Abstract
Mastitis, a disease that affects both dairy herds and humans, is recognized as the most common source of losses in the dairy industry. Antibiotics have been used for years as the primary treatment for mastitis. However, abuse of antibiotics has led to the emergence of resistant strains and the presence of drug residues and has increased the difficulty of curing this disease. In addition, antibiotics kill most of the microbes that are present in the digestive tract, leading to imbalances in the gut microbiome and destruction of the ecosystem that is normally present in the gut. Gut microbiota play an important role in the host’s health and could be considered the “second brain” of the body. In recent years, the gut microbiota and their metabolites, including lipopolysaccharide (LPS) and short-chain fatty acids (SCFAs), have been shown to participate in the development of mastitis. LPS is the main component of the cell walls of gram-negative bacteria. Overproduction of rumen-derived LPS injures the rumen epithelium, resulting in the entry of LPS into the blood and damaged liver function; once in the blood, it circulates into the mammary gland, increasing blood-barrier permeability and leading to mammary gland inflammation. SCFAs, which are produced by gut microbiota as fermentation products, have a protective effect on mammary gland inflammatory responses and help maintain the function of the blood-milk barrier. Recently, increasing attention has been focused on the use of probiotics as a promising alternative for the treatment of mastitis. This review summarizes the effects of the gut microbiome and its metabolites on mastitis as well as the current of probiotics in mastitis. This work may provide a valuable theoretical foundation for the development of fresh ideas for the prevention and treatment of mastitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis, a type of inflammation that occurs in the mammary gland, can be induced by mechanical irritation, microbial infection, and chemicophysical injury, and is a highly prevalent disease in dairy cows and humans. Mastitis is considered extremely important to the dairy industry worldwide because it causes economic losses due to reduced milk production, discarded milk, decreased likelihood of conception, premature culling, and treatment costs [40]. It is estimated that 15% of milk production per cow is lost due to the effect of mastitis [98]. In addition, mastitis is also a serious animal welfare issue because it is associated with pain, reduced well-being, and behavioral changes in the affected animals [73]. Antibiotics have long served as a major tool in the treatment of mastitis, but the cure rate is not very high, and side effects often occur. Thus, detailed knowledge of mastitis and identification of an effective solution to address the issue of how to prevent and treat mastitis is urgently needed to safeguard economic benefits within the dairy farming industry.
Gut microbiota, which are present in a large variety and in huge quantity, are an essential part of animals. The symbiotic relationship between host and bacteria is maintained by a highly intricate and extensive ecosystem [41, 59]. After Gordon found that gut microflora influence fat conservation, it was gradually realized that perturbations in gut microbiota not only contribute to intestinal disease but are also closely associated with certain metabolic illnesses such as diabetes, non-alcoholic fatty liver disease, colitis, obesity, and other diseases [39, 57, 123, 126]. A precious study showed that gut microbiota plays a protective role in chickens infected with influenza virus subtype H9N2 [128]. In addition, a significantly lower abundance of gut microbiota was observed in patients during the course of infection with Shiga toxin–producing E. coli (STEC) [32]. Furthermore, another study showed that gut microbiota protect against pneumonia induced by Streptococcus pneumoniae [97], suggesting that there may be a relationship between gut microbiota and infectious diseases such as mastitis. Many subsequent studies have proven that gut microbiota play a role in mastitis through their metabolites such as lipopolysaccharide (LPS) [48, 134] and short-chain fatty acids (SCFAs) [117, 118]. Rumen-derived LPS crosses the rumen epithelium and enters the bloodstream, subsequently passing through organs and tissues all over the body [35]. During the lactation stage, a great deal of the LPS in the blood enters the mammary gland, finally leading to mammary gland inflammation [134]. SCFAs, which are produced by bacterial fermentation of dietary fiber in the gut, have anti-inflammatory property; they have been shown to inhibit the production of pro-inflammatory cytokines and to decrease the pathological damage in mammary gland induced by LPS [118].
Sophisticated as an organism is, sophisticated coordination among tissues and organs ensures a well-arranged environment inside the body. In specialized compartments of the body, the movement of molecules between the blood and tissues is hindered by the so-called gatekeepers or barriers [96] such as the blood-milk barrier [120]. The commensal microflora provides the host with a barrier against the invasion of pathogens through maintaining the blood-milk barrier [103]. Evidence shows that changes in the gut microbiota can result in proliferation of specific pathogenic bacteria that produce higher levels of LPS within the digestive tract and that these bacteria then enter the mammary gland [132]. In addition, injecting LPS into the mammary gland decreases the threshold for PMN to cross the blood-milk barrier, triggering substantial PMN accumulation in the mammary gland [56]; this is clinically manifested as elevated SCC (somatic cell count) and increased susceptibility to mastitis. SCFAs are the most important energy source for the blood-tissue barrier [49]. Treatment with SCFAs was shown to inhibit the changes in the blood-milk barrier induced by LPS though regulating changes in associated tight junction proteins or HDACs [117, 118].
This review focuses on our current understanding of the role of the gut microbiota, especially its metabolites LPS and SCFAs, on the development of mastitis and on the role of probiotics in the treatment of mastitis. It may very well lead to a new approach to preventing and treating mastitis through modulation of the gut microbiome. It is hoped that this theory will also offer new insight into dealing with other infectious diseases.
Sources and species of milk microbiota
Milk is a highly nutritious food that can be obtained from mammals, including cows, goats, and humans. It has long been thought that the mammary gland is naturally free of resident bacteria and that the milk produced by a healthy mammary gland is germ-free; the observation that breast milk contains microorganisms was originally attributed to contamination by skin bacteria from the mother’s areola or bacteria present in the oral cavity of the offspring [116]. The use of traditional culture-dependent methods proved that the bacteria found in mammary gland teats belong to four main phyla, Firmicutes (76%), Proteobacteria (17.8%), Actinobacteria (4.9%), and Bacteroides (1.3%). In addition, the phyla Planctomycetes, Verrucomicrobia, Cyanobacteria, Chloroflexi, and unclassified bacteria were found by a clone library sequencing-based method to be present at low levels. In addition, some bacteria, such as Solobacterium, Clavibacter, and Arcanobacterium spp., were detected on the teat surface but were found in milk [114]. Raw cow’s milk has the potential to contain a large number of bacteria, including a significant LAB population that includes Lactococcus, Streptococcus, Lactobacillus, Leuconostoc, and Enterococcus spp. [24, 70]. Using a high-throughput DNA sequencing method, Masoud et al. identified a total of 256 bacterial species in milk: among these, Streptococcus thermophiles and Lactococcus lactis dominated, representing 43.7% and 19% of reads, respectively. Other microbiota in the milk, including Acinetobacter, Aeromonas, Brevibacterium, Corynebacterium, Lactobacillus, Pseudoalteromonas, Pseudomonas, and Staphylococcus, represented between 1.3 and 3.7% of the reads [71].
Over the last few years, however, with the development of detection methods based on more sensitive molecular methods of microbial identification, the theory that the healthy udder is germ-free has been challenged. In particular, the application of molecular DNA analysis has yielded results suggesting that commensal microbial communities exist within every mammary gland, healthy or not [81]. This may explain why some studies have found that some bacteria including Lactococcus, Lactobacillus, and Enterococcus as well as Leucobacter, Deinococcus, and Paracodus are present in milk but are not detected in the environment [112]. Bifidobacteria, a strict anaerobe, was present in milk, but it could not survive in the skin and is an unlikely source. In addition, microbiota from the mother’s intestine are also found in breast milk [45]. These findings suggest that microbiota present in other anatomical locations may enter the mammary gland in some way. Importantly, several studies have demonstrated the existence of an entero-mammary pathway through which some intestinal luminal microbiota are transported to the mammary gland through the mesenteric lymph nodes [86].
