Abstract
Clindamycin has high bioavailability together with good diffusion in bone tissue and could represent an alternative antibiotic compound for the treatment of bone and joint infections (BJIs). However, data regarding the efficacy and safety of clindamycin for BJIs are limited. A monocentric cohort study based on our medical dashboard, which prospectively recorded 28 characteristics for all hospitalized patients since July 2005, was performed. BJIs were selected, and then, all mono-microbial BJI managed with clindamycin-based therapy were included. Remission was defined as the absence of clinical and/or microbiological relapse after treatment. The duration of follow-up without relapse was determined retrospectively using computerized medical records. For 10 years, 196 BJIs, of which 80 (41%) were device-associated infections, were treated with clindamycin-based therapy. The bacterial causative agent was Staphylococcus aureus in 130 cases (66%), coagulase-negative staphylococci in 29 cases (15%), streptococci in 31 cases (16%) and other bacteria in 6 cases (3%). When used in combination therapy, clindamycin was mainly paired with fluoroquinolones (31%) or rifampin (27%). The mean duration of clindamycin treatment was 7.4 ± 3.2 weeks (range, 1–24). An AE was recorded for 9 (4.5%) patients. Remission was recorded for 111 (57%) patients, with a mean duration of clinical follow-up of 28 ± 24 months. Treatment failure occurred in 22 (11%) patients, 50 patients (25%) were lost to follow-up, and 8 (4%) required long-term suppressive therapy. Among the assessable patients, clindamycin-based therapy was efficient in 111/133 cases (83%) and thus represents a reliable and safe alternative treatment option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Community-acquired bone and joint infections (BJIs) are frequent, especially septic arthritis and pyogenic vertebral osteomyelitis (PVO) as well as bone infections resulting from pressure ulcers [1]. Additionally, an increasing number of patients currently benefit from joint prostheses due to aging, trauma and improved surgical techniques. However, BJI may complicate orthopaedic surgery, even without device implantation [2, 3]. The main pathogen involved in these BJIs in humans is Staphylococcus aureus, followed by coagulase-negative staphylococci and Streptococcus spp. Other bacteria from the skin bacterial flora have also been implicated in surgical infections, especially Propionibacterium acnes [4].
Several guidelines have been published regarding the use of antibiotic therapy in conjunction with surgery when necessary [5, 6]. These recommendations provide quite similar advice; for BJI caused by Staphylococcus spp., the assumed best treatment choice is combination therapy with a fluoroquinolone + rifampicin. However, adverse effects (AEs) are frequent because BJI require high doses of antibiotic and prolonged treatment durations [5,6,7].
We reported that between 20 and 30% of patients may present an AE related to antibiotic therapy for BJIs [8,9,10]. Thus, the search for alternative antibiotic therapies for patients with BJIs is of great clinical significance.
Clindamycin, a derivative of lincomycin, is an old antibiotic with excellent in vitro activity against most strains of Staphylococcus aureus [11]. Clindamycin diffuses well into bone, reaching good bone concentrations [12, 13]. To date, only a few clinical studies have examined the efficacy of clindamycin in treating human bone infections [14,15,16], which, in addition to its poorly estimated safety, prevents this antibiotic compound from being recommended in the national treatment guidelines. Therefore, our aim was to report our experience with a large cohort of patients with BJIs for whom antibiotic therapy included clindamycin.
Methods
Selection and characterization of patients
We conducted an observational study at the Nice Teaching Hospital, a tertiary care centre with only one infectious diseases department. This study was based on data collected through our medical dashboard beginning in July 2005.
Our medical database integrates 28 parameters for all hospitalized patients, including clinical diagnosis, microbiological information, antibiotic therapy, AEs and outcome [17].
We selected all of the recorded cases of BJI (septic arthritis, osteomyelitis and PVO) through June 2016 to characterize the patients treated with clindamycin that were adults and had a BJI diagnosis regardless of the mechanism involved. Chronic bone infection was defined as a history of disease >1 month.
In our practice, BJI diagnoses are made based on bone biopsy results that are obtained through invasive procedures or abscess drainage via radiological or surgical means.
BJI were defined as healthcare-associated (HA) if the diagnosis was established ≥48 h after hospital admission, ≤ 30 days after surgery or within 1 year after surgery involving foreign material implantation. In all other cases, the bone infections were classified as community-acquired (CA).
