Abstract
Persistent bacteraemia (PB) due to methicillin-resistant Staphylococcus aureus (MRSA) that fails to respond to glycopeptide therapy is a well-documented clinical problem. There are limited data on changes in agr functionality, vancomycin susceptibility and heteroresistance during MRSA PB. Thus, the frequency of these changes and their clinical significance remain unclear. Only patients with MRSA PB (≥7 days) from a prospective cohort of S. aureus bacteraemia were included. We collected isogenic paired strains and compared vancomycin MIC, vancomycin heteroresistance, and agr functionality between initial and final blood isolates. We also assessed the clinical outcome. A total of 49 patients had MRSA PB over 22 months. Bacteraemia persisted for a median of 13 days and most patients (98%) received glycopeptide as initial therapy. Among 49 isogenic pairs, only one pair showed a vancomycin MIC increase ≥2-fold by broth microdilution method, and only seven (14%) by E-test. Significant portions of initial isolates had vancomycin heteroresistance (49%) and agr dysfunction (76%). Development of vancomycin heteroresistance during PB occurred in four (16%) among 25 initial vancomycin-susceptible isolates, and acquisition of agr dysfunction occurred in two (16%) among 12 initial agr-functional isolates. Changes in the opposite direction occasionally occurred. These phenotypic changes during PB were not associated with mortality, whereas agr dysfunction of the initial isolates was significantly associated with mortality. During MRSA PB, phenotypic changes of MRSA isolates occurred occasionally under prolonged vancomycin exposure but were not significantly associated with clinical outcome. In contrast, initial agr dysfunction could be a predictor for mortality in MRSA PB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) infection still poses a substantial burden on healthcare systems. Among invasive MRSA infections, persistent MRSA bacteraemia that fails to respond to appropriate antibiotic therapy such as glycopeptide is a well-documented clinical problem often encountered in the management of S. aureus bacteraemia (SAB) [1, 2]. There are several reports examining clinical and microbiological factors associated with persistent MRSA bacteraemia [1–8]. Retention of infected devices, endovascular infection, methicillin resistance, and metastatic or multiple sites of infection are major clinical risk factors for persistent bacteraemia [1, 2, 4]. Vancomycin minimum inhibitory concentration (MIC) of 2 mg/L, vancomycin heteroresistance, agr dysfunction, and resistance to host defense cationic peptides have been suggested as microbiological factors for persistent MRSA bacteraemia [3, 5–8]. Although these microbiological characteristics of MRSA isolates have been regarded as contributing factors for persistent bacteraemia, they inversely could be acquired during persistent MRSA bacteraemia, particularly under prolonged vancomycin therapy. These changes, in turn, may hinder the clearance of MRSA bacteraemia and lead to glycopeptide treatment failure. It has been suggested that prolonged vancomycin exposure can induce phenotypic changes such as an increase in vancomycin minimum inhibitory concentration (MIC) or acquisition of vancomycin heteroresistance [9, 10]. However, there is currently little data on the frequency of phenotypic changes during vancomycin therapy for persistent MRSA bacteraemia, such as increased vancomycin MIC or acquisition of vancomycin heteroresistance and agr dysfunction [10]. This sole study was constrained by small sample size and mainly focused on microbiological features, not on associated clinical impact. Hence, both the frequency of phenotypic changes during persistent MRSA bacteraemia and their clinical significance have not been well defined. We thus evaluated how frequently phenotypic changes in sequential isolates occur during vancomycin therapy for persistent MRSA bacteraemia and assessed their clinical significance in a prospectively enrolled SAB cohort.
Materials and methods
Patients selection
The prospective observational cohort study of patients with SAB was performed at the Asan Medical Center, a 2700-bed teaching hospital in Seoul, Korea, from November 2008 to August 2010. All adult patients (aged ≥18 years) with SAB were enrolled and observed over a 12-week period. Patients were excluded if they had polymicrobial bacteraemia, or if they died or were discharged before positive blood culture results. In our hospital, almost all patients with SAB receive consultation from the Department of Infectious Diseases and are routinely recommended to undergo follow-up blood cultures at two- to three-day intervals until negative conversion and monitoring of vancomycin trough concentrations (just before the fourth dose and then at 3–4 day intervals). It is also recommended that vancomycin trough concentration be maintained at 15–20 mg/L in patients with MRSA bacteraemia. Among SAB patients, only those who had persistent MRSA bacteraemia, defined as bacteraemia for ≥7 days despite appropriate antibiotic therapy, were included in the analysis [1, 3]. This study was approved by the Asan Medical Center Institutional Review Board.