Although the detailed mechanism by which microbiota cross the intestinal barrier and reach the mammary gland has not been completely clarified, it is likely that immune cells, especially dendritic cells (DCs), are involved in the translocation of gut bacteria into breast milk [89]. DCs are able to take up nonpathogenic bacteria from the gut and subsequently carry them to the lactating mammary gland by opening the tight junctions between enterocytes without damaging the integrity of the epithelial barrier [25, 89]. Generally, the presence of occludin in DCs may be sufficient to loosen the epithelial tight junctions, and this destabilization is followed by the rapid formation of new junctions between the epithelial and the infiltrating DCs. When stimulated by target bacteria, DCs are recruited from the blood and are activated. They then upregulate the expression of occludin and distribute it to the cell surface and dendrites. This allows DCs to compete for epithelial occludin and open tight junctions. The infiltrating DCs then face the gut lumen and can directly sample bacteria [89]. DCs can retain live gut bacteria for several days and carry them to the mesenteric lymph nodes by passing through the lymphatic circulation; these bacteria can then be spread to distant organs, including the lactating mammary gland [67, 113]. In addition, research has suggested that the changes in hormone levels that occur in the later stages of pregnancy and during early lactation affect the expression of enterocyte tight junctions, leading to the taking up of gut microbiota by the mother due to an increasingly permeable intestine. These migrant gut bacteria are engulfed by lymphoid cells that home to the engorged breast and enter the milk, from which bacteria are released. There is evidence that numerous circulating lymphoid cells containing engulfed bacteria are present in lactating mothers, and labeled bacteria placed in the gut of lactating rodents were found in the animals’ breast milk [116]. In addition, the blood circulation was involved in the transfer of cells from gut-associated lymphoid tissue to the mammary gland. The bacteria and their genetic material were present in peripheral blood mononuclear cells and breast cells and were found in human lactation breast milk [25]. In addition, many studies have reported that live lactic acid bacteria are present in the blood of human subjects [47, 82]. Other studies have suggested that isolated Lactobacillus salivarius CECT 5713 and Lactobacillus gasseri CECT 5713 found in human milk have the ability to translocate across a Caco-2 cell monolayer mediated by DCs [91]. After pregnant mice received a labeled Enterococcus faecium M1a strain, this bacterium was isolated from milk samples [92].
Some recent studies have focused on the source of milk microbiota in ruminants. Using culture-independent methodology, the author tested the microbial composition and diversity of organisms derived from the milk, blood, and feces of healthy lactating cows. The results showed that Ruminococcus, Bifidobacterium, and the Peptostreptococcaceae family were present in all three types of samples from the same animals [129]. In addition, the results of a study comparing the microbial communities present in blood, feces, and uterine samples from the same cows at 0 and 2 days postpartum using deep sequencing and qPCR showed that major uterine pathogens such as Bacteroides, Porphyromonas, and Fusobacterium were some of the core genera present in blood and feces and in the vagina. In addition, uterine pathogens showed a strong and significant interaction with each other in the network of blood microbiota. These data indicate that blood harbors a unique microbiome that includes the main uterine pathogens and blood maybe as a route of transmission of uterine pathogens from the gut to the uterus in cows [44]. This evidence supports the existence of an endogenous entero-mammary pathway in ruminants; however, the detailed mechanism by which bacteria are transported to the circulation and to the mammary glands of ruminants is not clear.
Rumen microbiota
The rumen, the first compartment of the digestive tract of ruminants, is a very important digestive organ, and it contains a large amount of microbiota. The rumen microbiota are necessary for the proper physiological development of the rumen and for the animal’s ability to digest and convert plant mass into food products; they also participate in the development of many diseases, making them highly significant to the host [43]. Thus, it is necessary to understand the changes in rumen microbiota that occur during an animal’s lifetimes. During the first week after a calf’s birth, the relative proportions of the rumen are considerably smaller than those in the adult, and the rumen lacks some of its functional components. Thus, it is considered nonfunctional at this time. With the growth of the calf, the volume and function of the rumen gradually increase, and these changes are accompanied by development of the rumen microbiota [10, 53]. One study showed that the major bacteria present in the rumen of newborn calves are aerobic and facultative anaerobic microbial taxa. Subsequently, these bacteria are gradually replaced by exclusively anaerobic taxa when the animal is between 6 and 8 weeks of age. Recently, Jami et al. [43] used 16S rRNA to identify and characterize the overall bovine ruminal bacterial populations in 1-day-old calves to 2-year-old cows. Of the 15 phyla detected in the samples, Firmicutes, Bacteroidetes, and Proteobacteria were the dominant phyla regardless of the age of the animals. The percentage and composition of phyla in animals of different ages varied considerably, as shown by the fact that Firmicutes were more abundant in 1–3-day-old caves than in animals of other ages; the vast majority of the reads belonged to the genus Streptococcus. The Firmicutes decreased in the 2-month-old animals and gradually increased as the animals increased in age. The phylum Bacteroidetes was less abundant in 1–3-day animals than in older animals. Of the minor phyla present in the tested animals, Actinobacteria and Fusobacteria were present in animals of all ages and were more abundant in 1–3-day-old calves. Other phyla, including Tenericutes, Cyanobacteria, and TM7, were present in higher percentages in older animals than in newborn animals (Fig. 1) [43]. Li et al. also characterized the rumen microbiota of pre-ruminant calves fed milk replacer using pyrosequencing of hypervariable V3–V5 regions of the 16S rRNA gene and a whole-genome shotgun approach. In total, 15 bacterial phyla were detected in the rumen microbiota of 42-day-old animals. Among these, Bacteroidetes was the dominant phylum (74.8%), followed by Firmicutes (12.0%), Proteobacteria (10.4%), Verrucomicrobia (1.2%), and Synergistetes (1.1%). In rumen samples from 14-day-old animals, 170 bacterial genera were detected, while 45 genera were found in the core microbiome of pre-ruminant animals [63]. The composition of the ruminal microbiota of cows in early (76 to 82 days milk [DIM]), middle (151 to 157 DIM), and late (251 to 257 DIM) lactation has also been studied. The major phyla in the rumen of all groups were similar to those found in previous studies and included Bacteroidetes (49.42%), Firmicutes (39.32%), Proteobacteria (5.67%), and Tenericutes (2.17%); the most abundant genera included Prevotella (40.15%), Butyrivibrio (2.38%), Ruminococcus (2.35%), and Coprococcus (2.29%) as well as Succiniclasticum (2.28%). In addition, lower-efficiency cows (low GFE, high RFI) harbored a higher percentage of Anaerovibrio and Butyrivibrio, and more efficient cows (high GFE, low RFI) possessed total ruminal communities with a higher abundance of Coprococcus [46]. A similar study showed that M. elsdenii, C. catus, and Lachnospiraceae are more abundant in the microbiomes of efficient animals; these organisms are a source of the valuable SCFAs propionate and butyrate [11]. In addition, yaks (Bos grunniens) and Tibetan sheep (Ovis aries) possess adaptations for living in a high-altitude environment. Characterization of the rumen microbiomes of these animals has shown that the VFA-yielding pathway markedly enriched in high-altitude ruminants, as shown by significant upregulation of the expression of rumen microbial genes associated with VFA transport [136].
The effect of gut-derived LPS on the mastitis
The effect of gut-derived LPS on the mammary gland inflammatory response
Mastitis is one of the most prevalent diseases of cows, especially high-yield ones. In clinical practice, mastitis has been found to occur in cows at a high frequency during the early and peak lactation period. To make it possible to harvest better dairy products, dairy cows used in the dairy industry are always fed a highly concentrated diet. However, the use of concentrated feed may alter the composition and quantity of the ruminal microbiome; in other words, it may perturb the gut microbiota.
LPS, one of the main elements of the cell walls of gram-negative bacteria, is an important factor that induces inflammation. Because a large number of gram-negative bacteria are present in the gut, the gut microbiota constitute a huge reservoir of this endotoxin [29, 78]. Many studies have focused on exploring the mechanism of LPS-induced inflammation. Long-time feeding HC diet often results in lower ruminal pH, which may lead to subacute ruminal acidosis (SARA) [58]. A number of signs of SARA in dairy cows, including inflammation, the presence of an acute phase response, laminitis, and liver abscesses, have been attributed to the translocation of free LPS in gram-negative bacteria from the digestive tract to the interior circulation [35, 87]. In addition, one study showed that translocation of LPS from the gastrointestinal tract into the bloodstream could induce a systemic inflammatory response that activated the TLR4 signaling pathway and was accompanied by chromatin decompaction and demethylation of the proximal TLR4 promoter [18]. It is widely known that the liver is the main site for clearance of circulating LPS [33]. In animals undergoing a systemic inflammatory response induced by feeding of an HC diet, the hepatic clearance rate of LPS was elevated, but the percentage of removed LPS decreased, accounting for an increase in the total entry of LPS into the liver [17]. Furthermore, crossing of rumen-derived LPS into the bloodstream increases the levels of the inflammatory cytokines TNF-α, IL-1β, and IL-6 in peripheral blood after long-term feeding of an HC diet to dairy cows. The increased levels of LPS in the portal and hepatic veins further injure the hepatocytes and inhibit liver function, as shown by increases in the levels of TNF receptor–associated factor 6 (TRAF6), p-NF-κB, p38 MAPK, IL-1, and serum amyloid A (SAA) in the liver [35].
