs. + 9.4% and − 18.5 vs. − 2.7%, respectively, from 2018 to 2019. STGs were more likely than other ASP tools to improve the rate of OAT and to reduce the consumption of antibiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial stewardship programs (ASPs) involve a combination of tools and services aimed at the reduction of the overuse of antibiotics and the associated emergence of multidrug-resistant (MDR) bacteria [1,2,3]. Although ASPs are based on a variety of complementary approaches, the involvement of a dedicated multidisciplinary committee for appropriate use of antibiotics, as well as audits with feedback, is key requirements [3,4,5].
However, given the limited number of available experts in the field of antimicrobial stewardship and due to the multiplicity of the targets, human resources — and therefore financial support — need to be greatly increased [6, 7]. Indeed, ASPs are a resource-intensive process, thus prompting complementary approaches to be considered to improve the quality of the antibiotic prescriptions and to reduce the number of prescriptions.
Considering the challenges that come with antibiotic resistance and the scarcity of continuous medical education in the field of antibiotic use, repeated audits and feedback appear to be the cornerstones of efficient ASPs that improve antibiotic practices [5, 8, 9]. Accordingly, we have previously reported that repeated audits and providing feedback information were followed by improvement of the quality of antibiotic prescriptions when the feedback was individualized by immediate dedicated sessions referred to as “accompanied self-antibiotic reassessment” (ASAR) [10]. ASARs are constructive discussions with the physicians regarding their own patients and their own prescriptions, taking into account the local setting and the epidemiology of bacterial resistance, which allow the ASM team and the practitioners to issue simplified therapeutic guidelines (STGs). The main idea of STG is to provide non-expert clinicians with a short list of antibiotics chosen from the national recommendations, easier to remember than a large panel, and best suited to their activities performed and to the epidemiology of bacterial resistance observed in their respective institutions [11]. Accordingly, these STGs may have some differences between institutions, and for example, treatments of meningitis were not implemented in the institutions without emergency department or with surgical activities only. Importantly, we also showed that STGs reduced the antibiotic consumption [10]. However, we found that they were not implemented in all of the participating hospitals, as some local antimicrobial stewardship teams were not in favor of deviation from national directives, considering that use of STGs was not in keeping with established good practices. Therefore, our aim was to study the impact of STG on the quality of antibiotic prescriptions by comparison with other institutions that were at different levels of ASP implementation.
Methods
This was a prospective multicenter study involving several private hospitals, working under the same administration and that had the same electronic patient records (EPR) system. Part of our method has been reported elsewhere [5].
In all of these facilities, the same infectious diseases (ID) physician (P-MR) met with the key members of the antimicrobial stewardship team, including the head of administration, and they reported each available ASP tool implemented in the facility: 1. the existence of an internal antibiotic guideline (irrespective of the medium, paper and/or intranet), 2. information regarding antibiotic consumption defined as evidence of at least one annual specific session for discussion between the pharmacist and the physicians, 3 information regarding antimicrobial resistance defined as evidence of at least one annual specific session for discussion between the infection control nurse/doctor or microbiologist and the physicians, 4. the list of restricted access antibiotics and how the access was restricted, and 5. results of previous antibiotic audits carried out in the year before the study. These five items were retained as they form the cornerstone of all ASPs, which are aimed at achieving responsible antimicrobial use, monitoring, and surveillance [12].
Ethics
The antibiotic audits were sponsored by the French National Health Agency, and the patients or their relatives provided written consent for computerization of their anonymized personal data for hospitalization and clinical research purposes according to the French national ethics recommendations.
Antibiotic audits
At each institution and over several periods of 2 consecutive days, all of the patients receiving antibiotic therapies were included through EPR selection. The EPRs included the patient’s entire medical history, all laboratory and radiological results, and documentation of any additional treatments. As the audits were in real-time clinical activities, the same data were hence available to the ID specialists and the prescribers. Two audits were carried out in the institutions that did not use the STGs, and four audits were carried out in the institutions in which the STGs were in place. All of the audits were followed by immediate feedback to the voluntary prescribers.
Non-infectious syndromes were defined as an obvious other diagnosis that explained the clinical presentation and/or the associated inflammatory syndrome [5].
An unspecified diagnosis was defined as the absence of an identified diagnosis or a suspected diagnosis of infectious disease upon full perusal of the EPR.