Data collection and study definition
Demographic characteristics, underlying diseases or conditions, severity of underlying disease, severity of bacteraemia, source of infection, and clinical outcome were recorded. The Charlson comorbidity index was used to provide a composite score of comorbid conditions [11]. The severity of bacteraemia at the time of the first positive blood culture was assessed using the Pitt bacteraemia score [12].
The duration of bacteraemia was calculated as the number of days between the first and last positive blood culture. The type of infection causing SAB was defined by the surveillance criteria of the Centers for Disease Control and Prevention except for catheter-related infection, infective endocarditis, and vascular graft infection [13]. Catheter-related infection [14], the presence of infective endocarditis [15], and vascular graft infection [16] were defined according to widely accepted criteria. Bacteraemia was classified as “community-onset” if a blood culture performed within the first 48 hours after admission was positive, and was further subclassified as healthcare-associated or community-acquired bacteraemia [17]. SAB was defined as “hospital-acquired” if an initial positive blood culture was obtained >48 hours after admission. The clinical outcome measure was death within 12 weeks after onset of MRSA bacteraemia.
Microbiologic data and genotypic assays
Isogenic paired strains (the initial and final blood isolates from each study patient) were used for microbiological and molecular assessments. All MRSA isolates were identified by standard methods. Antimicrobial susceptibilities were determined using the MicroScan system (Dade Behring, West Sacramento, CA) and standard criteria of the Clinical and Laboratory Standards Institute. Methicillin resistance was confirmed by polymerase chain reaction (PCR) detection of the mecA gene. Vancomycin MICs of all serial MRSA isolates were determined using both broth microdilution (BMD) and E-test (AB Biodisk, Piscataway, NJ) on Mueller-Hinton agar according to the manufacturer’s instructions. All assays were performed in triplicate, and the most frequent concentration was chosen. Identification of heterogeneous vancomycin-intermediate S. aureus (hVISA) phenotype (vancomycin heteroresistance) was determined by population analysis profile (PAP) as previously described [18]. An isolate was identified as hVISA if the ratio of the area under the viable count–vancomycin curve (AUC) for the test isolate versus the AUC of the reference strain (Mu3; ATCC 700698) was ≥0.9.
The staphylococcal cassette chromosome mec (SCCmec) type, agr type, and agr functionality of isolates were determined using previously described methods [19–21]. We determined agr dysfunction by the level of δ-hemolysin production, measured by streaking the isolate adjacent to a β-hemolytic RN4220 strain (Remel, Lenexa, KS), because agr dysfunction results in deficient δ-hemolysin production [20, 22]. Multilocus sequence typing (MLST) was performed for all strains as described elsewhere [23], and sequence types were consistent in each isogenic pair.
Statistical analysis
Characteristics of patients and isolates are summarized using percentages or median and interquartile range (IQR). Categorical variables were compared using the χ2 test or the Fisher’s exact test as appropriate, and continuous variables were compared using Student’s t test or the Mann–Whitney U test. The time-to-event analyses were performed using Kaplan–Meier estimates and the log-rank test. To identify microbiological risk factors for 12-week mortality, significant variables in the univariate analysis were included in the multivariate analysis using penalized Cox regression model. All tests of significance were two-tailed, and P values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS version 21.0 (SPSS, Armonk, NY, USA) and R software version 3.1.2 (R Project for Statistical Computing, Vienna, Austria).
Results
Patients and isolates characteristics
A total of 235 patients were diagnosed with MRSA bacteraemia during the 22-month study period at our institution. Only the 49 patients with persistent MRSA bacteraemia and the isogenic paired strains isolated from these patients were included in the analysis. The median age of study patients was 65 years (IQR, 55–71 years) and the median duration of bacteraemia was 13 days (IQR, 8–18 days). Among these 49 cases, three (6%) were community-acquired, 13 (27%) were healthcare-associated, and 33 (67%) were hospital-acquired. Catheter-related infection was the most common site of infection (30 patients [61%]), followed by infective endocarditis (6 [12%]), skin and soft tissue infection (4 [8%]), vascular graft infection (4 [8%]), bone and joint infection (3 [6%]), and suppurative thrombophlebitis of varix (1 [2%]). All but one patient received glycopeptide (vancomycin in 92% and teicoplanin in 6% of patients) as initial therapy, whereas the remaining patient received linezolid. The median time from bacteraemia onset to initiation of appropriate antibiotic therapy was 1 day (IQR 0–2 days). Of 38 patients with an eradicable focus of MRSA bacteraemia, 32 (84%) underwent removal of the focus. Among 49 isogenic pairs, 35 (71%) were ST5-SCCmec II, and ten (20%) were ST72-SCCmec IV strains.
