Abstract
Introduction
Door-to-needle time (DNT) is an established predictor of outcome in acute ischemic stroke (AIS) patients treated with intravenous thrombolysis (IVT). Several strategies have been proposed to streamline in-hospital pathways, among which treatment at CT/MR bed.
Aim
To explore the impact of treatment at CT/MR bed, here defined as imaging area (IA), on functional outcome in stroke patients treated with IVT alone.
Methods
All AIS patients treated with IVT alone at our center in 2020, 2021, and 2022 were included. Patients with any previous disability were excluded. The cohort was divided into two groups, depending on the treatment site. One group received IVT at IA, the other at emergency room or stroke unit (non-IA). Regression analysis assessed the association between treatment site and 3-month outcome.
Results
A total of 327 patients who received IVT alone were included in the analysis. One hundred thirty-three (40.7%) were in the IA group and 194 (59.3%) in the non-IA group. The groups showed similar baseline characteristics. In the IA group, DNT was 45 min shorter. Despite similar rates of functional independence (mRS 0-2), the IA group showed higher rates of excellent outcome (mRS 0-1) compared to the non-IA group (60.1% vs 42.8%, p<0.01). Immediate treatment at IA was independently associated to excellent outcome (OR 1.78 [1.03–3.08]).
Conclusions
Thrombolytic treatment at IA lowers DNT and is an independent predictor of excellent outcome after AIS. Our study emphasizes the importance of immediate thrombolytic treatment at IA, soon after radiological eligibility is confirmed. Immediate treatment at IA should be a standard-of-care for AIS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intravenous thrombolysis (IVT) is an established treatment for acute ischemic stroke (AIS) and its effectiveness is strictly time-dependent [1]. Efforts to reduce treatment delays involve both the pre-hospital and in-hospital pathways. The door-to-needle time (DNT), meaning as the interval between hospital arrival and thrombolysis administration, is an important indicator of stroke care [2]. In the last decades, many strategies were proposed to lower DNT, as emergency medical service (EMS) pre-notification of a suspected stroke patient [3] a high-urgency code for transport and in-hospital management [4, 5], activation of a dedicated stroke team, treatment before availability of coagulation tests [6], and IVT administration directly at the emergency room [7], or at the imaging area (IA) [8].
Though starting IVT at IA, on CT/MR bed, results in a strong impact on DNT reduction, it is still not the standard-of-care in several comprehensive and primary stroke centers. Apart from organizational issues, a possible explanation is that the specific impact of administering IVT at IA on clinical outcome has rarely been highlighted. The aim of our study is to analyze the effects on functional outcome of IVT administration at the imaging area in a single-center population of stroke patients.
Methods
Study population
We retrospectively analyzed data from the local registry of acute stroke treatments at our center, including patients treated from January 1, 2020, to December 31, 2022.
The inclusion criteria for this study were as follows: age >18 years, pre-stroke excellent functional status, defined as a modified Rankin Scale score 0–1, IVT treatment with alteplase for acute ischemic stroke. In this analysis, we excluded the following: patients with previous disability, or undergoing endovascular thrombectomy (EVT), to reduce the heterogeneity of the sample and the influence of different predictors of outcome (e.g., stroke severity, door-to-groin and procedural times, final TICI score, number of passes, and collateral circulation status). All patients were treated according to current guidelines. [9] The study sample size was determined based on the tool developed by Johns et al. [10], setting a confidence level of 95% and statistical power of 90%, and further confirmed based on the tool developed by the Italian Health Institute (ISS) [https://www.epicentro.iss.it/strumenti/samplesize] setting confidence interval to 5.
Our internal protocol changed starting from October 1, 2021: all patients before this date were treated outside IA (emergency department or stroke unit), whereas after this date IVT was administered on CT/MR bed. All patients with AIS received a high-urgency (Red) code at hospital arrival, and were quickly transferred to the emergency room. In the meanwhile, the stroke team was activated. For patients treated at IA, the neurological evaluation and eventual hypertension management were performed in the emergency room, before moving to IA. If the neurological examination confirmed a vascular syndrome, IVT was administered directly on CT/MR bed, as soon as a brain hemorrhage was excluded. In patients beyond the 4.5-h time-window or with wake-up/unwitnessed stroke, IVT was started in IA, at the availability of CT perfusion or MR images, according to current guidelines [9].