Clindamycin was mostly prescribed in a combination therapy, the approach indicated by the national recommendations for Staphylococcus spp. BJI. For Streptococcus spp. and other susceptible bacteria, a single agent is suggested. The standard first combination therapy is levofloxacin + rifampicin, but due to frequent AEs, we recommend clindamycin as the first alternative. These antibiotics were always administered via the enteral route, with dosage variations for body weight: ≤ 70 kg, 750 mg of levofloxacin once a day +1800 mg of clindamycin/day, and >70 kg, 500 mg of levofloxacin twice a day +2400 mg of clindamycin/day. Rifampicin was prescribed at 20 mg/kg/d without exceeding 1200 mg/day. Our protocol suggests 6 weeks of antibiotic treatment for osteomyelitis, PVO, device-related septic arthritis, and 3 weeks for native joint septic arthritis. Day 1 of treatment being the day after surgery if performed. Once antimicrobial susceptibility testing results are available, enteral route is used as soon as infective endocarditis is ruled out and patient’s condition allows it even if the patient has bacteremia.
Outcome
Bone infection was determined to be in remission by the absence of clinical and/or microbiological relapse after antibiotic therapy discontinuation. Patients without follow-ups after 6 months were considered lost to follow-up. For the patients who experienced treatment failure, we distinguished clinically documented failures, relapses (same bacterium) and new infections (different bacterium). Death during the 6 first months of follow-up was classified as early death. Suspensive therapy was defined as antibiotic treatment administered without a treatment end date scheduled.
AEs that occurred during hospitalization were systematically reported in the medical dashboard as those that required changing the prescribed antibiotic. To describe the AEs that occurred during ambulatory care, we used computerized patient charts in which the therapeutic modifications were described. The responsibility of a given antibiotic was affirmed by the disappearance of the AE after cessation of this drug.
Microbiological studies
Aerobic and anaerobic cultures of bone biopsies were performed using Columbia blood agar and chocolate PolyViteX agar (CO2) and maintained for 5 days. Liquid media enriched cultures (Hemolinediphasic and/or Rosenow media) were also performed and maintained for 10 days. We used a manual method (API, BioMérieux) and an automated method (Phoenix, Becton Dickinson) to identify bacteria at the species level.
Statistical analysis
Data were analyzed with StatView software version 4.5, and statistical significance was established at α = 0.05. Continuous variables were compared using Student’s t-test or the Mann–Whitney non-parametric test. Proportions were compared via the χ2 statistic or Fisher’s exact test when appropriate. Univariate correlates and clinically significant variables (p < 0.1) were then entered into a stepwise logistic regression analysis.
Results
For 124 months, 196 patients with BJIs were treated with clindamycin-based antibiotic therapy. Figure 1 describes the selection of this cohort of patients from our medical dashboard. There were 127 men (67%) and 69 females, with a mean age of 64 ± 17 years. The diagnoses included 120 cases (61%) of osteomyelitis, 42 cases (22%) of PVO and 34 cases (17%) of septic arthritis. In 80 cases (41%), the infection was related to a surgical device. There were 143 chronic infections (73%), and device-related infections were more frequently chronic: 67/80 versus 76/116, p = 0.004.
The bacterial causative agents of these 196 cases of arthritis or osteomyelitis included 130 cases (66%) of Staphylococcus aureus, 29 cases (15%) of coagulase-negative staphylococci, 31 cases (16%) of streptococci and 6 cases (3%) of other bacteria. Among the 130 S. aureus strains, 10 (7.7%) had an inducible MLSB phenotype. Notably, 176 patients (90%) benefited from blood cultures, and 38/176 patients (22%) presented with bacteremia.
For pathogen-directed antibiotic treatment, clindamycin was prescribed as a first-line agent in 95 cases (48%), as a second-line agent after an AE due to another drug in 67 cases (35%) and as a third-line agent in 34 cases (17%). The main reason for clindamycin treatment that was prescribed as a secondary line of treatment was an AE to a previous antibiotic. Thus, 190 patients (97%) were discharged from the hospital with a clindamycin-based treatment after a mean hospitalization duration of 15 ± 9 days. Enteral antibiotic therapy using levofloxacin + clindamycin was prescribed in 61 cases (31%), rifampicin + clindamycin in 53 cases (27%), clindamycin + another compound in 37 cases (19%), clindamycin alone in 31 cases (16%) and clindamycin + amoxicillin in 14 cases (7%). Characteristics of patients treated with clindamycin alone are reported in Table 1.
The duration of antibiotic therapy was 7 ± 3 weeks, and the mean duration of clinical follow-up was 28 ± 24 months, with 81 patients (41%) continuing follow-up for at least 2 years after the cessation of antibiotic therapy.