Change of vancomycin susceptibility during persistent bacteraemia
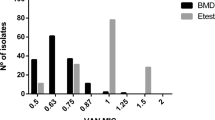
As shown in Fig. 1 and Table 1, only one isogenic pair showed a vancomycin MIC increase ≥2-fold by BMD and seven (14%) by E-test. Among the initial 49 isolates, 25 (51%) were vancomycin-susceptible S. aureus (VSSA) and 24 (49%) were hVISA. Acquisition of vancomycin heteroresistance was observed in four (16%) of 25 VSSA strains during persistent bacteraemia, whereas phenotypic change from hVISA to VSSA was observed in seven (29%) of 24 hVISA strains during persistent bacteraemia (Table 2). Change from VSSA to hVISA occurred only in ST5-MRSA.
Change of agr function during persistent bacteremia
Among 49 isogenic pairs, 14 (29%) were agr group I and 35 (71%) were agr group II. Of the initial 49 isolates, 37 (75%) showed agr dysfunction. Of the 12 initial isolates without agr dysfunction, two (17%) acquired agr dysfunction during persistent bacteraemia, whereas one of 37 initial isolates with agr dysfunction showed a phenotypic change to be agr functional during persistent bacteraemia (Table 2).
Outcome of patients with persistent bacteremia
When we compared the 12-week mortality, there were no significant differences between patients with and without changes in vancomycin MIC, development of hVISA, or acquisition of agr dysfunction (Table 3). In contrast, initial isolates with agr dysfunction were significantly associated with higher mortality than those with agr function (30% [11/37] vs. 0% [0/12], P = 0.045). Kaplan–Meier survival curves of 12-week mortality according to initial agr functionality are presented in Fig. 2 (P = 0.04). Underlying disease, the severity of infection, and duration of bacteraemia were comparable between groups with and without initial agr dysfunction (Table 4). However, hospital acquisition of bacteraemia, catheter-related infection, and receiving ICU care were significantly more frequent in patients with initial agr dysfunctional isolates than in patients with initial agr functional isolates. In addition, 34 (92%) of 37 initial isolates with agr dysfunction were ST5-SCCmec type II, whereas only one (8%) of 12 initial isolates with agr function were ST5-SCCmec type II. Initial agr dysfunction, ST5-MRSA and Charlson comorbidity index were included in a penalized Cox regression model to identify risk factors for 12-week mortality. Multivariate analysis revealed that initial agr dysfunction was the only significant prognostic factor for mortality (hazard ratio [HR], 14.3, 95% confidence interval [CI] 1.9–1820.5, P = 0.003).
Discussion
We assessed the phenotypic changes in isogenic paired MRSA strains isolated from persistent bacteraemia patients and the associated clinical outcomes. During glycopeptide therapy for persistent MRSA bacteraemia, phenotypic changes such as increased vancomycin MIC, development of vancomycin heteroresistance, and loss of agr function occurred occasionally, even in the opposite direction, but did not affect mortality. In contrast, agr dysfunction of initial isolates was significantly associated with adverse outcome.
In the present study, vancomycin MIC increased in only one pair (2%) as determined by BMD and seven pairs (14%) by E-test during vancomycin therapy for persistent MRSA bacteraemia. In addition, four pairs (16%) developed hVISA phenotype from initial VSSA strains. There are limited data on phenotypic changes such as increased vancomycin MIC and development of hVISA phenotype during persistent MRSA bacteraemia with glycopeptide therapy [10]. In a retrospective study, Lin et al. [24] reported that the frequency of vancomycin MIC increase (≥2-fold by agar dilution method) during persistent MRSA bacteraemia was 25% for subsequent isolates and 20% for the final isolates in 199 patients. Infrequent phenotypic changes of our data are consistent with another previous study, which found that only two pairs (9%) of isolates exhibited a slight increase of vancomycin MIC by E-test in brain-heart infusion agar during persistent bacteraemia and three isolates (14%) acquired heteroresistance out of 22 pairs during persistent or recurrent MRSA bacteraemia [10]. This discrepancy in the frequency of vancomycin MIC increase among studies might arise from the different vancomycin susceptibility testing methods. Given that duration of glycopeptide therapy in cases with phenotypic changes seemed to be longer than in cases without those changes (Table 3), the length of glycopeptide exposure also could contribute to inconsistent results among studies.