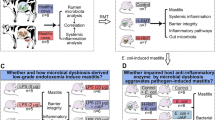
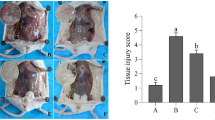
The rumen epithelium serves as an important biological barrier of immunity and acts as an important line of defense. Its main function is dependent on the rumen epithelium’s multicellular structure, which includes the stratum corneum, the stratum granulosum, the stratum spinosum, and the stratum basale, as well as on the tight junctions that exist in the stratum granulosum [34]. It is indicated that in ruminants fed HC diets, there is an increase in the level of the immunogenic compound LPS and abnormal depression of pH in the rumen, leading to damage to the expression or function of the rumen epithelium [85]. When the integrity of the rumen epithelium is damaged, LPS in the rumen or pathogenic bacteria attached to the rumen epithelium may translocate across it, further leading to a systemic inflammatory response [50]. Evidence for diet-induced systemic inflammation is associated with the HC diet–induced disruption of rumen epithelial barrier function [64] as well as with increased permeability of the rumen epithelium [54], which allows microbes and immunogenic compounds to enter the portal circulation [50] and further leads to systemic inflammation [135]. To study the mechanism through which feeding of an HC diet increases the permeability of the rumen epithelium, the author used GO to analyze the identified DEGs. The results showed that feeding of an HC diet induced the expression of genes associated with inflammation and that the levels of inflammatory genes, including IL-1β, IL-2, IL-22, CCL19, CCL8, CX3CR1, CXCL6, INHBE, LEPR, PRL, and TNFRSF9, were significantly increased in rumen epithelium. The level of LPS was also increased by feeding an HC diet. Increased levels of LPS are closely connected with the inflammatory response of the rumen epithelium, as shown by the fact that LPS treatment increased the expression of TNF-α, IL-8, and IL-6 [135]. Rumen epithelial bacteria are directly attached to the rumen epithelium and are thought to be important for adaptation to the daily diet. Studies in which PCR-DGGE was used showed that HC feeding changed the composition of the epithelial microbiota, increased the proportion of Bacteroidetes, and reduced the proportion of Firmicutes, changes that are associated with damage to the rumen epithelium [94]. Recent studies demonstrated that feeding mice a high-fat diet could lead to increased serum LPS, resulting in mammary gland inflammation [104]. Other studies showed that rumen-derived LPS increased the levels of pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8, and TLR4, NF-κB in the mammary gland during long-time feeding of a high-concentrate (HC) diet in cow [134], and increased the SCC in the cow mammary gland [48]. In our study, we also found that the serum LPS level was significantly increased in gut microbiota–depleted mice compared with that in wild-type mice. Fecal microbiota transplantation (FMT) to the gut of microbiota-depleted mice reversed this change. Changes in LPS levels were associated with changes in mammary gland inflammation. These unpublished results suggest that the development of mastitis in mice whose gut microbiota had been depleted was due in part to increased LPS levels.
These data suggest that changes in feeding or other stress factor lead to disturbances in the rumen microbiota. Changes in rumen microbiota cause the levels of LPS to increase significantly and lead to changes in the permeability of the rumen epithelium that allow LPS to pass through and enter tissues and organs via the bloodstream. The liver is the main organ responsible for the removal of LPS from the circulation. However, overproduction of LPS damages liver function and promotes the entry of LPS into the circulatory system through capillaries in tissues and organs, leading to chronic low-grade inflammation. Increased dairy production after birth increases blood flow; consequently, much more LPS enters the mammary gland, bringing about inflammation and increased susceptibility to mastitis [50].
The effect of gut-derived LPS on blood-milk barrier permeability
Dairy products harvested from cows suffering from mastitis contain increased somatic cell numbers (SCCs); SCC is the gold standard for the clinical diagnosis of mastitis as well as a reliable indicator of dairy quality [26]. Increased SCC corresponds to a reduction in the quantity and quality of dairy products. Based on previous research, the entry of a large volume of neutrophils (PMN), a major component of SCC, into mammary acini is the predominant reason for the elevation in SCC that occurs after infection of the mammary gland [83]. Therefore, the amount of PMN accessing mammary acini plays a paramount role in determining the severity and prognosis of mastitis. PMN must pass through the blood-milk barrier, which is made up of the vascular endothelium and the mammary epithelium, while crossing from the bloodstream to milk, a process that is under the control of the blood-milk barrier [133]. Disruption of the blood-milk barrier lowers the threshold against PMN crossing so that excessive numbers of PMN enter the mammary acini when the mammary gland has been irritated by pathogens. These PMN release high levels of inflammatory cytokines, leading to cytokine storm, the production of reactive oxygen free radicals, the activation of proteases, and consequent damage to the mammary gland [121] (Fig. 2).
The development of mastitis induced by pathogens. When pathogen escapes the defenses of the mammary gland teat, it enters the mammary gland and moves toward the milk pool. Macrophages are the primary immune cells that mediate the inflammatory responses of the body. They first contact and recognize the invading pathogen and then produce a large number of chemokines and some inflammatory cytokines. These further induce the accumulation of a large number of PMN in the infected mammary gland. In addition, these PMN release a large number of inflammatory mediators and ROS while removing pathogenic bacteria, leading to an inflammatory reaction in the mammary gland (mastitis). Importantly, to enter the mammary gland, PMN in the blood must cross a very important physiological barrier structure, the blood-milk barrier
The main components of the blood-milk barrier are tight junction proteins. These proteins form a specialized structure in the top membrane of the mammary epithelium; the structure is located between the mammary acini and controls the inward and outward passage of water molecules, ions, and bacteria [125, 137]. Various components, including occludin, zonula occludens-1 (ZO-1), and junctional adhesion molecules, make up the tight junctions present in mammals [61]. The blood-milk barrier, the blood-brain barrier (BBB), and the blood-testis barrier (BTB) share similar structures and functions. Some evidence suggests that the gut microbiota play an important role in the development of blood-tissue barriers such as BBB and BTB [15, 38, 74]. The maturation of the BBB and the BTB occurs slowly in germ-free mice and is accompanied by low expression of the tight junction proteins that constitute the BBB and BTB, such as claudin-5, occludin, and ZO-2. Fecal transplantation from healthy mice to germ-free mice increases the expression of these proteins [3, 15]. Research has shown that gut microbiota dysbiosis allows more LPS to enter the blood and that the expression of proteins relevant to tight junctions can be hindered by LPS, which finally devastates the blood-brain barrier [56, 117, 118]. When mastitis occurs in goats, cows, and other animals, the composition of occludin is altered, resulting in damage to the integrity of tight junctions [21]. In a mouse mastitis model, injection of LPS into the mammary gland was shown to cause changes in the expression of claudin-1, claudin-3, claudin-4, and claudin-7 that weaken the blood-milk barrier and lower the threshold for PMN crossing [56]. Our laboratory has assessed the effects of gut microbiota on blood-milk barrier permeability. The results demonstrated that the level of LPS was significantly increased in gut microbiota–depleted mice compared with wild-type mice, and these changes were accompanied by reduced expression of the tight junction proteins claudin-3 and occludin. However, administration of FMT to gut microbiota–depleted mice increased claudin-3 and occludin levels. FITC-albumin is an important factor that is used to evaluate the permeability of the blood-milk barrier [56]. We also tested the distribution of FITC-albumin in the alveolar lumen. We found that increased levels of LPS in microbiota-depleted mice caused increased distribution of FITC-albumin in the alveolar lumen and that FMT treatment of gut microbiota–depleted mice reversed these changes (unpublished observations). It is suggested that the increased levels of LPS caused by imbalances in the gut microbiota are closely associated with the function of the blood-milk barrier.
The role of SCFAs produced by gut microbiota on mastitis
The role of SCFAs produced by gut microbiota on mammary gland inflammatory response
Non-digestible carbohydrates, including cellulose, xylans, resistant starch, and inulin, are fermented to prove energy for the growth of microbiota and for the production of end products such as SCFAs [60, 108]. SCFAs are 1–6 carbons in length and are produced by fermentation of dietary fiber by the gut microbiota to butyrate, acetate, and propionate [2, 107]. It is well known that SCFAs provide approximately 70% of the energy source for ruminants, and they also serve as an important component of bovine milk [4, 42]. Although it has not been definitively determined through extensive bacterial isolation and metagenomics studies, some reports suggest that species differ greatly in their genetic makeup with respect to the enzymes that participate in SCFAs production [8, 20]. Acetate is the main product produced by enteric and acetogenic bacteria, and it is produced at higher levels than propionate and butyrate [75]. Propionate is produced from sugar molecules such as pentoses, hexoses, and rhamnose by three pathways, i.e., the succinate, acrylate, and propanediol pathways [88]. Usually, Bacteroidetes and some Firmicutes are good producers of propionate. This process occurs mainly via the succinate pathway [51]. Butyrate production is required for additional enzymatic processes such as the extension of acetyl-CoA by butyryl-CoA:acetate CoA-transferase. Some bacteria, including Roseburia, Eubacterium, and Anaerostipes species and Faecalibacterium prausnitzii, are good producers of butyrate because the enzymatic modification process is activated in these bacteria [65, 66].