Definitions of healthcare-associated infections (HCAI) and community-acquired ones were those used in a previous study [5].
Antibiotic treatment analysis
Optimal antibiotic therapy (OAT) was defined as a well-established diagnosis and appropriate antibiotic use, including the empirical treatment and its reassessment. Thus, the analysis of the antibiotic therapy was per patient, whatever the number of antibiotics used. Then, the whole antibiotic therapy was evaluated in accordance with STGs or with the internal guidelines or the national recommendations used as references.
Antibiotic reassessment was defined as any modification of the first-line treatment. Reassessment with antibiotic simplification was defined as a reduction in the number of the prescribed antimicrobials or a narrower spectrum of activity of the drug used as a second-line treatment.
An adverse outcome was defined as the persistence or worsening of clinical symptoms over 3 days of the antibiotic therapy.
The presence of an antibiotic consultant at the institution, who should be identified by the institution’s head administrative office, the availability of ID specialist advice, and the related recommendation in the EPR were systematically recorded.
Data consistency was ensured by the ID physician, who acted as the coordinator of the ASP in the hospital partnership network and who participated in all of the institutions’ audits, in close collaboration with the pharmacist and/or the infection control referee.
Simplified therapeutic guidelines
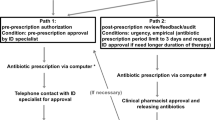
As described previously, the STGs included no more than 15 antibiotic drugs for most of the common infectious diseases reported by the participating hospitals, chosen in accordance with national guidelines, local microbiological data, and analysis of antibiotic consumptions [10]. The antibiotic treatment duration was the shortest time possible in order to limit antibiotic adverse effects and the emergence of MDR bacteria. Thus, the STGs avoided third-generation cephalosporins (TGC) drugs as well as fluoroquinolones in empirical treatments, instead favoring piperacillin/tazobactam in the context of HCAI. Carbapenem prescriptions were generally avoided, but prescribers retained the liberty to prescribe any antibiotic drug based on their own decision, irrespective of the STG recommendation. As an example, therapeutic propositions for urinary infections are available in Fig. 1.
Example of simplified therapeutic proposition for urinary infections. These propositions have to be compared with French guidelines in which community-acquired infections and healthcare-associated (HCA) ones are in separate documents [11]. Both empirical antibiotic therapies and antibiotic reassessment are described. In the real version, dosage and duration are indicated. Importantly, the ASM teams have to remember that STGs are the final result of a process including the analyses of microbial resistance, antibiotic consumptions, and real-time audit with immediate feedback at the prescriber’s level
Importantly, the introduction of STGs in the voluntary institutions resulted for other ASPs tools in a “dormant phase,” and notably, the list of restricted antibiotics was completely forgotten.
Antibiotic consumption
We measured antibiotic consumption at the institutional level using the national software Consores®, which all healthcare facilities are officially required to use to report their antibiotic consumption to the relevant health authorities (defined as daily doses per 1,000 days of hospitalization) [13]. The relative variation of antibiotic consumption from 2018 to 2019 was calculated as follows: (Q2019 − Q2018)/Q2018 × 100 (Fig. 2).
Relative variation of antibiotic consumption from 2018 to 2019. Data were available for seven hospitals with STGs and seventeen hospitals without this tool. In the latter, a median of three tools of ASP were in place. A Relative variation of total antibiotic consumption. B Relative variation of consumption of third-generation cephalosporins (TGC), fluoroquinolones (FQ), and piperacillin/tazobactam (Pip/taz)
Our primary outcome was to measure the impact of STGs on the quality of antibiotic therapies, determined by the rate of OAT. As one main goal of ASPs is to reduce the antibiotic consumption, our second outcome was to compare the latter before and after STGs’ implementation.
Statistical analysis
The data were analyzed with StatView software version 5.0, and statistical significance was established at α = 0.05. The continuous variables were compared with the Student’s t test or the Mann–Whitney test when appropriate. Proportions were compared with the χ2 or Fisher’s exact test when appropriate. Logistic regression was used to study the risk factors of OAT, and the results are presented as adjusted odds ratios (AORs) with their 95% confidence intervals (CIs). Variables were selected for the multivariate analysis based on the level of significance of the univariate association with OAT (p < 0.1). To determine the role of STGs in OAT, prescriptions after STGs implementations were audited through 2 other periods. Models were built up sequentially, starting with the variable most strongly associated with OAT and continuing until no other variable reached significance or altered the odds ratios of variables already in the model. When the final model was reached, each variable was dropped in turn to assess its effect.
