Abstract
Helicobacter pylori, a risk factor of cancer and chronic diseases, remains highly prevalent in China. This review aims to systematically evaluate the H. pylori-attributable burden for gastric cancer (GC), coronary heart disease (CHD), and ischemic stroke (IS) in the Chinese population. Helicobacter pylori prevalence was updated by pooling the results reported in studies across China. The population attributable fraction (PAF) was calculated based on the H. pylori prevalence 10 years ago and relative risks of specific disease by reviewing the prospective studies published from 2000 through 2015. In China, the nationwide average prevalence of H. pylori was estimated to be 42.06 % in the general population during 2009–2013. The fixed effects pooled relative risk (RR) of 1.89 [95 % confidence interval (CI): 1.57–2.26] was obtained for gastric cancer and H. pylori infection. Helicobacter pylori infection was responsible for around 37.38 % of noncardia GC, corresponding to about 105,536 cases in 2012. As for extra-gastric disorders, H. pylori infections had higher risk of CHD (RR = 1.55, 95 % CI: 1.37–1.76) and IS (RR = 1.54, 95 % CI: 1.42–1.66). About 23.15 % of CHD and 22.29 % of IS were attributable to H. pylori infection. The estimates of H. pylori-attributable burden reveal a great potential of reducing H. pylori-related chronic disease burden by H. pylori eradication. Large prospective studies are warranted to identify which H. pylori strains, which subtypes of the disease, and which subgroups of the population have the greatest risk of relevant diseases and the effect of H. pylori eradication on the prevention of H. pylori-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past four decades or so, increasing evidence has indicated that infectious agents play an important role in human cancer and impose a large burden on global health. One of the most principal agents was the bacterium Helicobacter pylori, which accounted for 32.5 % of the 2 million new cancer cases attributable to infection worldwide that occurred in 2008 [1].
As reported, a similar situation has been occurring in China. In 2005, H. pylori, one of the most highly prevalent infections in China, was responsible for 9.8 % and 9.2 % of infection-related cancer cases and deaths, respectively [2].
In terms of H. pylori, it is a Gram-negative spiral bacterium usually colonizing gastric mucosa. The seroprevalence of H. pylori infection was about 50 % worldwide and the majority of infected subjects remain asymptomatic. Around 10 % of H. pylori infections would develop chronic gastritis or gastroduodenal ulcer. Persistent infection could bring about inflammatory cell infiltration and DNA damage, and result in an imbalance between proliferation and apoptosis of the gastric epithelial cells and even by secreting toxins, including CagA, VacA, lipopolysaccharides, and hemolysin [3]. It is well known that whether an individual develops a specific H. pylori-related disease, the incidence, and prevalence of several H. pylori-associated diseases depend on the interaction between mutations and recombination frequently of mixed H. pylori strains and the capacity of the host immune response. In 1994, H. pylori infection was classified as a class 1 carcinogen by the International Agency for Research on Cancer (IARC) [4]. In addition to the well-known H. pylori-associated chronic gastritis, peptic ulcer, and duodenal ulcer, enormous epidemiological studies have demonstrated that H. pylori infections have an increased risk of gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and a decreased risk of esophageal adenocarcinoma [5]. Actually, screening and eradication for H. pylori have been recommended and accepted as a common clinical practice in the treatment of gastritis and ulcer in China. Additionally, evidence has accumulated that H. pylori (especially CagA-positive H. pylori) may play an inducing role in some extra-digestive conditions, such as cardiovascular diseases, including coronary heart disease and cerebral infarction [6, 7]. The common pathogenesis of both diseases is atherosclerosis, in the initiation, development, or persistence, of which, sustained infection, systematic inflammatory response, and possibly accompanying dyslipidemia, hyperhomocysteinemia, hypercoagulability, impaired glucose metabolism, or endothelial dysfunction may play an important role [8, 9]. Additionally, H. pylori infection may also contribute to the development of various other disorders, such as diabetes mellitus, iron deficiency anemia, chronic obstructive pulmonary disease (COPD), and so on [10–13]. Notably, the relation of H. pylori prevalence and diverse spectrum of extragastric diseases seem to be ambiguous, vary among different populations of diverse characteristics, and could be modified by other factors. Khamechian et al. reported an inverse correlation between childhood asthma and H. pylori in Kashan [14], whereas no inverse association was observed between H. pylori and adult asthma with peptic ulcers by Chang and Hu [15]. As pooled by Vasapolli et al. [16], the effect of H. pylori infection on the development of gastroesophageal reflux disease (GERD) may differ among diverse categories of GERD in populations of different characteristics. The potential underlying mechanisms remain to be elucidated, which may help stimulate personalized medicine.
In China, these H. pylori-associated diseases add a great burden to public health. The morbidity and mortality of gastric cancer in 2012 was estimated to be about 31.28/100,000 and 22.04/100,000, respectively [17]. Stroke and cardiovascular disease have been the leading cause of death and constituted a severe health and social problem. What’s more, the overall prevalence of H. pylori was as high as 54.76 % in the general population, which was revealed by a nationwide investigation covering 26,341 participants from 19 provinces and autonomous regions performed from January 2002 to June 2004 [18]. Seeing that a systematic quantitation of the disease burden attributable to H. pylori has not been performed in the Chinese population, this study aims to update the population attributable fraction (PAF) estimate of gastric cancer and evaluate the attributable burden of other H. pylori-related diseases, including coronary heart disease and ischemic stroke. Because of the lack of sufficient available literature on the Chinese population, this review will not address the possible disease burden of MALT, COPD, diabetes mellitus, and iron deficiency anemia attributable to H. pylori.