Because several reports implicated agr dysfunction as a cause of persistent MRSA bacteraemia [3, 5, 22], we assumed that agr dysfunction would be acquired in many cases of persistent MRSA bacteraemia. However, the acquisition of agr dysfunction was not common (17%, [2/12]), and change from agr dysfunction to agr function was observed in one isogenic pair (3% [1/34]). Initial infection caused by mixed MRSA populations with both traits could provide some explanation for unexpected changes from agr dysfunction to agr function or from hVISA to VSSA during glycopeptide therapy [20]. Although it has been suggested that agr dysfunction is associated with hVISA [22, 25], neither of the two isolates acquiring agr dysfunction during persistent bacteraemia developed hVISA phenotype in the present study. Interestingly, all four isolates that developed hVISA phenotype during persistent bacteraemia were initially agr dysfunctional. Therefore, we can postulate that agr dysfunctional isolates can more easily adapt to glycopeptide selection pressure during persistent bacteraemia than agr functional isolates [26].
In a recent retrospective study, elevated vancomycin MIC during persistent MRSA bacteraemia (in 25% of subsequent isolates) was associated with increased mortality [24]. However, we found that vancomycin MIC increase and development of hVISA phenotype during persistent MRSA bacteraemia were not significantly associated with mortality. Instead, agr dysfunction of initial isolates was significantly associated with mortality in our data. This result is partly consistent with that of Schweizer et al. [27], who demonstrated an independent association between agr dysfunction and mortality among severely ill patients with SAB (adjusted HR, 1.82; 95% confidence interval, 1.03–3.21). Although they did not quantify the duration of bacteraemia due to the retrospective nature of their study, they suspected that higher mortality by agr dysfunctional isolates may result from a longer duration of bacteraemia. However, in our study that all patients had persistent bacteraemia, there was no significant difference in the duration of bacteraemia between patients infected by agr dysfunctional and agr functional isolates. Interestingly, there were significant differences in several clinical characteristics (i.e., site of acquisition, site of infection, and ICU care during bacteremia) between these patient groups, which could affect clinical outcome. Sakoulas et al. [26] and Vuong et al. [28] suggested that the loss of agr function may confer survival advantages to MRSA, such as promoting biofilm formation and physiological changes that support colonization and survival in the nosocomial setting under glycopeptide selection pressure. Recently, Laabei et al. found that agr dysfunctional isolates are more fit in human serum, and as a result, are more associated with severe, invasive disease than agr function isolates [29]. These characteristics may explain the clinical features and increased mortality of our patients infected with agr dysfunctional isolates. Additional studies are needed to verify the impact of agr dysfunction on mortality in persistent MRSA bacteraemia.
Our study has some limitations. First, the majority of initial isolates already had agr dysfunction, which may have contributed to the lower acquisition rate (17%) of agr dysfunction than anticipated during persistent bacteraemia. A study performed in patients with a higher proportion of agr functional isolates may provide a better estimate of the acquisition rate of agr dysfunction during persistent MRSA bacteraemia. Second, this study was performed at a single tertiary care center, so a multicenter prospective study is warranted to generalize our findings. Despite these limitations, our findings provide some important implications for understanding the nature of persistent MRSA bacteraemia. To the best of our knowledge, this is the first relatively large-scale study to evaluate not only serial phenotypic changes during persistent MRSA bacteraemia but also their clinical significance on the outcome.
In summary, phenotypic changes such as increased vancomycin MIC, development of heteroresistance, and acquisition of agr dysfunction occurred occasionally, and changes in the opposite direction also occurred, during persistent MRSA bacteraemia, particularly under prolonged glycopeptide therapy. However, these changes did not significantly affect mortality. In contrast, initial agr dysfunction might be a predictor of poor outcome. Furthermore, as some clinical and microbiological characteristics (i.e., hospital-acquired bacteraemia, catheter-related infection, ICU care, and ST5-MRSA strain) were closely related to initial agr dysfunction, early aggressive treatment for patients with these characteristics may help improve outcome.