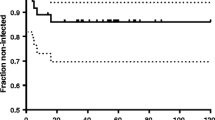
Figure 2 indicates the main clinical and therapeutic characteristics for these 196 patients as well as their final outcomes. Ten patients died. Early deaths occurred for 6 patients; only one was directly associated to BJI and was classified as treatment failure. Fifty (25.5%) patients were lost to follow-up. Among the assessable patients (those not lost to follow-up nor on suspensive therapy), therapeutic success was observed in 111/133 (83%) patients. Clinical failure was recorded for 8 patients, relapse for 8 patients and new infections for 6 patients. Characteristics of patients with therapeutic failure are reported in Table 2.
Overall, 45 AEs were observed (23%) among which 22 were associated with rifampicin. Nine AEs were observed in nine patients (4.5%) that were related to clindamycin, six cases of which occurred during hospitalization. In all cases, the clinical symptoms included nausea and/or diarrhea. Three cases occurred during the clinical follow-up: two rashes and one diarrhea. Clostridium difficile infection was systematically screened for patients with diarrhea, but no cases were identified.
Discussion
Our study provides additional data on the use of an efficient and safe clindamycin-based antibiotic therapy for BJIs associated with susceptible bacteria. The rate of therapeutic success was 83%. The rate of AEs was low, and all of the AEs disappeared quickly after clindamycin cessation.
The retrospective design is one limit of the study which needs to be considered when interpreting results regarding the relation between duration of treatment and the outcome. Patients considered at risk of failure are probably more prone to receive longer duration of treatment.
A few studies have previously reported similar results regarding the efficacy of clindamycin but with variable durations of treatment [14,15,16]. In 2008, Samad et al. reported a series of 56 patients with BJIs treated by various antibiotic combinations including clindamycin for a mean duration of 101 days [14]. The rate of recovery was 91%. In 2010, Zeller et al. reported a series of 56 patients with BJIs receiving intravenous clindamycin for a mean duration of 40 days. The rate of recovery was 87% (49/56) [15]. In 2011, Czekaj et al. reported a short series of 20 patients treated with clindamycin + rifampicin for non-bacteremic BJIs [16]. All patients recovered after durations of antibiotic therapy that ranged from 35 to 90 days. However, two subsequent studies highlighted the pharmacological interactions between clindamycin + rifampicin, which lead to lower clindamycin serum concentrations [18, 19].
As previously reported [2, 5, 20], we observed a trend towards chronic infection, often associated with surgical devices, and therapeutic failure. In contrast, the best combination therapy appeared to be clindamycin + levofloxacin.
AEs appeared to be rare, suggesting good tolerance of clindamycin in these clinical reports. We did not observe any cases of C. difficile colitis, which appeared to be very uncommon in the studies reported above.
Clindamycin should be considered for the treatment of BJIs as it is efficient and safe, especially when combined with fluoroquinolones.
References
Grammatico-Guillon L, Baron S, Gettner S, Lecuyer A-I, Gaborit C, Rosset P et al (2012) Bone and joint infections in hospitalized patients in France, 2008: clinical and economic outcomes. J Hosp Infect 82:40–48. https://doi.org/10.1016/j.jhin.2012.04.025
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet Lond Engl 364:369–379. https://doi.org/10.1016/S0140-6736(04)16727-5
Cook GE, Markel DC, Ren W, Webb LX, McKee MD, Schemitsch EH (2015) Infection in orthopaedics. J Orthop Trauma 29(Suppl 12):S19–S23. https://doi.org/10.1097/BOT.0000000000000461
Perry A, Lambert P (2011) Propionibacterium acnes: infection beyond the skin. Expert Rev Anti-Infect Ther 9:1149–1156. https://doi.org/10.1586/eri.11.137
La Société de Pathologie Infectieuse de Langue Française (SPILF), Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT), Groupe de Pathologie Infectieuse Pédiatrique (GPIP), Société Française d’Anesthésie et de Réanimation (SFAR), Société Française de Chirurgie Orthopédique et Traumatologique (SOFCOT), Société Française d’Hygiène Hospitalière (SFHH) et al (2009) Clinical practice recommendations. Osteoarticular infections on materials (prosthesis, implant, osteosynthesis). Med Mal Infect 39:815–863
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK et al (2015) 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46. https://doi.org/10.1093/cid/civ482
Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F (2001) Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect Dis 1:175–188. https://doi.org/10.1016/S1473-3099(01)00094-9