Materials and methods
Population attributable fractions
The PAF is an indicator applied to measure the proportion of the disease burden that would be theoretically avoided if the responsible exposure was eliminated or the individual was no longer susceptible to it. The PAFs of H. pylori-attributable diseases can be calculated by using the following formula originally proposed by Levin [19]:
where P is the prevalence of H. pylori in the Chinese population and RR is the corresponding relative risk (RR) of H. pylori infection and specific disease.
It remains to determine differential natural interval periods between H. pylori infection and the occurrence of various outcomes. However, what we may agree is that a long enough time is more practical for us to observe the health consequences of H. pylori infection and assess their impact on individuals and the whole population. Therefore, considering the interval suggested by other reports and the accumulation of possible confounding effect associated with the interval, we think that a latency time of 10 years could be reasonable between the exposure of H. pylori and the occurrence of gastric cancer and other chronic diseases. In the present study, the prevalence of H. pylori over the period 2002–2004 was used to estimate the PAFs of H. pylori-related diseases in 2014 or later.
Prevalence of H. pylori infection in China
In order to update the prevalence of H. pylori, we searched the PubMed, China National Knowledge Infrastructure (CNKI), and Wanfang databases. The search parameters were Helicobacter pylori, H. pylori, Campylobacter pylori, prevalence, and their combinations. Those studies conducted from 2005 to 2014 and reporting the prevalence of H. pylori in the Chinese population were included. Studies written in English and Chinese were both included. Moreover, the objective population was restricted to the general population or health examinees without digestive tract symptoms. Eventually, we pooled 55 qualified studies. Helicobacter pylori prevalence could be used subsequently to evaluate the PAFs of relevant diseases 10 years later.
Pooled relative risk of H. pylori infection and specific disease in the Chinese population
The association between H. pylori infection and specific disease (such as coronary heart disease and cerebral infarction) has long been debated. In order to estimate the PAFs of H. pylori-related diseases among the Chinese population, we pooled different articles to calculate the overall combined risk of specific disease from H. pylori infection targeting the Chinese population by meta-analysis.
Search strategy and selection criteria
We identified studies involving risk estimates of H. pylori and related diseases by researching the PubMed, CNKI, and Wanfang databases. The following keywords and their combinations were searched: Helicobacter pylori, H. pylori, Campylobacter pylori, gastric, stomach, cancer, carcinoma, tumor, neoplasm, infarction, stroke, brain attack, cerebrovascular, cardiovascular, and coronary heart disease. Additional studies were obtained by screening references of studies and reviews.
Inclusion criteria of literatures
Studies should focus on the Chinese population, be written in English or Chinese, and conducted from 2000/01/01 to 2015/03/01.
Studies should contain data on RRs, odds ratios (ORs), or hazard ratio (HRs) with corresponding 95 % confidence intervals (CIs) or relevant information that could be used to estimate the magnitude of the association between H. pylori infection and the outcomes. The types of the studies were not limited.
Exclusion criteria of literatures
Duplicate studies or those based on the same or overlapping data sets were excluded. Then, the articles with the largest sample size and the most detailed results were included preferentially.
Studies of patients with H. pylori-related diseases as controls were excluded.
Two independent reviewers would check and extract data from a given article. Conflicts on extracted data were resolved by reaching a consensus. The methodological quality of the included studies was assessed mostly based on the Newcastle–Ottawa Scale (NOS) [20]. When conducted, the NOS was revised by adding an extra item of “adjustment of confounding factors or not”. The studies awarded more than six points were regarded as high quality. All meta-analyses were performed by using the ‘metan’ command in Stata version 12.0 (StataCorp, College Station, TX, USA) with a two-sided p-value. We adopted a random effects model (if p for heterogeneity <0.05) by using the DerSimonian and Laird method (D&L method) [21] and a fixed effects model (if p for heterogeneity >0.05) to evaluate the pooled RRs and 95 % CIs. Meanwhile, potential publication bias was evaluated using a Begg’s test and Egger’s funnel plot. Heterogeneity among different studies was assessed by the Chi-squared test and calculated by I-squared (variation in RR attributable to heterogeneity).
H. pylori-associated disease morbidity and mortality data
The latest qualified data on the morbidity and mortality of gastric cancer in 2012 were derived from the National Central Cancer Registry (NCCR) and submitted by 193 cancer registries (74 cities and 119 counties) from 31 provinces, which covered about 198,060,406 population, accounting for approximately 16.43 % of the national population [17]. All cancer cases were classified according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) and the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). We use the data from the NCCR to estimate the gastric cancer burden attributable to H. pylori.
To date, there are no detailed and validated data on the nationwide morbidity of other chronic H. pylori-associated diseases, such as coronary heart disease and stroke.