Patients treated outside IA (non-IA) were transferred to the stroke unit or back to the emergency room, to receive IVT, after neuroimaging evaluation and eventual hypertension management. All patients received alteplase 0.9 mg/kg.
Data collection
Demographic and clinical information, such as time of symptoms onset or last-well-seen time (LWST); baseline National Institute of Health Stroke Scale (NIHSS) score; baseline Alberta Stroke Project Early Computed Tomography (ASPECT) score; door-to-imaging time (DTI) ; onset-to-imaging time; door-to-needle time (DNT) ; site of IVT starting; occurrence of hemorrhagic transformation; 2-h, 24-h, and discharge NIHSS score; and 3-month mRS score, were prospectively collected.
Clinical outcomes
Since in patients without large vessel occlusion (LVO) a high rate of functional independence at follow-up is expected, we set as main clinical outcomes both functional independence and excellent performance status, respectively, defined as mRS ≤2 and mRS ≤1, obtained during an in-person follow-up visit or by a telephone call to the patient or a close caregiver, at 90 ± 14 days after stroke. Secondary outcome measures were NIHSS scores at 2 h, at 24 h, and at discharge, symptomatic hemorrhagic transformation (sICH) according to SITS-MOST criteria [11], and overall mortality.
Statistical analysis
Continuous variables, such as age, pre-hospital delay (onset-to-arrival time, or last-well-seen-to-arrival time), DTI, DNT, onset-to-imaging time, and imaging-to-needle time, were expressed as mean values and standard deviations or as median and interquartile ranges, as appropriate.
Non-parametric variables, as NIHSS and ASPECT scores, were expressed as median and interquartile ranges.
Categorical variables, such as gender, arterial hypertension, diabetes mellitus, atrial fibrillation, site of IVT starting, sICH, functional independence, excellent performance status, and mortality at 3 months, were expressed as whole numbers and percentages.
The site of IVT starting was considered a binary variable: patients treated on CT/MR bed were considered the imaging area (IA) group, whereas patients treated at the emergency room or at the stroke unit were included in the non-IA group.
Etiology was divided in five categories (lacunar, large artery atherosclerosis, cardiac embolism, embolic stroke of undetermined source (ESUS), and other causes of stroke, including cancer-related stroke and dissections). Time was expressed in minutes.
For statistical purposes, our cohort was dichotomized in two samples depending on the site of IVT treatment beginning.
Student’s t-test was used to detect any significant differences in demographic and clinical variables among the two groups.
Afterwards, a stepwise forward logistic regression model was used to evaluate the odds ratio of excellent performance status and/or functional independence at 3 months for each independent variable. Among continuous variables, we used as independent variables age, baseline NIHSS and ASPECT scores, and door-to-imaging time. Among dichotomous variables, we used site of IVT beginning, gender, arterial hypertension, diabetes mellitus, smoking, atrial fibrillation, dyslipidemia, wake-up stroke, and occurrence of sICH; etiology was treated as a multinomial variable.
For each of these variables, except for etiology, the univariate logistic regression model was used; variables reaching a p value <0.1, corrected for age and gender, were included in a multiple logistic regression model, to estimate the effects on 3 months outcome. The effect size was measured as odds ratio (OR) and confidence intervals; the included independent variables were checked for collinearity to exclude possible confounders. In order to verify that treatment effect was consistent across all categories of the mRS, an ordinal logistic regression analysis was performed, with the same criteria and independent variables. For etiology, we applied a factorial analysis due to its multi-level categorization.
Statistical analysis was performed by using STATA 13 for iOS.
Results
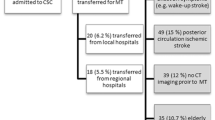
The estimated sample size to identify a significant effect ranged from 306 patients (according to ISS) to 330 (according to Johns et al.). Seven hundred forty-three consecutive patients with acute ischemic stroke received IVT during 2020, 2021, and 2022 and collected in our local stroke registry. A total of 357/743 patients also received EVT. Among the 386 patients treated only with IVT, 59 were excluded from this analysis: 51 with previous disability, defined as pre-stroke mRS ≥2, 6 with a final diagnosis of stroke mimic and 3 lost at follow-up. Therefore, 327 patients were included in the statistical analysis.
The demographic and clinical features of the entire sample are summarized in Table 1. The groups were matched for age, gender, risk factors, stroke clinical, and radiological severity at onset and prehospital delay. The two groups differed for the door-to-imaging time, lower in the IA group.
