Abstract
Responsible pathogens of chronic bone infections (CBI) are frequently resistant, requiring parenteral antimicrobial therapy. Therefore, adverse effects may be observed. We have determined the rate of adverse effects of antimicrobial therapy for CBI in a retrospective study of all patients receiving parenteral drugs via an implantable port. Patients from one medical ward (n = 89) and from one surgical ward (n = 40) between January 1995 and December 2005 were included in this study. The CBI included were 85 osteomyelitis (66%) and 44 prosthetic joint infections (34%). The main group of pathogens was Gram positive cocci (n = 144; 65%). The total duration of antibiotic treatment was 205 ± 200 days, including 133 ± 100 days for parenteral therapy. Thirty-three catheter-related complications were observed in 27 patients (21%). All complications led to hospitalization but none led to death. Twenty-one antibiotic-related complications occurred in 18 patients (16%), and one allergic reaction led to death. The mean duration of follow-up was 290 days. Remission was observed in 84 patients (65%). In multivariate analysis, adverse effects were mostly observed in the medical department. Adverse effects affect at least one third of the patients treated for CBI with parenteral antimicrobial therapy and are related to both the implantable port and the antibiotic compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic bone infections (CBI) are frequent due to the increasing incidence of trauma and prosthetic surgery [1–3]. CBI are commonly related to more than one bacteria and/or resistant pathogen(s), requiring several weeks of parenteral antibiotic treatment [1, 2]. Outpatient parenteral antimicrobial therapy (OPAT) has proved to be as effective as in-hospital treatment, but with reduced costs and improved quality of life for the patients [4, 5]. Therefore, bone and joint infections are among the leading causes of OPAT use [4–8].
Central venous access devices are proposed for prolonged antibiotic therapy [5]. An international OPAT registry based in Washington has been in place since 1997, gathering data from three countries (USA, UK, and Italy) [7]. Substantial differences among practices exist between countries, but overall, implantable ports are not commonly used (<2% of the cases) in these three countries [7]. In contrast, ports are of common practice in France for CBI management [9].
Port-related complications have already been studied, but mostly in oncology settings where ports are used for sequential short periods [10]. The frequency of port-related complications ranged from 0.1 to 1.6 per 1,000 catheter-days [6, 11–13]. In contrast, ports are used continuously for a long period in CBI. To the best of our knowledge, only one prospective study has studied adverse effects in 39 patients benefiting from OPAT for CBI, and has found a low rate of complications (5% allergy, no catheter-related complication) [4]. In other studies, antibiotic-related complications were associated with premature discontinuation of the antimicrobial course in 3–10% of the cases, whereas the rate of catheter-related complications was described in around 10% of the patients [5, 9]. However, adverse-event rates largely vary according to the antimicrobial administered and the type and duration of placement of the venous access device [5, 11].
In our clinical practice, adverse effects due to ports and/or antibiotic therapy seemed more frequent. Therefore, we performed a retrospective study to measure catheter- and antibiotic-related complication rates in all patients receiving OPAT for CBI.
Patients and methods
This is a retrospective and descriptive study of all patients receiving OPAT via an implantable port for a CBI between January 1995 and December 2005 in two departments: one medical ward (Infectious Diseases Department at Nice Teaching Hospital) and one surgical ward (Orthopaedics Surgery Department at Paris Ambroise Paré Teaching Hospital).
Patients were identified using computerised databases. Chronic bone infections included osteomyelitis and/or prosthetic joint infections. All infections were bacteriologically documented through bone biopsy.
The necessity of parenteral-administered antibiotics was defined by the presence of multi-drug-resistant organisms or allergy to the adequate oral antibiotic. All patients had an implantable port. Modalities of maintenance were left at the discretion of the teams. A physician specialised in infectious diseases and knowledgeable about OPAT evaluated all patients before and during therapy. Clinical monitoring was coordinated between the physician and home-care nurses but left at the discretion of the individual practitioner.