Results
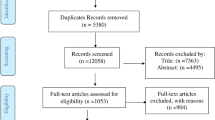
Of the 3616 publications retrieved from the databases, 48 were eventually included in the meta-analyses of risk estimates. A detailed identification of eligible studies is shown in Fig. 1. The data extracted from the 48 included studies and the NOS score are exhibited in Table 1. In addition, no statistical evidence of publication bias were found among studies by using Begg’s test (p = 0.07 for gastric cancer, p = 0.35 for coronary heart disease, p = 0.23 for ischemic stroke).
Helicobacter pylori prevalence in different periods in China are listed in Table 2. A significant declining trend from 58.07 % (1990–2002) to 42.06 % (2009–2013) is found among the average prevalence of H. pylori infection in China. The detailed information from 53 eligible studies on H. pylori prevalence are presented in Supplementary Table 1.
The forest plots and results of the heterogeneity test of the meta-analysis on gastric cancer, coronary heart disease, and ischemic disease are shown in Figs. 2, 3, and 4, respectively. For gastric cancer, the fixed effects pooled relative risk (RR) of 1.92 (95 % CI: 1.60–2.30) with no heterogeneity between studies (p = 0.09) was obtained for gastric cancer and H. pylori infection. For cardiovascular diseases, similar random effect pooled ORs were discovered, being 2.33 (95 % CI: 1.84–2.94) for coronary heart disease and 2.42 (95 % CI: 1.99–2.94) for ischemic stroke.
In order to investigate the relationship between H. pylori infection and the three related diseases more comprehensively, we conducted subgroup meta-analyses stratified by subtypes of disease, adjustment or not, detection of H. pylori, follow-up time, and study design separately. The detailed results of subgroup meta-analyses for gastric cancer, coronary heart disease, and ischemic disease are listed in Tables 3, 4, and 5, respectively. For gastric cancer, the subgroup meta-analysis involving six prospective studies with follow-up (8–11 years) adjusting for age, sex, and other factors revealed that H. pylori infections have a higher risk (RR: 1.89, 95 % CI: 1.57–2.26) of gastric cancer. Furthermore, similar RR (1.82, 95 % CI: 1.40–2.63) of noncardia cancer from H. pylori was obtained in another subgroup meta-analysis of two high-quality prospective studies. We found 19 case–control studies and two high-quality prospective studies assessing the relationship between H. pylori infection and coronary heart disease, the pooled OR and RR if which were 2.40 (95 %: 1.78–3.23) and 1.55 (95 %: 1.37–1.76), respectively. Similar risk estimates of H. pylori infection for ischemic disease were acquired by subgroup meta-analyses according to the study design. The pooled OR based on 18 case–control studies and the RR based on two high-quality prospective studies were 2.53 (95 %: 2.19–2.93) and 1.54 (95 %: 1.42–1.66), respectively.
The estimated PAFs of the diseases due to H. pylori infection or H. pylori eradication are shown in Table 6. Assuming that the time interval of H. pylori exposure and occurrence of related diseases is around 10 years, the PAFs of H. pylori-associated diseases in around 2014 are obtained by applying H. pylori prevalence data (54.76 %) during 2002–2004. Helicobacter pylori infection was estimated to account for 32.77 % of gastric cancer. When considering the gastric cancer by subsites, the estimated PAF for noncardia gastric cancer due to H. pylori infection was 37.38 %. The latest meta-analysis was based on eight cohort studies assessing the association between H. pylori eradication and gastric cancer incidence. The direct evidence showed that around 22.82 % of gastric cancer cases could be reduced by H. pylori eradication. Among the extragastric diseases in China, 23.15 % of coronary heart disease was attributable to H. pylori infection. In addition, H. pylori infection was responsible for 22.49 % of ischemic stroke cases. As a whole, with the prevalence of H. pylori decreasing from 54.76 % during 2002–2004 to 42.06 % during 2009–2013 across China, the three relevant diseases burden attributable to H. pylori would decline by about 5 % by 2024 or later.
Discussion
The present study is the first to systematically estimate the major disease burden attributable to H. pylori infection focusing on the Chinese population. In China, the nationwide average prevalence of H. pylori has declined steadily from 58.07 % (1990–2002) to 42.06 % (2009–2013) over the past two decades. We mainly pooled the high-quality Chinese prospective studies and validated the magnitude of the risk estimates by comparing with other relevant meta-analyses. We estimated that H. pylori infection may explain around 32.77 % of gastric cancer, 23.15 % of coronary heart disease, and 22.29 % of ischemic stroke during 2012–2014. With the decreasing prevalence of H. pylori, the overall attributable burden of the three H. pylori-related diseases may decline by about 5 % 10 years later.