Results
A total of 26 institutions were audited from April 2017 to March 2019, allowing analysis of the 1,028 patients receiving an antibiotic therapy, including 204 (20%) after STG implementation in seven hospitals. There were some structural differences between the institutions in terms of the number of beds (median, 170; range, 68 to 288) and the medical/surgical activities, with one institution performing only medical activities and another one undertaking only surgical activities. Of note, fifteen emergency departments and ten intensive care units were involved in these multicenter audits.
Antimicrobial stewardship program at baseline
The main characteristics of ASP implementation as well as the rate of OAT at baseline in these institutions are reported in Table 1. No ASP was implemented in 5 of the 26 hospitals (19.2%), and the ASPs of 4 of the 26 hospitals deployed the five considered tools. Regarding each ASP’s tool, 1. the guidelines were always full copies of the national ones, continuously available on the intranet, and were obviously weakly used; 2. reports of antibiotic consumption and antimicrobial resistance were rarely followed up as fewer than six physicians were present in these institutions that employed several dozen prescribers; 3. when there was a list of restricted access antibiotics, there was no real restriction for the prescribers except for the generation of a short list of items to justify the prescription, which resulted in an incomplete form filled by the nurses; and 4. the audits were mainly carried out on antibiotic prophylaxis, and most of the time there was no feedback. Thus, in 2017, the overall mean rate of OAT was 14.4%, and this was 10.0% in the seven institutions that had agreed to implement STGs.
Comparison of the number of tools of ASP in place in these institutions and the antibiotic consumption did not reveal any relationship: there was a median of 475, 320, 384, 402, and 366 DDD/1000 days of hospital stay with 1, 2, 3, 4, and 5 items, respectively, (p = 0.265). Additionally, we did not observe any correlation between the size of the institution, the medical or surgical specialties, and the number of tools already in place (data not shown).
There was no antibiotic referee in 9 (34.6%) of the 26 institutions.
Table 1 also shows the rate of OAT per institution at baseline; for institutions with STGs, the rate of OAT was 10.0%. There was no relationship with the ASP implementation according to the number of tools in place, with median rates of 15.3, 10.4, 16.8, 19.0, 7.0, and 21.4% for 1, 2, 3, 4, and 5 items, respectively, (p = 0.257). Also, the median rate of OAT was similar in clinics with or without internal guidelines, 13.9 vs 14.8% respectively.
Regarding the antibiotic treatments for 1,028 patients, a recommendation was obtained in 121 cases (11.8%), mainly from the antibiotic referee (101 recommendations, amounting to 83%). An antibiotic recommendation was obtained mostly for endocarditis (12/27 cases, i.e., 44.4%) or osteomyelitis and joint infections (17/53 cases, i.e., 32.0%) and far fewer in the other disease-related groups: urinary infections, 26/277 (9.3%), or pulmonary infections, 16/203 (7.8%), p < 0.001.
Risk factors for optimal antibiotic therapy
An OAT was noted in 176 cases (17%). It should be pointed out that the absence of a diagnosis in the patient’s file was encountered in 173/852 (20%) cases of non-optimal antibiotic therapy. Of note, the rate of OAT in institutions after STG implementation was 27.9%.
At the time of the audit, the duration of the antibiotic therapy was more than 3 days in 824 cases (80%). An antibiotic reassessment was noted in 289 cases (28%), being more frequently observed when STGs were used: 68/204 (33%) vs. 221/824 (27%), p = 0.063. The simplification of the treatment was observed in 121 cases (12%) and more frequently when the STGs were used: 37/68 (54%) vs. 84/221 (38%), p = 0.016.
