Abstract
The objective of this investigation was to identify risk factors for carbapenem-resistant Acinetobacter baumannii (CRAB) and its association with mortality. A population-based matched case–control study using the computerized database of Clalit Health Services (CHS) in the period between 2007 and 2012 was conducted. Hospitalized patients with CRAB colonization or infection were compared to hospitalized patients without evidence of A. baumannii, matched by age, ward of hospitalization, season, Charlson score, and length of hospitalization. Risk factors for CRAB isolation were searched for using multivariate analysis. Association of CRAB and other risk factors with mortality were assessed in the cohort. A total of 1190 patients with CRAB were matched to 1190 patients without CRAB. Low socioeconomic status was independently associated with CRAB isolation and CRAB bacteremia [odds ratio 2.18, 95 % confidence interval (CI) 1.02–5]. Other risk factors were invasive procedures and bacteremia with other pathogens prior to CRAB isolation, and various comorbidities. Among all patients, CRAB isolation was independently associated with increased mortality (hazard ratio 2.33, 95 % CI 2.08–2.6). Socioeconomic status is associated with health outcomes. Our population-based study revealed an almost doubled risk for CRAB in patients at lower socioeconomic status and an association with healthcare exposure. CRAB was associated with mortality and might become a risk indicator for complex morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter baumannii (A. baumannii) is an aerobic non-fermenter Gram-negative coccobacillus that has become a major nosocomial pathogen in the last two decades. Its ability to resist environmental stress and the rapid development of resistance to broad-spectrum antibiotics have made this pathogen one of the most prevalent causes of outbreaks in the setting of intensive care units (ICUs) [1, 2]. Ruiz et al. reported an increase in the rate of imipenem resistance from 1.3 % in 1991 to 80 % in 1996 in Spain [3]. Colistin, the only antibiotic with no resistance in that cohort, has recently been reported as having a high level of resistance in outbreaks in Italy and Greece [4].
Several recent studies investigated the epidemiology and morbidity associated with multidrug-resistant (MDR) A. baumannii [5–8]. Risk factors for carbapenem-resistant A. baumannii (CRAB) infection or colonization were prior use of carbapenems, aminoglycosides, or cephalosporins [9–11], and admission to the ICU and other wards with a high density of CRAB and correlates of intense healthcare exposure, such as intravenous catheters and mechanical ventilation [11–15]. The attributable mortality in patients with A. baumannii colonization or infection was reported in several studies. A systematic review reported an attributable mortality of 16–27 % in critically ill patients with infection or colonization compared to matched control patients without infection or colonization [16]. Most of the studies assessing risk factors and mortality of MDR A. baumannii included small cohorts or case–control designs, and differed regarding MDR definitions, the case definition (infection or colonization), and the control group selection.
The objectives of our study were to identify risk factors for CRAB colonization, infection, and bacteremia in hospitalized patients, and to assess the association between CRAB colonization or infection and mortality, in a large population-based study.
Methods
A matched case–control study, conducted using the computerized database of Clalit Health Services (CHS) between January 2007 and August 2012 was undertaken. The CHS is the largest health maintenance organization (HMO) in Israel, serving more than 4 million citizens, with different geographic and socioeconomic features. The database includes outpatient clinics, hospitalized patients, and patients residing in long-term care facilities (LTCFs) and nursing homes.
All hospitalized adult patients >18 years old were eligible for participation in the study. A case patient was defined as a patient from whom CRAB was isolated from any source, either as colonization or infection. Specimen sources were categorized as blood, respiratory, urine, and non-sterile sources such as wounds. A patient was included only once, at the time of the first CRAB isolation. CRAB was defined as A. baumannii resistant to imipenem or meropenem. In cases of discrepancies, imipenem resistance served as the reference. A control patient was matched to each case from all hospitalized patients in the CHS who did not have an A. baumannii isolated during the study period. Matching criteria included age (within 3 years of age), ward of hospitalization, season of hospitalization, Charlson’s comorbidity score (within 1.5 points), and the time at risk. To control for time at risk, control patients were hospitalized for at least the same number of days (within 3–5 days) as the days until the first culture of CRAB was recovered among cases. The index date was defined as the date of the first CRAB isolate in case patients, and the date corresponding to the same number of days post-admission in the matched control patient. A subgroup analysis for patients with CRAB bacteremia was predefined, in which case patients had CRAB bacteremia and control patients were matched using the same criteria to the bacteremic cases.