In terms of H. pylori prevalence in the general Chinese population, the prevalence of H. pylori during 2009–2013, compared with that which occurred 10 years ago, seemed to become weaker according to our estimates (58.07 % vs. 42.06 %, p < 0.01). Furthermore, the trend was consistently observed in several specific regions. As reported by Chen et al., the decreasing prevalence of H. pylori infection was observed during 1993–2003 in Guangzhou, Southern China (62.5 % vs. 49.3 %, p < 0.001) [72]. Another two regions, Muping County in Shandong [73.78 % vs. 50.95 %, p < 0.001) and Yanqing County in Beijing (55.35 % vs. 41.35 %, p < 0.01], both exhibited a significant decline in H. pylori prevalence during 1990–2006 [73]. In addition to the whole decreasing trend, the prevalence of H. pylori infection (42.06 %) during 2009–2013, which we estimated based on the data from 34 articles covering 149,391 individuals of 18 provinces or regions, was highly consistent with the H. pylori prevalence (42.39 %) demonstrated by the baseline data of a large community-based intervention trial involving 183,970 participants launched in Linqu County in 2011, a high-risk area of gastric cancer in China [74]. All these evidences suggested that our estimate of H. pylori prevalence was stable and convincing. The positive decline may be partly explained by H. pylori eradication, which is widely accepted as a common clinical practice in the treatment of chronic gastritis and peptic ulcers across China as recommended by the Fourth Chinese National Consensus Report on the management of H. pylori infection [75]. It may be more closely associated with the significant improvement of the economic level, living conditions, hygiene practices, education status, and people’s health awareness in China [76, 77]. All these advances have hugely benefitted the public health of China. With the declining prevalence of H. pylori in the recent 5–10 years, the proportion of corresponding diseases attributable to H. pylori infection may decrease in the future. Besides, it should be mentioned that further investigation on gender-, age-, and setting-specific (urban and rural) H. pylori prevalence would aid in more accurately estimating the attributable burden of H. pylori infection. However, the interpretation of the decreasing H. pylori prevalence and its impact remains to be further clarified.
Irrespective of gastric cancer subsites, the adjusted RR of gastric cancer associated with H. pylori was estimated to be 1.89 (95 % CI: 1.57–2.26) based on Chinese prospective studies. Actually, several risk estimates of gastric cancer from H. pylori-positive serology were yielded in previous meta-analyses conducted by the Helicobacter and Cancer Collaborative Group (OR: 3.12, 95 % CI: 2.23–4.35) [67], Xue et al. (OR: 2.56, 95 % CI: 1.85–3.55) [78], and Huang et al. (OR: 2.28, 95 % CI: 1.71–3.05) [79]. The pooled RRs were usually lower than the pooled ORs, which may be due to the difference in the interval between sample collection and cancer diagnosis [67]. Considering that the previous meta-analyses were mostly based on case–control studies and missed many literatures on the Chinese population, our present pooled RR was supposed to be more reliable to some extent in assessing the PAF of gastric cancer due to H. pylori in China.
Though the relationship between H. pylori and cardia cancer was conflicting, the association of noncardia cancer with H. pylori is widely recognized. Moreover, the magnitude of the risk of developing noncardia cancer associated with H. pylori infection has proven to be positively related with the interval between H. pylori detection and gastric cancer diagnosis. That may be because H. pylori tends not to colonize such areas as atrophy, intestinal metaplasia, or cancer [80]. Therefore, we further calculate the PAF of noncardia cancer due to H. pylori based on the relative risk (2.09, 95 % CI: 1.33–3.29) pooled by the meta-analysis of case–control studies nested within prospective cohorts when blood samples for H. pylori serology were collected 5–9.9 years before cancer diagnosis [67]. According to the global patterns of two major subsites of gastric cancer incidence in 2012, the average ratio of noncardia to cardia gastric cancer cases was 2:1 [81]. As suggested in the annual report by Chen et al., the year of 2012 witnessed around 420,489 cases due to gastric cancer in China [82], which indicated that approximately 105,536 noncardia gastric cancer cases may be attributable to H. pylori infection in China in 2012. The results demonstrated that taking the noncardia and cardia cancer as a whole may underestimate the burden of gastric cancer due to H. pylori infection. So the best choice was to estimate the attributable burden of gastric cancer by subsites.
Notably, compelling evidence suggested that H. pylori treatment had a positive effect on the morbidity and mortality of gastric cancer in China. The latest meta-analysis of cohort studies determined that H. pylori eradication was associated with a reduced risk of gastric cancer (RR: 0.46, 95 % CI: 0.32–0.66) [68]. A previous meta-analysis of randomized controlled trials also reinforced the eradication therapy of H. pylori as an effective strategy of preventing gastric cancer incidence (RR: 0.66, 95 % CI: 0.46–0.95) [83]. Another meta-analysis by Lee et al. also suggested that, after adjustment for baseline gastric cancer incidence, individuals with eradication of H. pylori infection had a lower incidence of gastric cancer (pooled incidence rate ratio = 0.53, 95 % CI: 0.44–0.64) [84]. By applying the relative risk of 0.46 (95 % CI: 0.32–0.66) to the calculation of PAF of gastric cancer due to H. pylori eradication, around 22.82 % of gastric cancer cases could be prevented by H. pylori eradication. Furthermore, by using an empirically calibrated natural history model, Yeh et al. [85] found that screening young adults for H. pylori and treating H. pylori infection could prevent 1 in every 4 to 6 cases of gastric cancer in China and would be considered cost-effective using the GDP per capita threshold in reducing the attributable burden due to H. pylori [86]. In China, the early screening and diagnosis of gastric cancer urgently remains to be improved. So a screen-and-treat strategy for H. pylori should be given priority to promote the prevention of gastric cancer.