In-hospital time-to-treatment and clinical outcomes in the two groups are described in Table 2. The median DNT in the entire sample was 71.1 min (IQR 42–100), significantly lower in the IA group than the non-IA group (44.4 vs 89.3; p<0.01). The rate of IVT with DNT ≤ 30 min, ≤ 45 min, and ≤ 60 min was significantly higher in the IA group (32.3% vs 3.6%; 65.4% vs 10.3% and 83.4% vs 28.3%, respectively; p<0.01). The IA group showed greater early neurological improvement, at 2 h (p<0.05), at 24 h (p<0.05), and at discharge (p<0.01). Though no differences were detected in the rate of functional independence at 3 months (mRS 0–2), IA group showed significantly higher rates of excellent outcome (60.1% vs 42.8%; p<0.01), defined as 3-month mRS 0–1 (Fig. 1). The rate of sICH was similar in the two groups.
In the univariate logistic regression analysis, baseline NIHSS score (OR 0.85 [0.81–0.90], z score −5.76), baseline ASPECTS (OR 1.77 [95% CI, 1.20–2.61], z score 2.90), history of hypertension (OR 0.54 [95% CI, 0.32–0.91], z score −2.26) and diabetes (OR 0.62 [95% CI, 0.35–1.07], z score −1.72), and site of IVT beginning (OR 2.06 [1.32–3.24], z score 3.18) were significantly associated to excellent outcome. Conversely, pre-hospital delay, door-to-imaging time, unwitnessed symptoms onset, and stroke etiology did not show any significant association with outcome.
In the multivariate logistic regression analysis, corrected by age and gender, age at stroke onset (OR 0.96 [95% CI, 0.94–0.98], z score −3.19], baseline NIHSS score (OR 0.86 [95% CI, 0.81–0.91], z score −4.54), and site of IVT beginning (OR 1.78 [95% CI, 1.03–3.08], z score 2.08) were significantly associated with excellent outcome (Table 3, supplementary material). Ordinal multiple logistic regression analysis confirmed the same results and showed a significant association between baseline ASPECT score and outcome (OR 0.75 [95% CI, 0.58–0.96], z score −2.22). Notably, IVT at IA was associated to reduced odds of unfavorable outcome (Table 4, supplementary material) (Fig. 2).
Discussion
Our study showed that starting IVT at IA, directly on the CT/MR bed, is associated to greater early neurological improvement and higher rate of excellent outcome in patients with AIS treated with IVT. This favorable effect is consistent across all categories of mRS. Treatment at IA significantly lowers in-hospital delays, increasing the proportion of patients receiving IVT within 30, 45, and 60 min from hospital arrival, as encouraged by current standards of excellence [12, 13]. There were no differences in safety outcomes, such as mortality or hemorrhagic transformation.
Six patients were excluded from the analysis as they received a final diagnosis of stroke mimic. Two of them were in IA group, whereas 4/6 were in the non-IA group. Therefore, the accuracy of the diagnostic process was not influenced by faster assessment and treatment.
Though the two groups did not differ in terms of functional independence at 3 months, as reasonably expected in patients with less severe strokes unrelated to LVO, our study showed that starting IVT at IA is an independent predictor of excellent outcome, improving the chances of full recovery.
These results confirm that developing protocols easily applicable in emergency settings can significantly influence outcome in a large subset of patients; in particular, starting IVT directly on the CT/MR bed appears to be one of the strategies leading to a greater reduction of DNT. A potential explanation may be avoiding multiple displacements and transfers, involving several professional figures and poorly cooperating patients.
Treatment delays are historically affected by the baseline NIHSS score, patients’ age and gender, and several other clinical characteristics, as atrial fibrillation, diabetes, and arterial hypertension [14, 15]. In our cohort, these variables were well-balanced in the two groups. Door-to-imaging time was significantly lower in patients treated at IA. We hypothesize, beyond an improvement over time in the hospital pathway, that the “treat-at-CT” protocol may play a positive “indirect effect”: all personnel involved in the management of acute stroke may become more aware that every single minute counts, speeding up all the stages of the stroke pathway. A potential limitation of our study is that a proportion of patients in the control group were managed in 2020, during the first COVID-19 outbreak, which has delayed stroke treatments worldwide, including our Region [16]. Despite that in our hospital the stroke-COVID patients were managed in a dedicated area with dedicated pathways and personnel (and therefore not included in this analysis), it is possible that, even if we struggled to guarantee the pre-pandemic level of care, the COVID-19 outbreak may have negatively affected stroke care, particularly the first part of in-hospital pathway.