Potential risk factors for CRAB colonization or infection included demographic features, comorbidities, immunosuppressive drugs, invasive procedures, recent bacteremia, and recent hospitalizations. Socioeconomic status was extracted from the outpatient clinic location to which the patient belonged. Comorbidities were defined as any chronic disease other than malignancies, including cardiovascular diseases, pulmonary diseases, cerebrovascular diseases, diabetes mellitus, hepatic diseases, and renal failure. Recent bacteremia was defined as positive blood cultures, excluding contaminations according to the Centers for Disease Control and Prevention (CDC) criteria [17], in the 30 days before CRAB isolation. We also searched for antibiotic use, steroid use, and immunosuppressive treatments in the 3 months before CRAB isolation. Data on demographic features and comorbidities, including malignancies and prior medications, were obtained from the community medical charts predating the relevant hospitalization. Information on invasive procedures and recent bacteremia was extracted from the database 3 months predating the CRAB isolation, and in the control group predating the index date. Risk factors for mortality were examined in the entire cohort. All data were extracted from the HMO’s electronic patient records that comprise inpatient admissions and outpatient visits.
Categorical variables were compared using the Chi-square or Fisher’s exact test, as appropriate. Continuous variables were analyzed using the t-test or the Mann–Whitney test after verifying whether the distribution was normal or not. A series of multivariable conditional logistic regression models was conducted to investigate the association between potential risk factors (independent variables) and CRAB isolation (dependent variable). Independent variables were selected based on a p-value ≤ 0.1 in the bivariate analyses and clinical relevance. Effect modifications/interactions between independent variables were tested. Odds ratios (ORs) and their corresponding 95 % confidence intervals (CIs) were calculated.
In order to identify variables associated with mortality, including CRAB carriage, we used Kaplan–Meier survival analysis with the log rank test. We then used the Cox proportional hazards model to determine hazard ratios and their confidence intervals.
All tests were two-tailed, and statistical significance was defined as a p-value <0.05. The analyses were performed using SAS version 9.3 and SPSS version 20. The study was approved by the Clalit Ethical Review Board. All data were anonymized before analysis.
Results
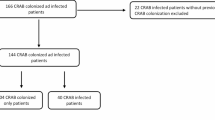
A total of 6998 patients had A. baumannii isolated between the years 2007 and 2012 in hospitals, LTCFs, nursing homes, and in the community. Of them, 4375 were hospitalized and 2196 of the hospitalized patients had CRAB colonization or infection. Of this population, 1190 hospitalized patients with CRAB colonization or infection (cases) could be matched to 1190 hospitalized control patients. The demographic and matching characteristics are described in Table 1.
Most of the patients in the case group (n = 1190) were hospitalized in medical wards (69 %), followed by surgery departments and ICUs (12 %). Patients with CRAB had a mean age of 73 ± 14 years (median 76, range 21–115) and slightly more than half were male (55.6 %). The mean Charlson score was 6.8 ± 3.2 (median 7, range 0–19). Twenty-one percent of the CRAB cases were isolated in the first 48 h of hospitalization and the median length of hospitalization prior to the recovery of CRAB was 8 days (range 0–70). Most of the CRAB isolates were from the respiratory tract (42.6 %), followed by blood (20 %), non-sterile sources, and urine. Of the CRAB isolates that were tested, 99 %, 48 %, 34 %, and 27.5 % were susceptible to colistin, ampicillin–sulbactam, amikacin, and trimethoprim–sulfamethoxazole, respectively. Less than 1 % were susceptible to piperacillin–tazobactam or ciprofloxacin.
Risk factors for CRAB infection on univariate analysis are detailed in Table 2. Compared to controls, patients with CRAB were at lower socioeconomic status, received more immunosuppressive drugs, and had more frequently chronic diseases other than malignancies. Healthcare exposure was greater among cases, as reflected by more invasive procedures, more frequently recurrent hospitalizations in the 6 months before the index date, and a higher frequency of bacteremia in the month before the index date. Antibiotics in the 3 months prior to CRAB isolation or the index date among controls were taken by 41 % of the patients in both groups. Data on antibiotic treatment in-hospital were unavailable.
In the multivariable analysis, a lower socioeconomic status, chronic diseases other than malignancies, immunosuppressive therapy (other than steroids), invasive procedures, and prior bacteremia were significant risk factors for CRAB isolation. Solid or hematological transplantations were associated with lower rates of CRAB isolation, with an adjusted OR of 0.15 (95 % CI 0.03–0.79).
The epidemiology of CRAB bacteremia was similar to that observed for all CRAB patients (71 % hospitalized in medical wards, 10.4 % surgery, and 13.7 % in the ICU). Similar susceptibility patterns were seen. The only significant risk factors for CRAB bacteremia were low socioeconomic status, invasive procedures, and bacteremia in the 30 days preceding the index date (Table 3).
The all-cause mortality for patients with CRAB was 73 % compared to 55 % among controls during the study period. On multivariate analysis of the overall cohort, CRAB isolation was associated with an increased mortality, with a hazard ratio (HR) of 2.33 (95 % CI 2.08–2.6). Other independent risk factors for mortality included comorbidities comprising malignancy, immunosuppressive treatment, and recurrent hospitalization in the 6 months preceding the index date (HR 1.33, 95 % CI 1.19–1.48) (Table 4).