When we compared the pooled RR/OR of coronary heart disease with that of ischemic stroke among H. pylori infections in the same subgroup, amazingly, a highly similar magnitude of risk estimates was found in each subgroup of prospective study, case–control study, adjustment, and anti-Hp IgG separately. The phenomenon could be partly explained by the common pathogenesis of atherosclerosis largely shared by CHD and IS. At the same time, we found that the RRs by pooling high-quality prospective studies were consistently lower than pooled ORs based on case–control studies for both CHD and IS. A similar finding was present in another meta-analysis of myocardial infarction (a subtype of CHD) due to H. pylori by Liu et al., in that the pooled RR (1.20, 95 % CI: 1.04–1.38) based on seven prospective studies was lower than the OR (1.95, 95 % CI: 1.46–2.60) based on 19 cross-sectional studies [69]. Moreover, as reported by Sun et al., H. pylori infection had a higher risk of CHD events revealed by pooling prospective studies that had less than 5 years of follow-up time (RR = 1.15, 95 % CI: 1.00–1.32) than those that had a follow-up time ≥10 years (RR = 1.04, 95 % CI: 0.87–1.24) [6]. The findings above reminded us that the magnitude of the risk estimates of CHD in H. pylori infections were negatively associated with the interval time between H. pylori detection and CHD diagnosis. We speculated that the true effect H. pylori infection had on CHD may be weakened or masked by the accumulation of age and other risk factors or comorbidity in long-term follow-ups, because previous meta-analyses have revealed that the association of H. pylori with myocardial infarction were likely age-dependent [69]. Just as suggested by the stratified analysis of a large prospective study involving 85,375 Chinese people, the effect of H. pylori infection on CHD tends to decrease with age, ranging from 1.67 (95 % CI: 1.07–2.61) for the ≤49 years age group to 1.32 (95 % CI: 1.07–1.63) for the >75 years age group [11]. As for other H. pylori-related diseases, these findings remain to be validated. Therefore, we should calculate the PAF of H. pylori infection with caution. We would be better off estimating CHD burden attributable to H. pylori based on high-quality prospective studies adjusting for other confounding factors. If not, we may overestimate the attributable burden. To date, there are insufficient direct evidences from the prospective studies and randomized controlled trials that H. pylori eradication can reduce the incidence of CHD and IS.
When it comes to ischemic stroke, we found that H. pylori infections conferred higher risk of ischemic stroke (RR = 1.53, 95 % CI: 1.41–1.66) in the Chinese population. The present subgroup meta-analysis also suggested that positive anti-H. pylori IgG (OR = 2.30, 95 % CI: 1.92–2.75) and 13C-urea breath test (OR = 2.59, 95 % CI: 2.22 –3.18) both have higher risk of IS. Apparently, the magnitude of relative risk based on prospective studies with longer latency periods between H. pylori detection and IS diagnosis appeared to be higher than that of ORs based on case–control studies. However, a previous meta-analysis of ten prospective studies determined that there was no association between cytotoxin-associated gene-A-positive/-negative/combined H. pylori infection and stroke [85]. This may be due to the heterogeneity across the relationship between H. pylori infection and subtypes of stroke. Notably, another meta-analysis including 13 case–control studies indicated that CagA-positive strains of H. pylori seemed to be associated with a higher risk of IS than H. pylori infections detected by anti-H. pylori IgG or 13C-urea breath test [70]. No CagA serology data are available for the present analysis and large prospective studies in the Chinese population are needed to address the relationship between IS/CHD and different strains of H. pylori, including CagA-positive strains. All these efforts might benefit the personalized prevention of H. pylori-related diseases.
To our knowledge, this is the first systematic assessment of the burden of H. pylori-associated diseases focusing on the Chinese population. The bias caused by genetic and geographical factors was reduced to some extent. Furthermore, our study comprehensively identified all relevant articles from the last 15 years or so. Equally important was the quality of the publications, which was ensured through strict inclusion and exclusion criteria of the studies and evaluating them using the NOS. The risk estimates of H. pylori-related diseases used to calculate the PAFs were based on subgroup meta-analyses of prospective studies, which might avoid the potential limitations of “reverse causality” and “recall bias”, and made the calculation of PAFs more persuasive. In addition, the prevalence of H. pylori in the last decade was estimated more accurately than ever due to large general populations and representative areas that the studies covered, and the wide acceptance of standard detection of H. pylori infection in clinical practice. However, several limitations should be acknowledged. First, although we managed to validate the risk estimates by deeply comparing them with the reported results from previous meta-analyses, more prospective studies among the Chinese population are needed to confirm it. Second, the overall prevalence of H. pylori across the country was estimated based on mainly three measures of H. pylori infection. The consistency among the different detections remains to be determined. Furthermore, the relationships between some other diseases (such as diabetes mellitus, iron deficiency anemia, and so on) and H. pylori infection are not sufficiently studied in China, so the overall burden of diseases due to H. pylori infection remains to be further estimated comprehensively and systematically.