Pulcini C, Couadau T, Bernard E, Lorthat-Jacob A, Bauer T, Cua E et al (2008) Adverse effects of parenteral antimicrobial therapy for chronic bone infections. Eur J Clin Microbiol Infect Dis 27:1227–1232. https://doi.org/10.1007/s10096-008-0570-y
Courjon J, Pulcini C, Cua E, Risso K, Guillouet F, Bernard E et al (2013) Antibiotics-related adverse events in the infectious diseases Department of a French teaching hospital: a prospective study. Eur J Clin Microbiol Infect Dis 32:1611–1616. https://doi.org/10.1007/s10096-013-1920-y
Danré A, Courjon J, Bernard E, Cua E, Mondain V, Roger P-M (2015) Safety of antibiotics combinations against staphylococcal bone and joint infections. Joint Bone Spine 82:134–135. https://doi.org/10.1016/j.jbspin.2014.03.021
Leigh DA (1981) Antibacterial activity and pharmacokinetics of clindamycin. J Antimicrob Chemother 7 Suppl A:3–9
Summersgill JT, Schupp LG, Raff MJ (1982) Comparative penetration of metronidazole, clindamycin, chloramphenicol, cefoxitin, ticarcillin, and moxalactam into bone. Antimicrob Agents Chemother 21:601–603
Norden CW, Shinners E, Niederriter K (1986) Clindamycin treatment of experimental chronic osteomyelitis due to Staphylococcus Aureus. J Infect Dis 153:956–959
El Samad Y, Havet E, Bentayeb H, Olory B, Canarelli B, Lardanchet J-F et al (2008) Treatment of osteoarticular infections with clindamycin in adults. Med Mal Infect 38:465–470. https://doi.org/10.1016/j.medmal.2008.06.030
Zeller V, Dzeing-Ella A, Kitzis M-D, Ziza J-M, Mamoudy P, Desplaces N (2010) Continuous clindamycin infusion, an innovative approach to treating bone and joint infections. Antimicrob Agents Chemother 54:88–92. https://doi.org/10.1128/AAC.01081-09
Czekaj J, Dinh A, Moldovan A, Vaudaux P, Gras G, Hoffmeyer P et al (2011) Efficacy of a combined oral clindamycin?Rifampicin regimen for therapy of staphylococcal osteoarticular infections. Scand J Infect Dis 43:962–967. https://doi.org/10.3109/00365548.2011.608082
Roger P-M, Farhad R, Leroux S, Rancurel S, Licari M, Bellissimo R et al (2008) Computerized management of a medical department, disease-related group management, clinical research and evaluations. Med Mal Infect 38:457–464. https://doi.org/10.1016/j.medmal.2008.06.027
Bernard A, Kermarrec G, Parize P, Caruba T, Bouvet A, Mainardi J-L et al (2015) Dramatic reduction of clindamycin serum concentration in staphylococcal osteoarticular infection patients treated with the oral clindamycin-rifampicin combination. J Inf Secur 71:200–206. https://doi.org/10.1016/j.jinf.2015.03.013
Curis E, Pestre V, Jullien V, Eyrolle L, Archambeau D, Morand P et al (2015) Pharmacokinetic variability of clindamycin and influence of rifampicin on clindamycin concentration in patients with bone and joint infections. Infection 43:473–481. https://doi.org/10.1007/s15010-015-0773-y
Farhad R, Roger P-M, Albert C, Pélligri C, Touati C, Dellamonica P et al (2010) Six weeks antibiotic therapy for all bone infections: results of a cohort study. Eur J Clin Microbiol Infect Dis 29:217–222. https://doi.org/10.1007/s10096-009-0842-1
Acknowledgements
We would like to thank Marie-Hélène Schiano, Emilie Leroux, Sophie Leroux and Stephanie Caravel for the implementation of the dashboard in our ward.
Funding
None.
Author information
Authors and Affiliations
Contributions
P-MR and JC performed the scientific literature search; P-MR was responsible for the study design. JC, ED, EC, EB and P-MR collected the data, and all of the authors interpreted the data. JC and P-MR analyzed the data. P-MR created the figures, and all of the authors were involved in the writing of the report.
Corresponding author
Ethics declarations
Competing interests
None declared.
Ethical approval
In France, ethical approval is not required for a non-interventional study. The medical dashboard used in the Infectious Diseases Department at Nice University Hospital is authorized by the French National Commission on Informatics and Liberty (registration number 1430722).
Informed consent
A signed consent form is obtained from each patient at our hospital to enable the use of the clinical data recorded during care for medical research.
Availability of data and material
The dataset analyzed during this study is not publicly available due to individual privacy issues, but it is available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Courjon, J., Demonchy, E., Cua, E. et al. Efficacy and safety of clindamycin-based treatment for bone and joint infections: a cohort study. Eur J Clin Microbiol Infect Dis 36, 2513–2518 (2017). https://doi.org/10.1007/s10096-017-3094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3094-5