In mammals, these metabolites are produced in varying ratios; approximately 60% is acetate, followed by propionate (~ 25%) and, to a much lesser degree, by butyrate (~ 15%) [27, 90]. In bovine ruminal fluid, approximately 50% is acetate, ~ 27% is propionate, and ~ 23% is butyrate [124]. SCFAs are absorbed in the colon and rumen epithelium, are transported to the portal vein, cross into the blood circulation, and are transported to other organs. SCFAs enter cells by passive diffusion and by carrier-mediated transport mediated by molecules such as SMCT1/SLC5a8 and MCTI/SLCI6a1 [62, 77, 127]. SMCT1, the sodium-coupled monocarboxylate transporter I, is required for cellular uptake of SCFAs and related organic acids such as lactate and pyruvate [77]. MCTI is an H+-coupled transporter of SCFAs and can also release organic acids. The transport of SCFAs by MCT1 is required to produce net chemical gradients of H+ and monocarboxylates across the membrane [36]. SMCTI and MCT1 are present in many cells, including colonocytes, DCs, kidney cells, brain cells, strial marginal cells, smooth muscle cells, and intestinal epithelial cells [31, 51, 52, 99, 131].
SCFAs modulate metabolic, nervous, inflammatory, and immunological functions primarily by activating G protein–coupled cell surface receptors (GPCR) such as GPR41, GPR43, and GPR109a as well as by inhibiting histone deacetylases (HDAC) [12, 28]. GPR41 and GPR43 are major receptors that can be activated by acetate, propionate, butyrate, and other SCFAs [16, 28], whereas GPR109a is mainly activated by butyrate [105, 106]. GPCR play an important role in immune responses regulated by SCFAs and are present on almost all cells in the immune system, including epithelial cells, neutrophils, and macrophages. Many studies have shown that SCFAs play an important role in the regulation of inflammation. There is evidence that butyrate and propionate inhibit LPS-induced TNF-α and nitric oxide synthase (NOS) expression through activating GPR41 and GPR43 receptors and GPR109A and that they inhibit HDACs in neutrophils [115]. Dietary fiber and its fermentation to SCFAs have been shown to have anti-inflammatory effects in asthmatic airways and this protective effect results in the upregulation of GPR41 and GPR43 gene expression [37]. Acetate-GPR43 interactions protect animals against dextran sulfate sodium (DSS)–induced colitis by inhibiting pro-inflammatory cytokine production by mononuclear cells [72]. Mice fed a high-fiber diet played increased circulating levels of SCFAs and were protected against allergic inflammation of the lung, whereas a low-fiber diet reduced circulating levels of SCFAs and aggravated this disease. Furthermore, treatment of mice with propionate improved the immunological environment in the lung and influenced the severity of allergic inflammation, and the protective effect of propionate was dependent on GRP41 [109]. Butyrate treatment inhibited inducible (iNOS), TNF-α, MCP-1, and IL-6 production by activation of GRP43 [80]. A role for GPR109A in immunity and inflammation has been suggested based on the observation that the expression of GPR109A is increased by treatment with cytokines such as IFNγ [95]. GPR109A protects against the inflammatory response in colonic macrophages and dendritic cells by inducing the differentiation of Treg cells and IL-10-producing T cells. Furthermore, GPR109A expression was shown to be associated with the butyrate-mediated induction of IL-8 in colonic epithelium [100]. Butyrate inhibited LPS-induced NF-κB activation in the colon of mice. The inhibitory effect of butyrate was dependent on GPR109A, shown by the fact that inhibition by butyrate did not occur in HCT116 cells transfected with GPR109A, which do not express [106]. Studies have also suggested that SCFAs exert anti-inflammatory properties by inhibiting HDACs in LPS-induced macrophages and dendritic cells. Treatment with butyrate and propionate was shown to inhibit TNF-α production through inhibition of activation of the NF-κB signaling pathway in LPS-stimulated mononuclear cells, and the effects of butyrate and propionate were similar to that of the HDAC inhibitor TSA [111]. In neutrophils, butyrate and propionate also inhibited TNF-α production and NF-κB signaling pathway activity through inhibiting HDACs after treatment of the cells with LPS [6].
In recent years, a protective effect of SCFAs on mastitis has been reported. Experiments conducted as part of a clinical investigation showed that the levels of some SCFAs in milk from clinical quarters differed from those in milk from control quarters. The data demonstrated that SCFAs levels were significantly lower and that the percentage of total neutral lipids was significantly higher in milk from clinical quarters compared with milk from control quarters [76]. Butyrate treatment reduced the internalization of S. aureus into bovine mammary epithelial cells (bMEC) by approximately 50% and increased the expression of tracheal antimicrobial peptide (TAP), β-defensin, and nitric oxide synthase (iNOS) [79]. Furthermore, it was also found that TSA, an HDAC inhibitor, inhibited pro-inflammatory cytokine production, suggesting that butyrate protects against LPS-induced mastitis through inhibition of HDAC [118]. Similarly, propionate was shown to reduce S. aureus internalization into bovine bMEC and to modulate antimicrobial peptide mRNA expression and propionate also conferred protection against LPS-induced mastitis by inhibiting pro-inflammatory cytokine production and NF-κB signaling pathway activation, as well as inhibiting HDACs in mice [117]. The results of another study suggested that treatment with acetate dose-dependently inhibited S. aureus internalization into bMEC by inhibiting activation of the NF-κB signaling pathway [119]. Recently, Shen et al. conducted important studies of the effects of butyrate on HC-induced damage to dairy goat mammary gland and rumen epithelium. They found that the molar proportion of propionate was increased in dairy cows fed a highly concentrated diet, whereas the proportional concentrations of other SCFAs were unchanged [124]. However, treatment with sodium butyrate significantly reduced rumen epithelium LPS levels, TNF-α, IL-1β, IL-6, MMP-2, and MMP-9 levels, MPO activity, and p-p65 expression induced by an HC diet. Furthermore, severe injury to the rumen epithelium induced by an HC diet was also ameliorated by dietary sodium butyrate [23]. Feeding lactating goats an HC diet induces an inflammatory response and apoptosis of cells in the mammary gland, whereas addition of sodium butyrate to the diet of lactating goats reduced the level of LPS and pro-inflammatory cytokines, subsequently inhibiting NF-κB and caspase-3 activation and eventually suppressing apoptosis of mammary gland cells [19]. This evidence suggests that supplementation of the diet with SCFAs plays an important role in maintaining mammary gland health.
The role of SCFAs produced by gut microbiota in blood-milk barrier function
SCFAs also serve as a critical energy source for host physiological barriers such as the gut barrier, the blood-brain barrier, the blood-testis barrier, and the blood-milk barrier. Butyrate is an energy source for colonocytes that can regulate gut epithelial barrier maintenance [102]. It can also induce colonic mucus secretion through promoting Muc2 and glycosyltransferase expression and promoting autophagy [122]. In type 1 diabetes, elevated circulating SCFAs levels improve symptoms by limiting the number of autoreactive T cells, inducting the numbers of Tregs, and enhancing the gut barrier [68]. In germ-free mice, the expression of claudin-5 and occludin in the BBB and the expression of occludin and ZO-2 in the BTB were lower than the levels found in wild-type mice; however, the protein components of the tight junctions in these barriers were restored to control levels by microbial colonization or by butyrate alone [15]. This may have occurred through epigenetic modification in the form of enhanced histone acetylation stimulated by butyrate [2, 3]. In addition, the gut microbiota disturbance caused by changes in the gut after exposure to provocative dietary agents favored low-grade systemic inflammation and altered SCFA utilization in the brain, changes that may lead to increased BBB permeability [69]. Recent reports have also indicated that SCFAs play an important role in blood-milk barrier function. It was shown that treatment with butyrate and propionate inhibited LPS-induced mastitis by restoring blood-milk barrier function and inhibiting the NF-κB signaling pathway as well as through HDAC inhibition [117, 118]. In our studies, we also found that the levels of butyrate, propionate, and acetate were significantly reduced in gut microbiota–depleted mice treated with ampicillin, neomycin sulfate, metronidazole, and vancomycin compared with those in wild-type mice, and decreases in the levels of SCFAs were associated with increased blood-milk barrier permeability. In addition, addition of sodium butyrate and sodium propionate to the diets of both gut microbiota–depleted mice and wild-type mice significantly inhibited the increase in blood-milk barrier permeability induced by S. aureus (unpublished observations). This evidence suggests that SCFAs maintain the blood-milk barrier in a way that protects against the development of mastitis.