Discussion
A. baumannii has emerged as a major threat in the healthcare setting in the past few decades worldwide [18]. In this population-based study, we sought to identify risk factors for CRAB colonization or infection. We found that patients of low socioeconomic status had an almost doubled risk of CRAB isolation compared to patients of high socioeconomic status. Other patients found to be at risk were patients who had chronic diseases or received immunosuppressive treatment, patients who underwent invasive procedures, or those who had bacteremia (with bacteria other than A. baumannii) in the 30 days prior to the index date. The last two factors were also found in the subgroup analysis for CRAB bacteremia. CRAB colonization and infection were independently associated with increased mortality (HR 2.33, 95 % CI 2.08–2.6).
Antibiotic exposure is one of the most frequently reported risk factors for MDR A. baumannii colonization or infection. Some studies found carbapenems to be a risk factor, but reported ORs varied across the studies [9, 12, 14, 19]. In other studies, the use of fluoroquinolones was associated with MDR A. baumannii infection, whereas several studies did not find an association between antibiotic use and MDR A. baumannii isolation [11]. Unfortunately, we did not have access to information on antibiotic use during hospitalization in our database and, therefore, could not support these results in the literature described above. However, we found that recent bacteremia with other pathogens was highly associated with CRAB isolation and also specifically with CRAB bacteremia, even after controlling for frequent confounders, such as length of hospitalization and baseline comorbidity score.
Low socioeconomic status has been described previously as a risk factor for antimicrobial resistance of Enterobacteriaceae, Mycobacterium tuberculosis, and other pathogens [19–21]. To our knowledge, low socioeconomic status has not been previously described as a risk factor for CRAB. Since both cases and controls were treated with antimicrobial agents in the community with the same frequency (40 %), antibiotic treatment is probably not the main explanation for this finding. As the socioeconomic status of the patients was extracted from their clinic affiliation, our results may be explained by healthcare disparities or by patients’ characteristics belong to those clinics. Unfortunately, these data were not available to us.
We found a lower rate of transplantations in the CRAB group as compared to the controls. This is allegedly an intriguing finding, as the patients with immunosuppressed therapy were found to be at high risk for CRAB recovery. We believe that this is due to the inclusion of patients with hematopoietic stem cell transplantation (HSCT) in the transplant group. Patients undergoing HSCT are neutropenic and exposed mainly to endogenic Gram-negative bacteria colonizing the gastrointestinal tract [22]. Because A. baumannii is a nosocomial environmental pathogen, it is not surprising that HSCT patients had a lower rate of CRAB than the controls [23].
The mortality rate in our study was very high (73 % in the study period and 55 % within the first year). CRAB isolation was an independent risk factor for death after adjustment to other risk factors, albeit without clinical data on the severity of illness during hospitalization. The attributable mortality of A. baumannii has been a matter of continuing debate. Other causes for death, such as severity of illness before A. baumannii infection and inappropriate antibiotic use in cases of multidrug resistance, make it difficult to attribute the mortality to CRAB. Most studies agree with ours that CRAB carriage is associated with higher mortality [23].
The strength of our study is in its size and diversity of patients included. We searched for CRAB isolation in a very large population database, which included more than half of Israeli citizens. We selected the control group without A. baumannii from the source population using strict matching criteria. This control group optimally represents the base population at risk of exposure to CRAB [24, 25]. Despite matching, we found statistically significant differences in matching variables between cases and controls; however, these were minor differences of little clinical significance. We evaluated the risk factors for CRAB isolation and not specifically CRAB infection. Playford et al. described the importance of colonization pressure for CRAB acquisition [11]. Therefore, we believe that it is important to identify the burden of the patients with CRAB, either infected or colonized, in order to contain the spread of this worrisome pathogen.
Our study had some limitations. We included only 1190/2196 hospitalized patients with CRAB in our analyses, due to our strict matching criteria. The excluded patients with CRAB were younger and had less comorbidities (data not shown). We believe that strict matching was necessary for valid estimation of risk factors and outcomes of CRAB. Because we did not have information on clinical signs and symptoms nor about antibiotic use, infection was not an exclusion criteria in the control group. This could lead to underestimation of certain risk factors. We also could not match for disease severity during hospitalization, although we can assume that the results were not biased in a distorted way.
In conclusion, patients with comorbidities and patients with previous healthcare exposure are at an increased risk of CRAB colonization or infection. Curiously, low socioeconomic status was also associated with such risk. Further research should determine whether patients’ characteristics or healthcare disparities explain this association. CRAB was independently associated with more than double the hazard for death. It remains to be determined whether the screening of patients at risk for CRAB colonization or infection is beneficial.