Table 2 shows the factors associated with the OAT identified by univariate and multivariate analysis. Notably, osteomyelitis, joint infections, and endocarditis were associated with an OAT, since these diagnoses (n = 80) were associated with a higher rate of therapeutic recommendations. Furthermore, as the STGs had been devised to reduce the use of FQ and to choose piperacillin/tazobactam in HCAI, these antibiotics were differentially distributed between OAT and other antibiotic treatments. Regarding ASP tools, the multivariate analysis showed that the STGs were the main risk factor for OAT: AOR [95% CI] 2.21 [1.51–3.22]. No single ASP tool was significantly associated with OAT, even when the combination of three of them significantly increased its rate: AOR 1.75 [1.24–2.48]. Importantly, an OAT was protective of an adverse clinical outcome, AOR 0.17 [0.06–0.49], but we did not find any relationship between the combination of three items and clinical outcomes (data not shown).
Antibiotic consumption
Finally, we studied the antibiotic consumption in these hospitals using the national software Consores®. Although antibiotic consumption is meant to be measured in all hospitals in France, we were only able to obtain this information for seven institutions with STGs and seventeen institutions without these combined tools. Figure 1 shows that there was a trend towards more of a decrease in the relative variation of consumption of TCG and FQ from 2018 to 2019 in hospitals with STGs compared to the others. Interestingly, despite the fact that piperacillin/tazobactam was promoted in STGs for HCAI in surgery, the relative increase in the use of this drug was similar in both groups of institutions.
Discussion
Our study shows that ASP implementation was heterogeneous between the hospitals, and that among the various tools of the ASP, the STGs were the main factor associated with an OAT, leading to nearly threefold the baseline rate of OAT in the institutions that used them. Furthermore, OAT was associated with a higher rate of favorable clinical outcomes. Importantly, these results were obtained despite that the other ASP tools have been set aside.
A number of limitations of our study need to be pointed out. First, 1028 antibiotic treatments could appear as a low number for 26 audited institutions, but some of them had a low recruitment of infected patients. Second, we did not search for a specific impact of each tool in place in the ASP. However, several previous studies have reported that audits and feedback are the most efficient way to improve the quality of antibiotic prescriptions, followed by pre-prescription authorization [9, 14]. Indeed, STGs have been reported to be the result of successive audits with feedback in real-time [10]. Thirdly, a favorable outcome was defined as short-term clinical improvement, which may not be synonymous with sustained recovery. However, this association between a favorable outcome and STGs precluded to complete the study with interrupted time series analysis for ethical reasons.
In our study, there was no ASP in 19.2% of the participating institutions, and an incomplete ASP implementation was the rule. Our results are in accordance with a recent national online survey in France showing that 16% (15/95) of the public and private hospitals had not initiated an ASP and that the main tools were not systematically in place [15]. In a Dutch survey, the implementation of an ASP was not present in 6% of the participating institutions (4/63), but a list of restricted antibiotics and bedside consultations was present in only 64% and 56% of the latter, respectively [16]. The reason for these results appears to be the lack of ID and infection control specialists [6, 7]. Moreover, in the participating private hospitals, the bacteriology lab is often an independent organization, for which the hospital only pays for part of their expenditures. Thus, by not being fully part of these institutions, the microbiologists were not able to ascertain what information may improve the quality of the antibiotic prescriptions.
A rate of OAT close to 30% in institutions with STGs could still be considered to be low.
However, our definition of an OAT was restrictive as it combined a precise diagnosis and the proposed drugs in the STGs, including empirical ones and reassessment, and it has not been used to date. This result indicates an effective use of STGs, contrasting with the absence of impact of the internal guidelines observed at baseline between institutions with or without the latter, showing a similar rate of OAT. Accordingly, the simplification of the antibiotic therapy was more frequently observed when the STGs were implemented. In other words, STGs were associated with behavioral changes.
The tripling of the baseline rate of OAT in the institutions with STGs was obtained despite that other previous tools in place before STGs have been set aside. This was made possible because the practice of real-time audits indicated that non-expert practitioners often did not fully know what to do with these tools. The translation of antibiotic consumption in DDD into change in daily prescriptions is difficult for them. Also, as many empirical antibiotic therapies appear to be efficacious [5], the physicians are not helped in the process of therapeutic simplification, notably in the absence of bacteriological data. Accordingly, a previous large study found that 66% of the broad-spectrum antibiotics remained unchanged by the 5th day of therapy [17]. In contrast, the process of simplification, with STGs containing fewer than fifteen drugs, was devised through antibiotic audits and real-time feedback in which the physicians were directly involved, leading to the perception by the latter that this tool allowed adequate antibiotic therapies to be provided to their patients [10].