References
Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M (2007) Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med 167(17):1861–1867
Khatib R, Johnson LB, Sharma M, Fakih MG, Ganga R, Riederer K (2009) Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand J Infect Dis 41(1):4–9
Fowler VG Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS (2004) Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 190(6):1140–1149
Chong YP, Park SJ, Kim HS, Kim ES, Kim MN, Park KH, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS (2013) Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine 92(2):98–108
Park SY, Chong YP, Park HJ, Park KH, Moon SM, Jeong JY, Kim MN, Kim SH, Lee SO, Choi SH, Woo JH, Kim YS (2013) agr Dysfunction and persistent methicillin-resistant Staphylococcus aureus bacteremia in patients with removed eradicable foci. Infection 41(1):111–119
Neuner EA, Casabar E, Reichley R, McKinnon PS (2010) Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis 67(3):228–233
Wang JL, Lai CH, Lin HH, Chen WF, Shih YC, Hung CH (2013) High vancomycin minimum inhibitory concentrations with heteroresistant vancomycin-intermediate Staphylococcus aureus in meticillin-resistant S. aureus bacteraemia patients. Int J Antimicrob Agents 42(5):390–394
Seidl K, Bayer AS, Fowler VG Jr, McKinnell JA, Abdel Hady W, Sakoulas G, Yeaman MR, Xiong YQ (2011) Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother 55(2):575–582
Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK (2006) Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50(9):3039–3047
Khosrovaneh A, Riederer K, Saeed S, Tabriz MS, Shah AR, Hanna MM, Sharma M, Johnson LB, Fakih MG, Khatib R (2004) Frequency of reduced vancomycin susceptibility and heterogeneous subpopulation in persistent or recurrent methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 38(9):1328–1330
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Ramphal R, Wagener MM, Miyashiro DK, Yu VL (1991) Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 115(8):585–590
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16(3):128–140
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49(1):1–45
Durack DT, Lukes AS, Bright DK (1994) New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 96(3):200–209
Darouiche RO (2004) Treatment of infections associated with surgical implants. N Engl J Med 350(14):1422–1429
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797
Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP (2001) A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 47(4):399–403
Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, Nesme X, Etienne J, Vandenesch F (2002) Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun 70(2):631–641
Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP (2008) agr function in clinical Staphylococcus aureus isolates. Microbiol (Reading, England) 154(Pt 8):2265–2274
Oliveira DC, de Lencastre H (2002) Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 46(7):2155–2161
Chong YP, Kim ES, Park S-J, Park K-H, Kim T, Kim M-N, Kim S-H, Lee S-O, Choi S-H, Woo JH, Jeong J-Y, Kim YS (2013) Accessory gene regulator (agr) dysfunction in Staphylococcus aureus bloodstream isolates from South Korean patients. Antimicrob Agents Chemother 57(3):1509–1512
Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG (2000) Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38(3):1008–1015
Lin SH, Liao WH, Lai CC, Liao CH, Tan CK, Wang CY, Huang YT, Hsueh PR (2010) Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia in a tertiary care hospital in Taiwan. J Antimicrob Chemother 65(8):1792–1798
Sakoulas G, Eliopoulos GM, Moellering RC Jr, Wennersten C, Venkataraman L, Novick RP, Gold HS (2002) Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother 46(5):1492–1502
Sakoulas G, Moellering RC Jr, Eliopoulos GM (2006) Adaptation of methicillin-resistant Staphylococcus aureus in the face of vancomycin therapy. Clin Infect Dis 42(Suppl 1):S40–50
Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, McGregor JC, Thom KA, Perencevich EN (2011) Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother 55(3):1082–1087
Vuong C, Saenz HL, Gotz F, Otto M (2000) Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis 182(6):1688–1693
Laabei M, Uhlemann A-C, Lowy FD, Austin ED, Yokoyama M, Ouadi K, Feil E, Thorpe HA, Williams B, Perkins M, Peacock SJ, Clarke SR, Dordel J, Holden M, Votintseva AA, Bowden R, Crook DW, Young BC, Wilson DJ, Recker M, Massey RC (2015) Evolutionary trade-offs underlie the multi-faceted virulence of staphylococcus aureus. PLoS Biol 13(9), e1002229
Acknowledgements
We sincerely thank Su-Jin Park, Hee Sueng Kim, So Young Kim, Mi Young Kim, and Sujin Lee for supporting the data collection.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by a grant (grant number: 2015-616) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Disclosure of potential conflicts of interest
We have no conflicts of interest to declare.
Ethical approval
This study was approved by the Institutional Review Board of Asan Medical Center (No. 2008-0274).
Informed consent
The IRB waived the requirement for informed consent in view of the observational nature of the study, and the patient records were anonymized and deidentified.
Rights and permissions
About this article
Cite this article
Kim, T., Kim, E.S., Park, S.Y. et al. Phenotypic changes of methicillin-resistant Staphylococcus aureus during vancomycin therapy for persistent bacteraemia and related clinical outcome. Eur J Clin Microbiol Infect Dis 36, 1473–1481 (2017). https://doi.org/10.1007/s10096-017-2956-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2956-1