Effect of probiotics on mastitis
Effect of probiotics on monogastric mastitis
According to the concept of mastitis as a manifestation of dysbiosis, i.e., an imbalance of the gut or rumen microbiota, the use of probiotics to re-equilibrate the microbiota appears as a possible corrective measure. Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer health benefits on the host [5]. Scientists have isolated various strains of probiotics from milk, and the presence of these organisms is thought to be protective against mammary gland infections or mastitis.
The use of probiotics to treat mastitis in breastfeeding women has been reported. Live culture treatment has the potential to be as effective at eliminating chronic subclinical infections as treatment with an antibiotic; 15 of 25 cases treated with the culture and 18 of 25 cases treated with an antibiotic did not exhibit clinical signs of the disease following treatment [55]. After 3 weeks of receiving Lactobacillus fermentum CECT5713 or Lactobacillus salivarius CECT5714, the mean bacterial counts in milk from the probiotic group were lower than those in milk from the control group; in addition, women receiving the probiotics experienced less pain and less frequent recurrence of mastitis than those assigned to the antibiotic group [7]. In a prevention experiment, it was shown that the incidence of mastitis in women who received Lactobacillus salivatius PS2 from approximately week 30 (25%) was lower than that in the control group (57%). When mastitis occurred, the bacterial counts in the milk from the probiotic group were markedly lower than those in milk from the control group [30]. In addition, a 16-week clinical trial showed that women receiving Lactobacillus fermentum CECT5716 during lactation showed a decrease of 51% in the incidence of clinical mastitis. In addition, oral administration of Lactobacillus fermentum CECT5716 resulted in lower levels of Staphylococcus spp. than those in the milk of women in the control group [84].
Effect of probiotics on ruminant mastitis
The use of probiotics to treat bovine mastitis in the dairy industry has also been widely studied. Lactococcus lactis DPC 3147 is a food-grade organism that exhibits broad-spectrum antimicrobial activity against mastitis-causing pathogens in vitro [93]. Lactococcus lactis DPC 3147 combined with a bismuth-based treatment has a protective effect on mastitis caused by Streptococcus dysgalactiae and S. aureus in dry cows [93, 110]. Others also found that the use of resuspended freeze-dried Lactococcus lactis is as effective as an antibiotic in curing clinical mastitis [55]. Treatment of the mammary glands of uninfected animals with the lactococcal culture produced an immunomodulatory effect. It was shown that Lactococcus lactis treatment resulted in substantial recruitment of PMN and lymphocytes to the infused quarters [22]. In addition, infusion with a live culture of Lactococcus lactis DPC 3147 leads to a rapid and considerable innate immune response, as shown by the increased expression of immune-related genes and high SCC levels. However, the immune response was short-lived, and SCC returned to pre-infusion levels within 1 week [9]. The use of single-molecule, real-time sequencing technology (SMRT) to measure changes in the bacterial community after treatment of cows with probiotic lactic acid bacteria (LAB) showed that LAB treatment reduced the number of mastitis-causing bacteria and improved the microbial environment of the cow teat. The results also suggested that SCC levels were lower after LAB treatment than after treatment with a commercial disinfectant [130]. Some isolated probiotics from milk have been shown to inhibit the growth of mastitis-causing bacteria in vitro, possibly through the production of bacteriocins [1]. Bouchard et al. isolated 165 lactic acid bacteria (LAB) from the bovine teat canal. Among these, they found that ten non-redundant LAB possess the ability to inhibit the mastitis-causing bacteria Staphylococcus aureus, Escherichia coli, and Streptococcus uberis and to reduce the colonization capacities of bovine mammary epithelial cells (bMEC), as well as possessing immunomodulatory properties. In addition, three strains exhibited high colonization capacity and moderate surface hydrophobicity, and nine strains exhibited anti-inflammatory properties in E. coli-stimulated bMEC [14]. Lactobacillus casei strains such as BL23 protect against invasion of bMEC by S. aureus by inhibiting the adhesion and internalization of S. aureus in a strain-dependent manner, suggesting that the inhibitory role of BL23 in S. aureus depends on interactions between L. casei cell surface components and bMEC [13]. Further research showed that the mutants serA1, srtA2, serC1, and srtC2 and a double mutant (srtA1-srtA2) of L. casei BL23 reduced its inhibitory capacity, especially in the case of the srtA2 mutant. In addition, a lower internalization capacity of L. casei srtA2 into bMEC was found. This proves the important role of sortase A2 in the inhibition of S. aureus internalization by L. casei BL23 [101]. To date, a large amount of evidence indicates that probiotics may provide an effective treatment measure and an alternative to antibiotics in the treatment of mastitis. Thus, the efficacy of probiotics in the treatment of mastitis should be evaluated.
Conclusion and Perspective
Mastitis is a benign inflammatory condition of the mammary gland with heterogeneous histopathological findings. Mastitis is recognized as one of the most common diseases affecting dairy herds. Bovine mastitis causes huge financial losses to the dairy industry due to reduced yield and milk quality, death, and treatment costs. Antibiotics have been used for years as the primary treatment for mastitis. However, the abuse of antibiotics has led to the emergence of resistant strains and the presence of drug residues, increasing the difficulty of curing this disease. In addition, administration of antibiotics kills most of the microbes in the digestive tract, leading to imbalances in the composition of the gut microbiota and destroying the normal exosystem within the digestive tract.
Gut microbiota play an important role in the development of mastitis, although the detailed mechanism of their action has not been reported. On one hand, changes in factors such as feeding will lead to rumen microbiota imbalance and reduced pH, impairing the integrity of the rumen epithelium and leading to entry of LPS into the rumen or the translocation of pathogenic bacteria across the rumen epithelium into the blood. When the liver is overloaded with endotoxins, substantial amounts of endotoxins escape detoxification, and flow into the capillary vessels pass through the bloodstream into organs and tissues all over the body, resulting in subclinical endotoxemia, a chronic systemic low-grade inflammation; under these conditions, the LPS circulating in the blood enters the mammary gland, especially during lactation. The increased level of LPS in the mammary gland damages the blood-milk barrier, lowering the threshold for PMN to cross the barrier. When stimulated in the mammary gland, a large number of PMN enter the gland and increase the susceptibility to mastitis. On the other hand, SCFAs, the end products of gut microbiota fermentation, are also involved in the development of mastitis. Although the detailed mechanism of the effect of SCFAs on mastitis has not been elucidated, SCFA treatment inhibits the development of mammary gland inflammation and reversed the blood-milk barrier permeability induced by LPS or S. aureus through inhibition of HDACs (Fig. 3). In addition, probiotic treatment has a protective effect against mastitis in both humans and animals. This suggested to us that probiotics or drugs that regulate the gut-mammary gland axis by increasing the production of SCFAs and inhibiting LPS may represent a new and promising direction in mastitis treatment. Hopefully, this theory will offer new insight into dealing with other infectious diseases.