The higher rate of OAT associated with the STGs is due, at least in part, to the higher rate of a precise diagnosis being specified in the patient’s medical records, leading to more antibiotic reassessments with simplification. These results confirm our previous study that showed the importance of clinical management in ensuring the quality of antibiotic therapies [5]. Of note, it has been shown that there is an absence of a relationship between ASP implementation and the quality of the antibiotic therapy at the bedside [4]. Once again, the reason for such a relationship between STGs and a precise diagnosis in the patient’s file is most likely that the former were based on immediate feedback, allowing for some shortcomings in the patient’s file to be noted by the prescribers. Clearly, an unknown diagnosis is a barrier to the antibiotic reassessment, the latter being based on the accuracy of the former. Indeed, a number of reports have revealed a high rate of unknown diagnoses in case of patients with sepsis, from 10 to > 30% [18,19,20]. Consequently, unknown diagnoses were associated with unnecessary antibiotic therapies and/or unfavorable outcomes [5, 21,22,23,24]. Further studies should address the therapeutic challenges involved in antibiotic recommendations for patients with sepsis of unknown origin.
Lastly, our study revealed a trend towards less antibiotic consumption in the institutions using the STGs compared to the other institutions, which is in accordance with previous findings showing a significant decrease in critical antibiotics use (e.g., amoxicillin/clavulanic acid, third-generation cephalosporins, and fluoroquinolones) [10]. The absence of a significant difference in antibiotic consumption was linked to an institution with STGs in which the antibiotic consumption increased by 90% due to the departure of several prescribers between 2018 and 2019. Also, in the other institutions without STGs, the practitioners had benefited from the feedback from two rounds of antibiotic audits. These observations illustrate the importance of repeated audits and feedback every year for all physicians. Of note, two hospitals had modified their organization and function, making it difficult to compare antibiotic consumption from 1 year to the next.
Conclusion
STGs were the main tool of ASPs associated with OAT, the latter being associated with better clinical outcomes. STGs were also associated with a trend towards lower antibiotic consumption. Therefore, our results suggest that STGs could be included in the core of ASPs.
Data availability
The data used during the current study is available from the corresponding author on reasonable request.
Code availability
StatView software version 5.0.
Change history
29 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10096-022-04406-7
References
Pollack LA, Srinivasan A (2014) Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 59(S3):97–100
Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ et al (2016) Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51-77
Schuts EC, Hulscher MEJL, Mouton JW, Verduin CM, Cohen Stuart JWT, Overdiek HWPM et al (2016) Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 16(7):847–856
Étienne P, Roger PM, Brofferio P, Labate C, Blanc V, Tiger F et al (2011) Antimicrobial stewardship program and quality of antibiotic prescriptions. Med Mal Inf 41(11):608–612
Roger PM, Montéra E, Lesselingue D, Troadec N, Charlot P, Simand A et al (2019) Risk factors for unnecessary antibiotic therapy: a major role for clinical management. Clin Infect Dis 69(3):466–472
Le Coz P, Carlet J, Roblot F, Pulcini C (2016) Human resources needed to perform antimicrobial stewardship teams’ activities in French hospitals. Med Mal Infect 46(4):200–206
Ten Oever J, Harmsen M, Schouten J, Ouwens M, Van der Linden PD, Verduin CM et al (2018) Human resources required for antimicrobial stewardship teams: a Dutch consensus report. Clin Microbiol Infect 24(12):1273–9
Roger PM, Demonchy E, Risso K, Courjon J, Leroux S, Leroux E et al (2017) Medical table: a major tool for antimicrobial stewardship policy. Med Mal Infect 47(5):311–318
Tamma PD, Avdic E, Keenan JF, Zhao Y, Anand G, Cooper J et al (2017) What is the more effective antibiotic stewardship intervention: preprescription authorization or postprescription review with feedback? Clin Infect Dis 64(5):537–543