However, door-to-imaging time was not independently associated with outcome, confirming that the decision to start IVT at imaging area remains crucial to improve neurological recovery and functional outcome.
Some components of our stroke pathway are certainly improvable. Emergency medical system prenotification of a potential stroke patient is still poorly developed. This intervention is particularly important in limited supply of radiological equipment, such as in small hospitals [17]. A direct-to-CT protocol may be desirable. However, it may affect the recognition of potential life-threatening stroke mimics by the emergency physicians and may even increase chaos in conditions of Emergency Department overcrowding and understaffing, as it is currently happening in Italy.
A direct-to-angio protocol has recently been suggested for AIS patients [18]. This protocol would be applicable only in a selected population of patients with LVO admitted within 6 h after symptom onset, considered for EVT. However, this approach is probably far from wide clinical use, as it requires the constant availability of an angiographer equipped with a flat-panel computed tomography, and dedicated to neurological emergencies. Other intrinsic limitations of our study are the relatively small sample size, its observational and retrospective nature, and the unblinded outcome assessment, which confer our results a low level of evidence.
Notwithstanding, our analysis presents some strengths. The demographic and clinical characteristics were well-balanced. The rate of patients lost at follow up was less than 1%, increasing the strength of our analysis. The rate of stroke mimics was not influenced by a faster diagnostic and treatment pathway, accounting for less than 2% of the entire cohort. Noteworthy, safety concerns about starting intravenous thrombolysis outside a monitored area, before blood tests are available, were overcome. In our analysis, in fact, the rate of severe hemorrhagic transformation did not differ according to treatment area. Indeed, earlier treatment was associated with greater neurological improvement and more chances of excellent outcome. Although the construct “time is brain” is well known, to our knowledge, this study is the first highlighting that treatment at CT/MR bed is an independent predictor of excellent outcome in stroke. One of the reason for treatment outside imaging area was care taken by a non-stroke neurologist. This highlights the importance of building a dedicated team as it happens for other neurological diseases (e.g., multiple sclerosis [19]) or other time-dependent conditions (e.g., trauma team [20]). Alternatively, a more intensive education for non-stroke neurologists is necessary to guarantee the highest level of care and give patients the greatest chances of recovery.
In conclusion, immediate thrombolytic treatment at CT/MR bed is an independent predictor of excellent outcome after stroke and should be a standard-of-care for AIS.
References
Emberson J, Lees KR, Lyden P et al (2014) Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384(9958):1929–1935. https://doi.org/10.1016/S0140-6736(14)60584-5
Ahmed N, Wahlgren N, Grond M et al (2010) Implementation and outcome of thrombolysis with alteplase 3-4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 9(9):866–874. https://doi.org/10.1016/S1474-4422(10)70165-4
Lin CB, Peterson ED, Smith EE et al (2012) Emergency medical service hospital prenotification is associated with improved evaluation and treatment of acute ischemic stroke. Circ Cardiovasc Qual Outcomes 5(4):514–522. https://doi.org/10.1161/CIRCOUTCOMES.112.965210
Vidale S, Arnaboldi M, Bezzi G et al (2016) Reducing time delays in the management of ischemic stroke patients in Northern Italy. Int J Cardiol 15(215):431–434. https://doi.org/10.1016/j.ijcard.2016.03.232
Candelaresi P, Lattuada P, Uggetti C et al (2017) A high-urgency stroke code reduces in-hospital delays in acute ischemic stroke: a single-centre experience. Neurol Sci 38(9):1671–1676. https://doi.org/10.1007/s10072-017-3046-y
Rost NS, Masrur S, Pervez MA, Viswanathan A, Schwamm LH (2009) Unsuspected coagulopathy rarely prevents IV thrombolysis in acute ischemic stroke. Neurology 73(23):1957–1962. https://doi.org/10.1212/WNL.0b013e3181c5b46d