For each patient, demographic, epidemiologic and microbiologic data, therapeutic modalities used and their complications, and outcome were recorded when reviewing the medical charts.
Antibiotic treatment
Treatment was managed by home-infusion companies experienced in OPAT. Patients were seen at home every day by a nurse. Antibiotic solutions were prepared by the nurse at the patient’s home. Oral antibiotics (such as fluoroquinolones or rifampicin) were used in addition to parenteral antibiotics when required.
The type of antibiotic and duration of therapy (parenteral and total durations) were recorded. Duration of parenteral antibiotic therapy went from the first day the antibiotic was given in the hospital until the last day it was given at home. A course of antibiotic treatment included all antibiotics prescribed for an infectious episode. Recurrence of the infection could lead to another course of treatment for the same patient. The total number of antibiotics (parenteral and oral) prescribed for each patient was also recorded.
Complications of parenteral antibiotic treatment
Catheter-related complications and antibiotic-related complications were recorded. A catheter-day was defined as the use of a catheter for one day to deliver intravenous therapy.
Catheter-related complications were categorised as perioperative complications (pneumothorax and haemorrhage) and long-term complications (infection, thrombosis confirmed by ultrasound and doppler, extravasation, migration of the tip of the catheter, clotting). Infection of the catheter was classified as pocket infection or catheter-related bacteraemia/fungemia according to the literature [10, 11].
Antibiotic-related complications included complications of parenteral-administered antibiotics only. In the Nice Teaching Hospital, all charts were reviewed with a pharmacology specialist when adverse effects related to treatment were observed. Severity was scored according to National Cancer Institute criteria [14].
Laboratory monitoring and surveillance of adverse effects
The frequency of blood specimens and follow-up visits to the prescribing physician were determined on an individual practitioner basis. Systematic laboratory data included total leukocytes count, absolute neutrophil counts, platelet counts, serum liver markers, and creatinine levels. Patients were included only if they had laboratory data from the beginning until the end of their course of treatment.
Clinical efficacy
Follow-up duration was defined as the number of days between the last day of antibiotic therapy and the last medical consultation. A patient was considered to be in remission if he/she did not have any symptoms at the last available consultation. Treatment failure was defined by recurrence or persistence of symptoms despite antibiotic therapy.
Statistical analysis
Statistical analysis was performed using SPSS software version 11 (SPSS Inc., Chicago, IL). Univariate analysis was performed using the chi-square test and nonparametric Mann-Whitney test when appropriate. Multivariate analysis used multiple logistic regression. A two-sided P < 0.05 was considered statistically significant.
Results
One hundred twenty-nine patients were included in this study. Mean age (±SD) was 54 ± 18 years, and there were 91 males (71%). Eighty-nine (69%) were from the medical department and 40 (31%) from the surgical department.
CBI included 85 osteomyelitis (66%) and 44 prosthetic joint infections (34%). The most frequently infected sites were leg (28%), knee (21%), hip (20%), and foot (10%).
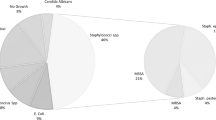
The bone infection involved more than one bacterium in 64 (50%) cases. The bacteria identified were S. aureus (n = 75), coagulase negative staphylococci (n = 47), streptococci or enterococci (n = 22), Pseudomonas aeruginosa (n = 39), and enterobacteriacae (n = 29).
Antibiotic treatment
The mean (±SD) total number of antibiotics (parenteral and oral) per patient was 4.1 ± 2.1, representing 1.7 ± 1.0 courses of treatment per patient. The mean (±SD) total duration of antibiotic treatment was 205 ± 200 days, including 133 ± 100 days for parenteral therapy.
Sixty-one patients (47%) received parenteral-only antibiotic treatment. Total catheter-days were 16,742.
Parenteral antibiotics prescribed were vancomycin in 92 cases (71%), teicoplanin in 27 (21%), ceftazidim in 22 (17%), imipenem in 19 (15%), other beta lactams in 36 (28%), aminoglycosides in 30 (23%), and fosfomycin in 23 (18%) cases.