The effect of gut microbiota on mastitis. The gut microbiota metabolites LPS and SCFAs are thought to be closely associated with the development of mastitis. Stressors, including feed alterations, disturb the regular gut microbiota, leading to the propagation of pathogenic bacteria that continually release LPS into the bloodstream through the rumen epithelium which may have been damaged by exposure to a lower pH. The gradually increasing levels of LPS injure the function of the liver, and LPS then enters the bloodstream. In lactating cows, the increased blood flow within the mammary gland enables much more LPS to enter the mammary gland tissue, decreasing the threshold for PMN crossing of the blood-milk barrier and finally increasing the SCC of the milk, a clinical diagnostic criterion for mastitis. In addition, SCFAs, especially butyrate and propionate, are the main energy source for the blood-milk barrier. Reducing the presence of SCFA-producing bacteria damages the function of the blood-milk barrier and further promotes the accumulation of PMN in the mammary gland when the mammary gland is stressed
References
Aguilar C, Vanegas C, Klotz B (2011) Antagonistic effect of Lactobacillus strains against Escherichia coli and Listeria monocytogenes in milk. J Dairy Res 78:136–143
Al-Asmakh M, Hedin L (2015) Microbiota and the control of blood-tissue barriers. Tissue Barriers 3:e1039691
Al-Asmakh M, Stukenborg JB, Reda A, Anuar F, Strand ML, Hedin L, Pettersson S, Soder O (2014a) The gut microbiota and developmental programming of the testis in mice. PLoS One 9:e103809
Alva-Murillo N, Ochoa-Zarzosa A, Lopez-Meza JE (2012) Short chain fatty acids (propionic and hexanoic) decrease Staphylococcus aureus internalization into bovine mammary epithelial cells and modulate antimicrobial peptide expression. Vet Microbiol 155:324–331
Amir LH, Griffin L, Cullinane M, Garland SM (2016) Probiotics and mastitis: evidence-based marketing? Int Breastfeed J 11:19
Aoyama M, Kotani J, Usami M (2010) Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26:653–661
Arroyo R, Martin V, Maldonado A, Jimenez E, Fernandez L, Rodriguez JM (2010) Treatment of infectious mastitis during lactation: antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin Infect Dis 50:1551–1558
Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ (2000) Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol 66:1654–1661
Beecher C, Daly M, Berry DP, Klostermann K, Flynn J, Meaney W, Hill C, McCarthy TV, Ross RP, Giblin L (2009) Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1beta and IL-8 gene expression. J Dairy Res 76:340–348
Beharka AA, Nagaraja TG, Morrill JL, Kennedy GA, Klemm RD (1998) Effects of form of the diet on anatomical, microbial, and fermentative development of the rumen of neonatal calves. J Dairy Sci 81:1946–1955
Ben Shabat SK, Sasson G, Doron-Faigenboim A, Durman T, Yaacoby S, Miller MEB, White BA, Shterzer N, Mizrahi I (2016) Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J 10:2958–2972
Bhutia YD, Ganapathy V (2015) Short, but smart: SCFAs train T cells in the gut to fight autoimmunity in the brain. Immunity 43:629–631
Bouchard DS, Rault L, Berkova N, Le Loir Y, Even S (2013) Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei. Appl Environ Microbiol 79:877–885
Bouchard DS, Seridan B, Saraoui T, Rault L, Germon P, Gonzalez-Moreno C, Nader-Macias FM, Baud D, Francois P, Chuat V, Chain F, Langella P, Nicoli J, Le Loir Y, Even S (2015) Lactic acid bacteria isolated from bovine mammary microbiota: potential allies against bovine mastitis. PLoS One 10:e0144831
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Guan NL, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S (2014a) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278:11312–11319
Chang G, Zhang K, Xu T, Jin D, Seyfert HM, Shen X, Zhuang S (2015a) Feeding a high-grain diet reduces the percentage of LPS clearance and enhances immune gene expression in goat liver. BMC Vet Res 11:67
Chang GJ, Zhuang S, Seyfert HM, Zhang K, Xu TL, Jin D, Guo JF, Shen XZ (2015b) Hepatic TLR4 signaling is activated by LPS from digestive tract during SARA, and epigenetic mechanisms contribute to enforced TLR4 expression. Oncotarget 6:38578–38590
Chang G, Yan J, Ma N, Liu X, Dai H, Bilal MS, Shen X (2018) Dietary sodium butyrate supplementation reduces high-concentrate diet feeding-induced apoptosis in mammary cells in dairy goats. J Agric Food Chem 66:2101–2107
Charrier C, Duncan GJ, Reid MD, Rucklidge GJ, Henderson D, Young P, Russell VJ, Aminov RI, Flint HJ, Louis P (2006) A novel class of CoA-transferase involved in short-chain fatty acid metabolism in butyrate-producing human colonic bacteria. Microbiology 152:179–185
Chen YH, Xu X, Sheng MJ, Zheng Z, Gu Q (2011) Effects of asymmetric dimethylarginine on bovine retinal capillary endothelial cell proliferation, reactive oxygen species production, permeability, intercellular adhesion molecule-1, and occludin expression. Mol Vis 17:332–340
Crispie F, Alonso-Gomez M, O'Loughlin C, Klostermann K, Flynn J, Arkins S, Meaney W, Ross RP, Hill C (2008) Intramammary infusion of a live culture for treatment of bovine mastitis: effect of live lactococci on the mammary immune response. J Dairy Res 75:374–384
Dai H, Liu X, Yan J, Aabdin ZU, Bilal MS, Shen X (2017) Sodium butyrate ameliorates high-concentrate diet-induced inflammation in the rumen epithelium of dairy goats. J Agric Food Chem 65:596–604
Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blumer N, von Mutius E, Bufe A, Gatermann S, Renz H, Holst O, Heine H (2007) Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol 119:1514–1521
Donnet-Hughes A, Perez PF, Dore J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ (2010) Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nutr Soc 69:407–415
Dufour S, Frechette A, Barkema HW, Mussell A, Scholl DT (2011) Invited review: effect of udder health management practices on herd somatic cell count. J Dairy Sci 94:563–579
Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, Flint HJ (2004) Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr 91:915–923
Eberle JAM, Widmayer P, Breer H (2014) Receptors for short-chain fatty acids in brush cells at the “gastric groove”. Front Physiol 5:152
Elin RJ, Wolff SM (1976) Biology of endotoxin. Annu Rev Med 27:127–141
Fernandez L, Cardenas N, Arroyo R, Manzano S, Jimenez E, Martin V, Rodriguez JM (2016) Prevention of infectious mastitis by oral administration of Lactobacillus salivarius PS2 during late pregnancy. Clin Infect Dis 62:568–573
Findeisen HM, Kahles FK, Bruemmer D (2013) Epigenetic regulation of vascular smooth muscle cell function in atherosclerosis. Curr Atheroscler Rep 15:319
Gigliucci F, von Meijenfeldt FAB, Knijn A, Michelacci V, Scavia G, Minelli F, Dutilh BE, Ahmad HM, Raangs GC, Friedrich AW, Rossen JWA, Morabito S (2018) Metagenomic characterization of the human intestinal microbiota in fecal samples from STEC-infected patients. Front Cell Infect Microbiol 8:25
Gozho GN, Plaizier JC, Krause DO, Kennedy AD, Wittenberg KM (2005) Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J Dairy Sci 88:1399–1403
Graham C, Simmons NL (2005) Functional organization of the bovine rumen epithelium. Am J Physiol Regul Integr Comp Physiol 288:R173–R181
Guo J, Chang G, Zhang K, Xu L, Jin D, Bilal MS, Shen X (2017) Rumen-derived lipopolysaccharide provoked inflammatory injury in the liver of dairy cows fed a high-concentrate diet. Oncotarget 8:46769–46780
Halestrap AP, Wang X, Poole RC, Jackson VN, Price NT (1997) Lactate transport in heart in relation to myocardial ischemia. Am J Cardiol 80:17A–25A
Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG (2017) Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients 9(1)
Harrington M (2015) For lack of gut microbes, the blood-brain barrier ‘leaks’. Lab Anim 44:14–14
He CX, Shan YJ, Song W (2015) Targeting gut microbiota as a possible therapy for diabetes. Nutr Res 35:361–367
Hertl JA, Grohn YT, Leach JD, Bar D, Bennett GJ, Gonzalez RN, Rauch BJ, Welcome FL, Tauer LW, Schukken YH (2010) Effects of clinical mastitis caused by gram-positive and gram-negative bacteria and other organisms on the probability of conception in New York State Holstein dairy cows. J Dairy Sci 93:1551–1560
Hooper LV, Gordon JI (2001) Commensal host-bacterial relationships in the gut. Science 292:1115–1118
Itavo LCV, dos Santos GT, Jobim CC, Voltolini TV, Ferreira CCB (2000) Evaluation of orange peel silage with different additives by rumen fermentation parameters and energy contribution from volatile fatty acids. Rev Bras Zootec 29:1491–1497