To conclude, in the past 20 years, China has witnessed a steady decline in the prevalence of H. pylori. The risk estimates of H. pylori-related diseases reinforce H. pylori as a common risk factor of various chronic diseases. The PAFs of these diseases above due to H. pylori infection remind us that H. pylori treatment might be helpful in reducing the burden of these diseases and provide potential opportunities for prevention and treatment. Large high-quality prospective studies are warranted to identify which H. pylori strains, which subtypes of the disease, and which subgroups of the population are at greatest risk of relevant diseases (especially cardiovascular diseases and diabetes mellitus) and the effect of H. pylori eradication on the prevention of H. pylori-related diseases. Randomized controlled trials integrating the best biologic, epidemiologic and economic data, and mathematical simulation models are urgently needed to assess the cost-effectiveness ratios of H. pylori screening and treatment.
References
de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D et al (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13:607–615
Xiang W, Shi JF, Li P, Wang JB, Xu LN, Wei WQ et al (2011) Estimation of cancer cases and deaths attributable to infection in China. Cancer Causes Control 22:1153–1161
Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M et al (1996) cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A 93:14648–14653
Møller H, Heseltine E, Vainio H (1995) Working group report on schistosomes, liver flukes and Helicobacter pylori. Int J Cancer 60:587–589
Zullo A, Hassan C, Ridola L, Repici A, Manta R, Andriani A (2014) Gastric MALT lymphoma: old and new insights. Ann Gastroenterol 27:27–33
Sun J, Rangan P, Bhat SS, Liu L (2016) A meta-analysis of the association between Helicobacter pylori infection and risk of coronary heart disease from published prospective studies. Helicobacter 21:11–23
Cremonini F, Gabrielli M, Gasbarrini G, Pola P, Gasbarrini A (2004) The relationship between chronic H. pylori infection, CagA seropositivity and stroke: meta-analysis. Atherosclerosis 173:253–259
Vijayvergiya R, Vadivelu R (2015) Role of Helicobacter pylori infection in pathogenesis of atherosclerosis. World J Cardiol 7:134–143
Chmiela M, Gajewski A, Rudnicka K (2015) Helicobacter pylori vs coronary heart disease—searching for connections. World J Cardiol 7:187–203
Huang WS, Tseng CH, Lin CL, Tsai CH, Kao CH (2014) Helicobacter pylori infection increases subsequent ischemic stroke risk: a nationwide population-based retrospective cohort study. QJM 107:969–975
Lai CY, Yang TY, Lin CL, Kao CH (2015) Helicobacter pylori infection and the risk of acute coronary syndrome: a nationwide retrospective cohort study. Eur J Clin Microbiol Infect Dis 34:69–74
Monzón H, Forné M, Esteve M, Rosinach M, Loras C, Espinós JC et al (2013) Helicobacter pylori infection as a cause of iron deficiency anaemia of unknown origin. World J Gastroenterol 19:4166–4171
Marietti M, Gasbarrini A, Saracco G, Pellicano R (2013) Helicobacter pylori infection and diabetes mellitus: the 2013 state of art. Panminerva Med. 55:277–281
Khamechian T, Movahedian AH, Ebrahimi Eskandari G, Heidarzadeh Arani M, Mohammadi A (2015) Evaluation of the correlation between childhood asthma and Helicobacter pylori in Kashan. Jundishapur J Microbiol 8, e17842
Chang SS, Hu HY (2014) No inverse relationship between Helicobacter pylori infection and adult asthma with peptic ulcer disease. Hepatogastroenterology 61:529–534
Vasapolli R, Malfertheiner P, Kandulski A (2016) Helicobacter pylori and non-malignant upper gastrointestinal diseases. Helicobacter 21(Suppl 1):30–33
Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J (2016) National cancer incidence and mortality in China, 2012. Chin J Cancer Res 28:1–11
Zhang WD, Hu FL, Xiao SD, Xu ZM (2010) Prevalence of Helicobacter pylori infection in China. Mod Digest Interv 15:265–270
Levin ML (1953) The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum 9:531–541
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al (2010) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Kamangar F, Qiao YL, Blaser MJ, Sun XD, Katki H, Fan JH et al (2007) Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer 96:172–176
Epplein M, Zheng W, Xiang YB, Peek RM Jr, Li H, Correa P et al (2012) Prospective study of Helicobacter pylori biomarkers for gastric cancer risk among Chinese men. Cancer Epidemiol Biomarkers Prev 21:2185–2192
Ma JL, Zhang L, Pan KF, Liu WD, Feng GS, You WC (2005) Helicobacter pylori and the progression of gastric cancer: a 10-year cohort study. Natl Med J China 85:2758–2761
Zhang X, Xue L, Xing L, Wang J, Cui J, Mi J et al (2012) Low serum pepsinogen I and pepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric cancer: 14-year follow up result in a rural Chinese community. Int J Cancer 130:1614–1619
Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR et al (2002) Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case–control study in China. World J Gastroenterol 8:1103–1107