Surgical treatment
A total of 119 patients underwent surgery (92%). Patients underwent on average 2.3 ± 1.6 operations as part of their surgical care for CBI.
Catheter-related complications
Thirty-three catheter-related complications were observed in 27 patients (21%). Details of the catheter-related complications are presented in Table 1. Catheter-related complications occurred 100 ± 79 (mean ± SD) days after insertion of the port. All complications led to hospitalisation, but none led to death. Complications led to placement of a new catheter for 13 of 27 patients (48%).
Antibiotic-related complications
Twenty-one antibiotic-related complications occurred in 18 patients (16%). One patient died from an allergic reaction. Details of the antibiotic-related complications are presented in Table 2.
Clinical efficacy
Mean duration of follow-up was 290 days after the end of antibiotic treatment. Remission was observed for 84 patients (65%). Treatment failure occurred for 27 patients (21%), 13 requiring amputations. Two patients died, one from an allergic reaction as indicated above, and a second one from a cause unrelated to CBI. Twelve patients (9%) were lost to follow-up and four are still under treatment.
Comparison between the two departments
Since some differences occurred between these two departments in terms of frequency of adverse effects, we performed a comparative analysis, which is presented in Table 3. Multivariate analysis confirmed that adverse effects were statistically related to the Nice department and to the use of teicoplanine. Lastly, taking into account these results, we compared the medical department (Nice) to the surgical one (Paris, see Table 4). Because our study was not specifically designed for that purpose, we conducted an univariate analysis only. The main differences were that patients were older in Nice and underwent fewer operations, but antibiotic treatment durations were longer. Catheter- and antibiotic-related complications were also more frequent in Nice. However, it should be noted that the rate of recovery was not different between these two departments.
Discussion
Chronic bone infections are the second most frequent cause of hospitalisation in our department after pulmonary infections, representing 12% of admissions from July 2005 to April 2007. Due to the frequency of antibiotic-resistant bacteria, parenteral therapy is widely used and adverse affects should be considered.
Our study was performed in two different university hospitals, involving a large number of patients with confirmed CBI requiring an implantable port for prolonged parenteral antibiotic therapy.
One hundred twenty-nine patients were included in an 11-year period. Adverse effects were frequent, involving at least one third of the patients, and were made up of 33 catheter-related complications, including 16 infections, and 21 antibiotic-related complications among which 17 were related to glycopeptides. To the best of our knowledge, this is the second published report specifically describing adverse effects of parenteral therapy for CBI. Adverse effects appeared frequently and are potentially harmful as measured through severity score according to National Cancer Institute criteria [14].
The methodology used in previous studies is highly variable, and comparisons are therefore difficult. As an example, Hoffman-Terry et al. published adverse effects of OPAT in 256 patients, but only 59% presented with CBI [6]. Retrospective studies are the most frequent, and probably only the most severe complications are reported in the medical charts [15]. Therefore, large variations of adverse effects is reported: allergic reactions are observed in 4–12% of the patients [4, 6, 9], nephrotoxicity in 8–20% [9], and neutropenia in 5–35% [9].
In published studies, OPAT rarely used ports [7, 8]. In contrast, ports are regularly used in France. Our rate of port-related complications is in line with previous studies [6, 11–13].
In multivariate analysis, adverse effects also appeared linked to glycopeptide use (Table 3), which requires intra-venous perfusion. In line with this result, the univariate analysis comparing Nice to Paris indicated that treatment duration was related to drug adverse-effects (Table 4). Since the optimal antibiotic therapy for CBI has not been determined and the rate of recovery was not different between these two departments, our study suggests the possibility of reducing parenteral drug use and possibly the duration of antibiotic treatment.