Jami E, Israel A, Kotser A, Mizrahi I (2013a) Exploring the bovine rumen bacterial community from birth to adulthood. ISME J 7:1069–1079
Jeon SJ, Cunha F, Vieira-Neto A, Bicalho RC, Lima S, Bicalho ML, Galvao KN (2017) Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 5(1):109
Jeurink PV, van Bergenhenegouwen J, Jimenez E, Knippels LMJ, Fernandez L, Garssen J, Knol J, Rodriguez JM, Martin R (2013) Human milk: a source of more life than we imagine. Benefic Microbes 4:17–30
Jewell KA, McCormick CA, Odt CL, Weimer PJ, Suen G (2015) Ruminal bacterial community composition in dairy cows is dynamic over the course of two lactations and correlates with feed efficiency. Appl Environ Microbiol 81:4697–4710
Jimenez E, Fernandez L, Marin ML, Martin R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodriguez JM (2005) Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol 51:270–274
Jin D, Chang G, Zhang K, Guo J, Xu T, Shen X (2016a) Rumen-derived lipopolysaccharide enhances the expression of lingual antimicrobial peptide in mammary glands of dairy cows fed a high-concentrate diet. BMC Vet Res 12:128
Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–671
Khafipour E, Krause DO, Plaizier JC (2009a) A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci 92:1060–1070
Kim CH, Park J, Kim M (2014) Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw 14:277–288
Klasvogt S, Zuschratter W, Schmidt A, Krober A, Vorwerk S, Wolter R, Isermann B, Wimmers K, Rothkotter HJ, Nossol C (2017) Air-liquid interface enhances oxidative phosphorylation in intestinal epithelial cell line IPEC-J2. Cell Death Discov 3:17001
Klein RD, Kincaid RL, Hodgson AS, Harrison JH, Hillers JK, Cronrath JD (1987) Dietary fiber and early weaning on growth and rumen development of calves. J Dairy Sci 70:2095–2104
Klevenhusen F, Hollmann M, Podstatzky-Lichtenstein L, Krametter-Frotscher R, Aschenbach JR, Zebeli Q (2013) Feeding barley grain-rich diets altered electrophysiological properties and permeability of the ruminal wall in a goat model. J Dairy Sci 96:2293–2302
Klostermann K, Crispie F, Flynn J, Ross RP, Hill C, Meaney W (2008a) Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: comparison with antibiotic treatment in field trials. J Dairy Res 75:365–373
Kobayashi K, Oyama S, Numata A, Rahman MM, Kumura H (2013a) Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. PLoS One 8:e62187
Koukias N, Buzzetti E, Tsochatzis EA (2017) Intestinal hormones, gut microbiota and non-alcoholic fatty liver disease. Minerva Endocrinol 42:184–194
Krause KM, Oetzel GR (2006) Understanding and preventing subacute ruminal acidosis in dairy herds: a review. Anim Feed Sci Technol 126:215–236
Kuznetsova TA, Makarenkova ID, Koneva EL, Aminina NM, Yakush EV (2015) Effect of probiotic product containing bifidobacteria and biogel from brown algae on the intestinal microflora and parameters of innate immunity in mice with experimental drug dysbacteriosis. Vopr Pitan 84:73–79
LeBlanc JG, Chain F, Martin R, Bermudez-Humaran LG, Courau S, Langella P (2017) Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact: 16(1):79
Lee JF, Zeng Q, Ozaki H, Wang LC, Hand AR, Hla T, Wang E, Lee MJ (2006) Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem 281:29190–29200
Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JKV, Plass C, Markowitz SD (2003) SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci U S A 100:8412–8417
Li RW, Connor EE, Li CJ, Baldwin RL, Sparks ME (2012) Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ Microbiol 14:129–139
Liu JH, Xu TT, Liu YJ, Zhu WY, Mao SY (2013) A high-grain diet causes massive disruption of ruminal epithelial tight junctions in goats. Am J Physiol Regul Integr Comp Physiol 305:R232–R241
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12:661–672
Macfarlane S, Macfarlane GT (2003) Regulation of short-chain fatty acid production. Proc Nutr Soc 62:67–72
Macpherson AJ, Uhr T (2004) Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662–1665
Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, Krishnamurthy B, Nefzger CM, Macia L, Thorburn A, Baxter AG, Morahan G, Wong LH, Polo JM, Moore RJ, Lockett TJ, Clarke JM, Topping DL, Harrison LC, Mackay CR (2017) Erratum: gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 18:1271
Marungruang N, Arevalo Sureda E, Lefrancoise A, Westrom B, Nyman M, Prykhodko O, Fak Hallenius F (2018) Impact of dietary induced precocious gut maturation on cecal microbiota and its relation to the blood-brain barrier during the postnatal period in rats. Neurogastroenterol Motil 30:e13285
Masoud W, Takamiya M, Vogensen FK, Lillevang S, Abu Al-Soud W, Sorensen SJ, Jakobsen M (2011) Characterization of bacterial populations in Danish raw milk cheeses made with different starter cultures by denaturating gradient gel electrophoresis and pyrosequencing. Int Dairy J 21:142–148
Masoud W, Vogensen FK, Lillevang S, Abu Al-Soud W, Sorensen SJ, Jakobsen M (2012) The fate of indigenous microbiota, starter cultures, Escherichia coli, Listeria innocua and Staphylococcus aureus in Danish raw milk and cheeses determined by pyrosequencing and quantitative real time (qRT)-PCR. Int J Food Microbiol 153:192–202
Masui R, Sasaki M, Funaki Y, Ogasawara N, Mizuno M, Iida A, Izawa S, Kondo Y, Ito Y, Tamura Y, Yanamoto K, Noda H, Tanabe A, Okaniwa N, Yamaguchi Y, Iwamoto T, Kasugai K (2013) G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm Bowel Dis 19:2848–2856
Medrano-Galarza C, Gibbons J, Wagner S, de Passille AM, Rushen J (2012) Behavioral changes in dairy cows with mastitis. J Dairy Sci 95:6994–7002
Michel L, Prat A (2016) One more role for the gut: microbiota and blood brain barrier. Ann Transl Med 4:15
Miller TL, Wolin MJ (1996) Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol 62:1589–1592
Miller RH, Bitman J, Bright SA, Wood DL, Capuco AV (1992) Effect of clinical and subclinical mastitis on lipid composition of teat canal keratin. J Dairy Sci 75:1436–1442
Miyauchi S, Gopal E, Fei YJ, Ganapathy V (2004) Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+−coupled transporter for short-chain fatty acids. J Biol Chem 279:13293–13296
Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C (2012) Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 108:801–809
Ochoa-Zarzosa A, Villarreal-Fernandez E, Cano-Camacho H, Lopez-Meza JE (2009) Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb Pathog 47:1–7
Ohira H, Fujioka Y, Katagiri C, Mamoto R, Aoyama-Ishikawa M, Amako K, Izumi Y, Nishiumi S, Yoshida M, Usami M, Ikeda M (2013) Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb 20:425–442
Oikonomou G, Machado VS, Santisteban C, Schukken YH, Bicalho RC (2012) Microbial diversity of bovine mastitic milk as described by pyrosequencing of metagenomic 16s rDNA. Plos One 7(10):e47671
Ouwehand AC, Saxelin M, Salminen S (2004) Phenotypic differences between commercial Lactobacillus rhamnosus GG and L-rhamnosus strains recovered from blood. Clin Infect Dis 39:1858–1860
Oviedo-Boyso J, Valdez-Alarcon JJ, Cajero-Juarez M, Ochoa-Zarzosa A, Lopez-Meza JE, Bravo-Patino A, Baizabal-Aguirre VM (2007) Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J Inf Secur 54:399–409
Paricio-Talayero JM, Baeza C (2018) Re: “Oral administration to nursing women of lactobacillus fermentum CECT5716 prevents lactational mastitis development: a randomized controlled trial” by Hurtado et al. (Breastfeed Med 2017;12:202-209). Breastfeed Med 13(6):453-454
Penner GB, Steele MA, Aschenbach JR, McBride BW (2011) Ruminant nutrition symposium: molecular adaptation of ruminal epithelia to highly fermentable diets. J Anim Sci 89:1108–1119
Perez PF, Dore J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A (2007) Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119:e724–e732
Plaizier JC, Khafipour E, Li S, Gozho GN, Krause DO (2012) Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim Feed Sci Technol 172:9–21
Reichardt N, Duncan SH, Young P, Belenguer A, Leitch CM, Scott KP, Flint HJ, Louis P (2014) Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 8:1323–1335
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P (2001a) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361–367
Richards JL, Yap YA, McLeod KH, Mackay CR, Marino E (2016) Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clin Transl Immunol 5:e82
Rinkinen M, Jalava K, Westermarck E, Salminen S, Ouwehand AC (2003) Interaction between probiotic lactic acid bacteria and canine enteric pathogens: a risk factor for intestinal Enterococcus faecium colonization? Vet Microbiol 92:111–119
Rodriguez JM (2014) The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr 5:779–784