Hsu WY, Lin CH, Lin CC, Sung FC, Hsu CP, Kao CH (2014) The relationship between Helicobacter pylori and cancer risk. Eur J Intern Med 25:235–240
Liu XH (2005) Relation between Helicobacter pylori infection and risk factors of coronary heart disease. J Pract Diagn Ther 19:881–883
Lu SY, Guo QH, Liu JS, Xie SZ, Li L, Sun YC et al (2003) The relative study of coronary heart disease and Helicobacter pylori. Med J Chin People Health 15:67–68
Qiao ZF, Hong Y, Zhou L (2010) Investigation on the association between Helicobacter pylori infection and coronary heart disease. Hainan Med J 21:38–40
Wang C, Liu YB, Han ML (2014) Research on correlation between Helicobacter pylori infection and coronary heart disease. Chin J Nosocomiol 24:3526–3527
Wang YP, Yan SQ, Tian HN, Jing H (2008) Correlation between Helicobacter pylori infection and coronary heart disease. Chin J Diffic Compl Cases 7:74–76
Lai YQ, Yang XL, Li M, Hu H, Yang G (2005) Study on relationship between Helicobacter pylori IgG antibody components and coronary heart disease. Chin J Pract Intern Med 25:241–242
Wang Y, Shen J, Yan S, Tian H, Jing H (2008) Correlation between Helicobacter pylori infection and coronary heart disease. Chin J Diffic Compl Cases 7:74–76
Cao PL, Liu XQ, Tang QZ, Liu Y, Wang XQ (2011) Correlation between different Helicobacter pylori genotypes and coronary heart disease. China Med Her 24–26
Chuai Y, Song H, Xue M, Ning X, He Z, Li Y et al (2012) Relationship between Helicobacter pylori infection and coronary artery disease. Chin Heart J 24:57–59
Dian Q, Guo T, Liu Z, Liu R, Li C (2014) Correlation between coronary stenosis and lipid metabolism and Helicobacter pylori infection. Chin J Nosocomiol 24:6144–6146
Zhang J, Xun Y, Gai J, Li Y (2008) The relationship between Helicobacter pylori infection and risk factors associated with coronary heart disease. Chin J Prim Med Pharm 15:1686
Wang WY, Yang PH, Cao YY, Liu F, Ma B (2013) Relation between Helicobacter pylori infection and coronary heart disease. Chin Heart J 21:609–610
Guan XR, Jiang LX, Ma XH, Wang LF, Quan H, Li HY (2010) Respiratory syncytial virus infection and risk of acute myocardial infarction. Am J Med Sci 340:356–359
Liu MY (2011) Relationship between Helicobacter pylori infection and coronary heart disease. Chin J Nosocomiol 21:1599–1601
Deng X, Chen X, Li B (2005) Matching case–control study of the relation between Helicobacter pylori and coronary heart disease. J Nanhua Univ(Med Ed) 33:499–500
Qu W (2012) Research on the correlation of Helicobacter pylori infection in patients with coronary heart disease. China Med Herald 9:49–50
Tian F, Hu D, Sun X, Ge J, Ma J, Wang J et al (2004) The relation between coronary heart disease and chronic infection of microorganism. Tianjin Med J 32:554–556
Zeng Z, Huang MH, Liang YJ, Zhang Q, Jiang T, Liu YG (2000) Preliminary exploration of the association between Helicobacter pylori infection and coronary heart disease. China J Mod Med 10:27–28
Liu DN, He ZY, Li JS (2003) Relation between Helicobacter pylori infection and acute myocardial infarction. Chin J Arterioscler 11:541–544
Lu YL, Pan WQ (2015) Association between Hp infection and first cardio-cerebrovascular events in elderly hypertensive patients. Chin J Geriatr Heart Brain Vessel Dis 17:130–132
Hao R, Tian J (2013) Studies on correlation between acute cerebral infarction and Helicobacter pylori. Pharma Care Res 13:404, 432, 466
Yu Z (2011) Correlation between cerebral infarction and Helicobacter pylori infection. Guide China Med 09:285–287
Shu AM, Zhou YA (2006) The study on relationship between acute cerebral infarction and chronic Helicobacter pylori (Hp) infection with 13C-UREA-Breath test. J Hebei North Univ (Med Ed) 23:3–6
Zhang A, Wang Y, Zhu K (2009) Relationship between the infection of Helicobacter pylori and ischemic stroke. J Neurol Neurorehabil 6:187–190
Wang YS, Jiao J, Wang G (2003) Analysis of Helicobacter pylori infection in patients with acute myocardial infarction patients. Chin J Gerontol 23:716–717
Wei ZH, He D, Zeng ZJ (2014) Situation of Helicobacter pylori infection in patients with cerebral infarction and its effect on high-sensitivity C-reactive protein and blood lipid metabolism. Guangxi Med J 36:335–337
Wu HQ, Zhang X, Tang Y (2012) Association of Helicobacter pylori infection and serum homocysteine level in patients with cerebral infarction. J Zhejiang Univ (Med Sci) 41:89–92
Wu XX, Huang ZY, Huang DH (2003) Study on the relationship between Helicobacter pylori infection and cerebral infarction. Chin J Prim Med Pharm 10:599–600
Zhang X (2007) Study on the relationship between Helicobacter pylori infection and cerebral infarction. Med Inf 20:872–873
Zhang Q, Zhang RB, Meng X (2008) Relationship between Helicobacter pylori infection and transient ischemic attack as well as cerebral infarction. Shandong Med J 48:29–30
Zhao M (2012) Correlation between Helicobacter pylori infection and cerebral infarction. Chin J Nosocomiol 22:3273–3274
Dou YC, Li X, Leng X (2008) Prevalence of H. pylori infection in cerebral infarction patients and the association between H. pylori infection and cerebral infarction. Chin J Lab Diagn 12:352–354