References
Villegas MV, Hartstein AI (2003) Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol 24(4):284–295
Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5(12):939–951
Ruiz J, Núñez ML, Pérez J, Simarro E, Martínez-Campos L, Gómez J (1999) Evolution of resistance among clinical isolates of Acinetobacter over a 6-year period. Eur J Clin Microbiol Infect Dis 18(4):292–295
Agodi A, Voulgari E, Barchitta M, Quattrocchi A, Bellocchi P, Poulou A et al (2014) Spread of a carbapenem- and colistin-resistant Acinetobacter baumannii ST2 clonal strain causing outbreaks in two Sicilian hospitals. J Hosp Infect 86(4):260–266
Falagas ME, Kopterides P (2006) Risk factors for the isolation of multi-drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: a systematic review of the literature. J Hosp Infect 64(1):7–15
Wadl M, Heckenbach K, Noll I, Ziesing S, Pfister W, Beer J et al (2010) Increasing occurrence of multidrug-resistance in Acinetobacter baumannii isolates from four German University Hospitals, 2002–2006. Infection 38(1):47–51
Abbo A, Carmeli Y, Navon-Venezia S, Siegman-Igra Y, Schwaber MJ (2007) Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur J Clin Microbiol Infect Dis 26(11):793–800
Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J et al (2007) Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 13(1):97–103
del Mar Tomas M, Cartelle M, Pertega S, Beceiro A, Llinares P, Canle D et al (2005) Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk-factors for colonisation and infection. Clin Microbiol Infect 11(7):540–546
Lee SO, Kim NJ, Choi SH, Hyong Kim T, Chung JW, Woo JH et al (2004) Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case–control study. Antimicrob Agents Chemother 48(1):224–228
Playford EG, Craig JC, Iredell JR (2007) Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect 65(3):204–211
Corbella X, Montero A, Pujol M, Domínguez MA, Ayats J, Argerich MJ et al (2000) Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol 38(11):4086–4095
Tacconelli E, Cataldo MA, De Pascale G, Manno D, Spanu T, Cambieri A et al (2008) Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. J Antimicrob Chemother 62(5):1130–1137
Routsi C, Pratikaki M, Platsouka E, Sotiropoulou C, Nanas S, Markaki V et al (2010) Carbapenem-resistant versus carbapenem-susceptible Acinetobacter baumannii bacteremia in a Greek intensive care unit: risk factors, clinical features and outcomes. Infection 38(3):173–180
Levin AS, Mendes CM, Sinto SI, Sader HS, Scarpitta CR, Rodrigues E et al (1996) An outbreak of multiresistant Acinetobacter baumanii in a university hospital in São Paulo, Brazil. Infect Control Hosp Epidemiol 17(6):366–368
Falagas ME, Bliziotis IA, Siempos II (2006) Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case–control studies. Crit Care 10(2):R48
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36(5):309–332
Villar M, Cano ME, Gato E, Garnacho-Montero J, Miguel Cisneros J, Ruíz de Alegría C et al (2014) Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: a reappraisal. Medicine 93(5):202–210
Bonelli RR, Moreira BM, Picão RC (2014) Antimicrobial resistance among Enterobacteriaceae in South America: history, current dissemination status and associated socioeconomic factors. Drug Resist Updat 17(1–2):24–36
Kristiansson C, Grape M, Gotuzzo E, Samalvides F, Chauca J, Larsson M et al (2009) Socioeconomic factors and antibiotic use in relation to antimicrobial resistance in the Amazonian area of Peru. Scand J Infect Dis 41(4):303–312
Kirby A, Herbert A (2013) Correlations between income inequality and antimicrobial resistance. PLoS One 8(8):e73115
Mackall C, Fry T, Gress R, Peggs K, Storek J, Toubert A (2009) Background to hematopoietic cell transplantation, including post transplant immune recovery. Bone Marrow Transplant 44(8):457–462
Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J et al (2009) Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control Hosp Epidemiol 30(12):1186–1192
Harris AD, Karchmer TB, Carmeli Y, Samore MH (2001) Methodological principles of case–control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin Infect Dis 32(7):1055–1061
Harris AD, Samore MH, Lipsitch M, Kaye KS, Perencevich E, Carmeli Y (2002) Control-group selection importance in studies of antimicrobial resistance: examples applied to Pseudomonas aeruginosa, Enterococci, and Escherichia coli. Clin Infect Dis 34(12):1558–1563
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
All authors have no conflicts of interest relevant to this article to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Larissa German is a Research Consultant, Calgary, Alberta, Canada.
Rights and permissions
About this article
Cite this article
Henig, O., Weber, G., Hoshen, M.B. et al. Risk factors for and impact of carbapenem-resistant Acinetobacter baumannii colonization and infection: matched case–control study. Eur J Clin Microbiol Infect Dis 34, 2063–2068 (2015). https://doi.org/10.1007/s10096-015-2452-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2452-4