Roger PM, Peyraud I, Vitris M, Romain V, Bestman L, Blondel L et al (2020) Impact of simplified therapeutic guidelines on antibiotic prescriptions: a prospective multicentre comparative study. J Antimicrob Chemother 75(3):747–755
Roger PM, Michelangeli C, Girard D et al (2019) Streamlined guidelines for antibiotic therapies are required for greater efficacy. Med Mal Infect 49:363–366
Mendelson M, Morris AM, Thursky K, Pulcini C (2020) How to start an antimicrobial stewardship programme in a hospital. Clin Microbiol Infect 26(4):447–453
Boussat S, Demoré B, Lozniewski A, Aissa N, Rabaud C (2012) How to improve the collection and analysis of hospital antibiotic consumption: preliminary results of the ConsoRes software experimental implementation. Med Mal Infect 42(4):154–160
Spivak ES, Cosgrove SE, Srinivasan A (2016) Measuring appropriate antimicrobial use: attempts at opening the black box. Clin Infect Dis 63(15):1639–1641
Binda F, Tebano G, Kallen MC, Tebano G, Pulcini C, Murri R et al (2020) Nationwide survey of hospital antibiotic stewardship programs in France. Med Mal Infect 50(5):414–422
Kallen MC, Ten Oever J, Prins JM, Kullberg BJ, Schouten JA, Hulscher ME (2018) A survey on antimicrobial stewardship prerequisites, objectives and improvement strategies: systematic development and nationwide assessment in Dutch acute care hospitals. J Antimicrob Chemother 73(12):3496–3504
Braykov N, Morgan DJ, Schweizer ML, Uslan DZ, Kelesidis T, Weisenberg SA et al (2014) Assessment of empirical therapy in six hospitals: an observational cohort study. Lancet Infect Dis 14(12):1220–1227
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310
Tsertsvadze A, Royle P, Seedat F, Cooper J, Crosby R, McCarthy N (2016) Community-onset sepsis and its public health burden: a systematic review. Syst Rev 5:81. https://doi.org/10.1186/s13643-016-0243-3
Lopansri BK, Miller Iii RR, Burke JP, Levy M, Opal S, Rothman RE et al (2019) Physician agreement on the diagnosis of sepsis in the intensive care unit: estimation of concordance and analysis of underlying factors in a multicenter cohort. J Intens Care 7:13. https://doi.org/10.1186/s40560-019-0368-2
Roger PM, Martin C, Taurel M, Fournier JP, Nicole I, Carles M et al (2002) Motives for the prescription of antibiotics in the emergency department of the university hospital center in Nice. A prospective study. Press Med 31(2):58–63
Coon ER, Maloney CG, Shen MW (2015) Antibiotic and diagnostic discordance between ED physicians and hospitalists for pediatric respiratory illness. Hosp Peds 5(3):111–8
Aillet C, Jammes D, Fribourg A, Léotard S, Pellat O, Etienne P et al (2018) Bacteraemia in emergency departments: effective antibiotic reassessment is associated with a better outcome. Eur J Clin Microbiol Infect Dis 37(2):325–331
Casaroto E, Marra AR, Camargo TZS, de Souza ARA, de Almeida CES et al (2015) Agreement on the prescription of antimicrobial drugs. BMC Infect Dis 30(15):248. https://doi.org/10.1186/s12879-015-0992-y
Funding
This study was carried out as part of our routine work.
Author information
Authors and Affiliations
Contributions
P-M. R, V. D., and A. R. contributed to the study design. P-M. R. and F. M. contributed to the statistical analysis; E. M., P-M. R., A. R., and V. D. contributed to the writing of the article; P-M. R., D. R., O. P., and M-J. M. contributed to the study design and patient inclusion.
Corresponding author
Ethics declarations
Ethics approval
The antibiotic audit was sponsored by the French National Health Agency.
Consent to participate
The patients or their relatives provided written consent for computerization of their personal data for hospitalization purposes and clinical research. In accordance with national directives, patient privacy was protected as no personal data were extracted or copied from the computerized chart.
Consent for publication
All authors have read the paper and consent to its publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: “V3” was incorrectly added at the end of the main title of the originally published article.
Rights and permissions
About this article
Cite this article
Roger, PM., Espinet, A., Ravily, D. et al. Simplified therapeutic guidelines: the main tool of antimicrobial stewardship programs associated with optimal antibiotic therapy. Eur J Clin Microbiol Infect Dis 41, 375–383 (2022). https://doi.org/10.1007/s10096-021-04317-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04317-z