Duan HL, Shi LX, Chen YC, Wang YL (2005) A study on relationship between Helicobacter pylori infection and cerebral hemorrhage and cerebral infarction. J North China Coal Med Coll 7:557–558
Su Z, Pang Y (2007) Relation of Helicobacter pylori infection and coronary heart disease. J Pract Med 23:3019–3021
Zhou GA, Zhang FY, Wang AM (2013) The influence of Helicobacter pylori infection to cerebral infarction of different subtype. J Commun Med 11:33–34
Li XH, Yin L, Liu P (2007) The relationship between cytotoxin-associated gene-A positive Helicobacter pylori strains infection and cerebral infarction. Int J Cerebrovasc Dis 15:455–459
Xu F, Zhu B (2013) Study on correlation between Helicobacter pylori infectis and high sensitive C reactive protein in patients with ischemic stroke. Chin J Nosocomiol 23:3645–3646
Yang X, Gao Y, Zhao X, Tang Y, Su Y (2011) Chronic Helicobacter pylori infection and ischemic stroke subtypes. Neurol Res 33:467–472
Wang KJ, Wang RT (2003) Meta-analysis on the epidemiology of Helicobacter pylori infection in China. Chin J Epidemiol 19–22
Helicobacter and Cancer Collaborative Group (2001) Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 49:347–353
Doorakkers E, Lagergren J, Engstrand L, Brusselaers N (2016) Eradication of Helicobacter pylori and gastric cancer: a systematic review and meta-analysis of cohort studies. J Natl Cancer Inst 108. pii: djw132
Liu J, Wang F, Shi S (2015) Helicobacter pylori infection increase the risk of myocardial infarction: a meta-analysis of 26 studies involving more than 20,000 participants. Helicobacter 20:176–183
Wang ZW, Li Y, Huang LY, Guan QK, Xu DW, Zhou WK et al (2012) Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta-analysis. J Neurol 259:2527–2537
Zhang S, Guo Y, Ma Y, Teng Y (2008) Relationship between the cytotoxin-associated gene-A status of H pylori strains and cerebral infarction in European Caucasians and Chinese Han: a meta-analysis. World J Gastroenterol 14:1286–1292
Chen J, Bu XL, Wang QY, Hu PJ, Chen MH (2007) Decreasing seroprevalence of Helicobacter pylori infection during 1993–2003 in Guangzhou, southern China. Helicobacter 12:164–169
Zhang DH, Zhou LY, Lin SR, Ding SG, Huang YH, Gu F et al (2009) Recent changes in the prevalence of Helicobacter pylori infection among children and adults in high- or low-incidence regions of gastric cancer in China. Chin Med J (Engl) 122:1759–1763
Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K et al (2016) A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 65:9–18
Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD et al (2013) Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis 14:211–221
Everhart JE (2000) Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am 29:559–578
Shi RH, Xu SF, Zhang HJ, Ding YB, Sun GQ, Huang XY et al (2008) Prevalence and risk factors for Helicobacter pylori infection in Chinese populations. Helicobacter 13:157–165
Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM (2001) Association of H. pylori infection with gastric carcinoma: a meta analysis. World J Gastroenterol 7:801–804
Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH (2003) Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 125:1636–1644
Karnes WE Jr, Samloff IM, Siurala M, Kekki M, Sipponen P, Kim SW et al (1991) Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology 101:167–174
Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I (2015) Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 64:1881–1888
Chen WQ, Zheng RZ, Zeng HM, Zhang SW, He J (2015) Annual report on status of cancer in China, 2011. Chin J Cancer Res 27:2–12
Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P (2014) Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 348:g3174
Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS et al (2016) Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology 150:1113–1124.e5
Yeh JM, Kuntz KM, Ezzati M, Goldie SJ (2009) Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer 124:157–166
Rothenbacher D, Brenner H (2003) Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: recent developments and future implications. Microbes Infect 5:693–703
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
The authors have no competing interests.
Ethical approval
Not required.
Informed consent
All authors approved the final version of the manuscript.
Electronic supplementary material
Below are the links to the electronic supplementary material.
Supplementary Table 1
The detailed information from the studies on the prevalence of H. pylori across China (DOC 169 kb)
Supplementary Table 2
References cited in Table 2 (DOC 978 kb)
Rights and permissions
About this article
Cite this article
Jiang, J., Chen, Y., Shi, J. et al. Population attributable burden of Helicobacter pylori-related gastric cancer, coronary heart disease, and ischemic stroke in China. Eur J Clin Microbiol Infect Dis 36, 199–212 (2017). https://doi.org/10.1007/s10096-016-2810-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2810-x