Ryan MP, Flynn J, Hill C, Ross RP, Meaney WJ (1999a) The natural food grade inhibitor, lacticin 3147, reduced the incidence of mastitis after experimental challenge with Streptococcus dysgalactiae in nonlactating dairy cows. J Dairy Sci 82:2625–2631
Sadet-Bourgeteau S, Martin C, Morgavi DP (2010) Bacterial diversity dynamics in rumen epithelium of wethers fed forage and mixed concentrate forage diets. Vet Microbiol 146:98–104
Schaub A, Futterer A, Pfeffer K (2001) PUMA-G, an IFN-gamma-inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor superfamily. Eur J Immunol 31:3714–3725
Schlingmann B, Molina SA, Koval M (2015) Claudins: gatekeepers of lung epithelial function. Semin Cell Dev Biol 42:47–57
Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJTH, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, de Vos WM, van der Poll T, Wiersinga WJ (2016) The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65:575–583
Schukken YH, Hertl J, Bar D, Bennett GJ, Gonzalez RN, Rauch BJ, Santisteban C, Schulte HF, Tauer L, Welcome FL, Grohn YT (2009) Effects of repeated gram-positive and gram-negative clinical mastitis episodes on milk yield loss in Holstein dairy cows. J Dairy Sci 92:3091–3105
Shimozono M, Scofield MA, Wangemann P (1997) Functional evidence for a monocarboxylate transporter (MCT) in strial marginal cells and molecular evidence for MCT1 and MCT2 in stria vascularis. Hear Res 114:213–222
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi HD, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139
Souza RFS, Jardin J, Cauty C, Rault L, Bouchard DS, Bermudez-Humaran LG, Langella P, Monedero V, Seyffert N, Azevedo V, Le Loir Y, Even S (2017) Contribution of sortase SrtA2 to Lactobacillus casei BL23 inhibition of Staphylococcus aureus internalization into bovine mammary epithelial cells. PLoS One 12(3):e0174060
Spiljar M, Merkler D, Trajkovski M (2017) The immune system bridges the gut microbiota with systemic energy homeostasis: focus on TLRs, mucosal barrier, and SCFAs. Front Immunol 8:1353
Stecher B, Chaffron S, Kappeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, Macpherson AJ, Hardt WD (2010) Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog 6(1):e10000711
Subbaramaiah K, Howe LR, Bhardwaj P, Du BH, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang PY, Kopelovich L, Hudis CA, Dannenberg AJ (2011) Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res 4:329–346
Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG (2005) (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280:26649–26652
Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V (2009a) GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69:2826–2832
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064
Tremaroli V, Backhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166
Twomey DP, Wheelock AI, Flynn J, Meaney WJ, Hill C, Ross RP (2000) Protection against Staphylococcus aureus mastitis in dairy cows using a bismuth-based teat seal containing the bacteriocin, lacticin 3147. J Dairy Sci 83:1981–1988
Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, Kotani J (2008) Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res 28:321–328
Vacheyrou M, Normand AC, Guyot P, Cassagne C, Piarroux R, Bouton Y (2011) Cultivable microbial communities in raw cow milk and potential transfers from stables of sixteen French farms. Int J Food Microbiol 146:253–262
Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC (1999) Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804–808
Verdier-Metz I, Gagne G, Bornes S, Monsallier F, Veisseire P, Delbes-Paus C, Montel MC (2012) Cow teat skin, a potential source of diverse microbial populations for cheese production. Appl Environ Microbiol 78:326–333
Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R (2011) Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 22:849–855
Walker WA, Iyengar RS (2015) Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res 77:220–228
Wang J, Wei Z, Zhang X, Wang Y, Yang Z, Fu Y (2017a) Propionate protects against lipopolysaccharide-induced mastitis in mice by restoring blood-milk barrier disruption and suppressing inflammatory response. Front Immunol 8:1108
Wang JJ, Wei ZK, Zhang X, Wang YN, Fu YH, Yang ZT (2017b) Butyrate protects against disruption of the blood-milk barrier and moderates inflammatory responses in a model of mastitis induced by lipopolysaccharide. Br J Pharmacol 174:3811–3822
Wei ZK, Xiao C, Guo CM, Zhang X, Wang YN, Wang JJ, Yang ZT, Fu YH (2017) Sodium acetate inhibits Staphylococcus aureus internalization into bovine mammary epithelial cells by inhibiting NF-kappa B activation. Microb Pathog 107:116–121
Wellnitz O, Wall SK, Saudenova M, Bruckmaier RM (2014) Effect of intramammary administration of prednisolone on the blood-milk barrier during the immune response of the mammary gland to lipopolysaccharide. Am J Vet Res 75:595–601
Wellnitz O, Zbinden C, Huang X, Bruckmaier RM (2016) Short communication: differential loss of bovine mammary epithelial barrier integrity in response to lipopolysaccharide and lipoteichoic acid. J Dairy Sci 99:4851–4856
Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA (2003) Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 52:1442–1447
Xie YQ, Xiao M, Ni YL, Jiang SF, Feng GZ, Sang SG, Du GK (2018) Alpinia oxyphylla Miq. Extract prevents diabetes in mice by modulating gut microbiota. J Diabetes Res 4;2018:4230590
Xu T, Tao H, Chang G, Zhang K, Xu L, Shen X (2015) Lipopolysaccharide derived from the rumen down-regulates stearoyl-CoA desaturase 1 expression and alters fatty acid composition in the liver of dairy cows fed a high-concentrate diet. BMC Vet Res 11:52
Xu CM, Li XM, Qin BZ, Liu B (2016) Effect of tight junction protein of intestinal epithelium and permeability of colonic mucosa in pathogenesis of injured colonic barrier during chronic recovery stage of rats with inflammatory bowel disease. Asian Pac J Trop Med 9:143–147
Xu J, Chen N, Wu Z, Song Y, Zhang YF, Wu N, Zhang F, Ren XH, Liu Y (2018) 5-Aminosalicylic acid alters the gut bacterial microbiota in patients with ulcerative colitis. Front Microbiol 9:1274
Yanase H, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T (2008) Cellular expression of a sodium-dependent monocarboxylate transporter (Slc5a8) and the MCT family in the mouse kidney. Histochem Cell Biol 130:957–966
Yitbarek A, Weese JS, Alkie TN, Parkinson J, Sharif S (2018) Influenza A virus subtype H9N2 infection disrupts the composition of intestinal microbiota of chickens. FEMS Microbiol Ecol 94(1)
Young W, Hine BC, Wallace OAM, Callaghan M, Bibiloni R (2015) Transfer of intestinal bacterial components to mammary secretions in the cow. PeerJ 3:e888
Yu J, Ren Y, Xi X, Huang W, Zhang H (2017) A novel lactobacilli-based teat disinfectant for improving bacterial communities in the milks of cow teats with subclinical mastitis. Front Microbiol 8:1782
Yurchenko V, Constant S, Bukrinsky M (2006) Dealing with the family: CD147 interactions with cyclophilins. Immunology 117:301–309
Zebeli Q, Ametaj BN (2009) Relationships between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows. J Dairy Sci 92:3800–3809
Zhang CK, Zhai SM, Wu L, Bai YH, Jia JB, Zhang Y, Zhang B, Yan B (2015) Induction of size-dependent breakdown of blood-milk barrier in lactating mice by TiO2 nanoparticles. PLoS One 10(4):e0122591
Zhang K, Chang G, Xu T, Xu L, Guo J, Jin D, Shen X (2016a) Lipopolysaccharide derived from the digestive tract activates inflammatory gene expression and inhibits casein synthesis in the mammary glands of lactating dairy cows. Oncotarget 7:9652–9665
Zhang R, Zhu W, Mao S (2016c) High-concentrate feeding upregulates the expression of inflammation-related genes in the ruminal epithelium of dairy cattle. J Anim Sci Biotechnol 7:42
Zhang ZG, Xu DM, Wang L, Hao JJ, Wang JF, Zhou X, Wang WW, Qiu Q, Huang XD, Zhou JW, Long RJ, Zhao FQ, Shi P (2016d) Convergent evolution of rumen microbiomes in high-altitude mammals. Curr Biol 26:1873–1879
Zhao GJ, Li D, Zhao Q, Lian J, Hu TT, Hong GL, Yao YM, Lu ZQ (2016) Prognostic value of plasma tight-junction proteins for Sepsis in emergency department: an observational study. Shock 45:326–332
Funding
This work was supported by a grant from the National Natural Science Foundation of China (nos. 31602122, 31472248).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, X., Li, S., Fu, Y. et al. Targeting gut microbiota as a possible therapy for mastitis. Eur J Clin Microbiol Infect Dis 38, 1409–1423 (2019). https://doi.org/10.1007/s10096-019-03549-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03549-4