Adverse effects were recorded more frequently in Nice than in Paris. That may reflect the role of our clinical pharmacist, to whom all suspected drug adverse effects have been referred for investigations for more than 20 years. No such cooperation was in place in the surgical ward. Also, physicians and associations of visiting nurses had different line care and practices. One limitation of our study in comparing these two departments is that comorbid conditions such as diabetes or vascular diseases were not recorded. The latter could explain the different rate of adverse effects between medical and surgical departments. In contrast, it has been reported that different ports appeared without different complication rates [16]. Obviously, the impact of all these factors needs to be studied prospectively.
Conclusion
Adverse effects related to parenteral antibiotic treatment of CBI, using OPAT via a port, are frequent and related to clinical practice. Our study highlights the need for continuous monitoring of adverse effects and standardised care for CBI.
References
Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi:10.1056/NEJMra040181
Bernard L, Hoffmeyer P, Assal M et al (2004) Trends in the treatment of orthopaedic prosthetic infections. J Antimicrob Chemother 53:127–129. doi:10.1093/jac/dkh033
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364:369–379. doi:10.1016/S0140-6736(04)16727-5
Bernard L, El H, Pron B et al (2001) Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: evaluation of efficacy, tolerance and cost. J Clin Pharm Ther 26:445–451. doi:10.1046/j.1365-2710.2001.00380.x
Tice AD, Rehm SJ, Dalovisio JR et al (2004) Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis 38:1651–1672. doi:10.1086/420939
Hoffman-Terry ML, Fraimow HS, Fox TR et al (1999) Adverse effects of outpatient parenteral antibiotic therapy. Am J Med 106:44–49. doi:10.1016/S0002-9343(98)00362-3
Esposito S, Noviello S, Leone S et al (2004) Outpatient parenteral antibiotic therapy (OPAT) in different countries: a comparison. Int J Antimicrob Agents 24:473–478. doi:10.1016/j.ijantimicag.2004.06.004
Tice A (2001) The use of outpatient parenteral antimicrobial therapy in the management of osteomyelitis: data from the Outpatient Parenteral Antimicrobial Therapy Outcomes Registries. Chemotherapy 47(suppl 1):5–16. doi:10.1159/000048563
Galperine T, Ader F, Piriou P et al (2006) Outpatient parenteral antimicrobial therapy (OPAT) in bone and joint infections. Med Mal Infect 36:132–137. doi:10.1016/j.medmal.2006.01.002
Kurul S, Saip P, Aydin T (2002) Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol 3:684–692. doi:10.1016/S1470-2045(02)00905-1
O’Grady NP, Alexander M, Dellinger EP et al (2002) Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol 23:759–769. doi:10.1086/502007
Nathwani D, Tice A (2002) Ambulatory antimicrobial use: the value of an outcomes registry. J Antimicrob Chemother 49:149–154. doi:10.1093/jac/49.1.149
Barbut F, Soukouna S, Lalande V et al (2004) Totally implantable venous access ports: frequency of complications and analysis of bacterial contamination after ablation. Pathol Biol (Paris) 52:566–574. doi:10.1016/j.patbio.2004.07.020
National Cancer Institute (2003) Cancer therapy evaluation program common terminology criteria for adverse events, version 3.0. Available at: http://ctep.cancer.gov/forms/CTCAEv3.pdf. Accessed on 10 October 2007
Talfer S, Conessa C, Herve S, Roguet E, de Rotalier P, Poncet JL (2003) Complications with totally implantable venous access device. A retrospective of 116 cases. Presse Med 32:1263–1268
Hartkamp A, van Boxtel AJ, Zonnenberg BA, Witteveen PO (2000) Totally implantable venous access devices: evaluation of complications and a prospective comparative study of two different port systems. Neth J Med 57:215–223. doi:10.1016/S0300-2977(00)00083-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pulcini, C., Couadau, T., Bernard, E. et al. Adverse effects of parenteral antimicrobial therapy for chronic bone infections. Eur J Clin Microbiol Infect Dis 27, 1227–1232 (2008). https://doi.org/10.1007/s10096-008-0570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0570-y