Xian Y, Smith EE, Zhao X et al (2014) Strategies used by hospitals to improve speed of tissue-type plasminogen activator treatment in acute ischemic stroke. Stroke 45(5):1387–1395. https://doi.org/10.1161/STROKEAHA.113.003898
Siarkowski M, Lin K, Li SS, Al Sultan A, Ganshorn H, Kamal N, Hill M, Lang E (2020) Meta-analysis of interventions to reduce door to needle times in acute ischaemic stroke patients. BMJ Open Qual 9(3):e000915. https://doi.org/10.1136/bmjoq-2020-000915
Berge E, Whiteley W, Audebert H et al (2021) European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 6(1):I-LXII. https://doi.org/10.1177/2396987321989865
Johns H, Campbell B, Turc G, Churilov L (2023) Power analysis for ordinal analyses of the modified Rankin scale and an online and downloadable tool for practical use. Stroke 54(7):1750–1760. https://doi.org/10.1161/STROKEAHA.122.041260
Wahlgren N, Ahmed N, Dávalos A et al (2007) Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 369(9558):275–282. https://doi.org/10.1016/S0140-6736(07)60149-4
Ringelstein EB, Chamorro A, Kaste M et al (2013) European Stroke Organisation recommendations to establish a stroke unit and stroke center. Stroke 44(3):828–840. https://doi.org/10.1161/STROKEAHA.112.670430
Powers WJ, Rabinstein AA, Ackerson T et al (2019) Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 50(12):e344–e418. https://doi.org/10.1161/STR.0000000000000211
Fonarow GC, Smith EE, Saver JL et al (2011) Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation 123(7):750–758. https://doi.org/10.1161/CIRCULATIONAHA.110.974675
Tong X, Wiltz JL, George MG et al (2018) A decade of improvement in door-to-needle time among acute ischemic stroke patients, 2008 to 2017. Circ Cardiovasc Qual Outcomes 11(12):e004981. https://doi.org/10.1161/CIRCOUTCOMES.118.004981
Candelaresi P, Manzo V, Servillo G et al (2021) The impact of Covid-19 lockdown on stroke admissions and treatments in Campania. J Stroke Cerebrovasc Dis 30(1):105448. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105448
Thortveit ET, Bøe MG, Ljøstad U, Mygland A, Tveiten A (2014) Organizational changes aiming to reduce iv tPA door-to-needle time. Acta Neurol Scand 130(4):248–252. https://doi.org/10.1111/ane.12204
Galecio-Castillo M, Vivanco-Suarez J, Zevallos CB et al (2022) Direct to angiosuite strategy versus standard workflow triage for endovascular therapy: systematic review and meta-analysis. J Neurointerv Surg 16:neurintsurg-2022-018895. https://doi.org/10.1136/neurintsurg-2022-018895
Gallien P, Gich J, Sánchez-Dalmau BF, Feneberg W (2014) Multidisciplinary management of multiple sclerosis symptoms. Eur Neurol 72(Suppl 1):20–25. https://doi.org/10.1159/000367620
Dehli T, Uleberg O, Wisborg T (2018) Trauma team activation - common rules, common gain. Acta Anaesthesiol Scand 62(2):144–146. https://doi.org/10.1111/aas.13013
Acknowledgements
The authors would like to acknowledge all the professionals involved in stroke care at our hospital, who actively contributed to the improvement of the stroke chain of recovery.
Author information
Authors and Affiliations
Contributions
Drs. De Mase, Spina, and Candelaresi had full access to all data in the study and take responsibility for the integrity of the data.
Concept and design: De Mase, Spina, Candelaresi.
Drafting of the manuscript: De Mase, Spina, Candelaresi. Collection of data: De Mase, Spina, Candelaresi, Servillo, Barbato, Leone, Giordano, Guarnieri, Muto Massimo, Alfieri, Longo, Di Iorio.
Critical revision of the manuscript for important intellectual content: De Mase, Spina, Candelaresi, Servillo, Barbato, Leone, Giordano, Guarnieri, Muto Massimo, Alfieri, Longo, Di Iorio, Muto Mario, Andreone.
Supervision: Candelaresi, Andreone.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was not sought for the present retrospective study because every patient was treated according to the current standard of care. This study was completed in accordance with the Helsinki Declaration as revised in 2013.
Informed consent
All patients or relatives have given written or verbal consent to the clinical procedures and to inclusion in the internal register for subsequent retrospective analysis.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 17 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Mase, ., Spina, E., Servillo, G. et al. Effects of immediate thrombolytic treatment in imaging area on functional outcome in patients with acute ischemic stroke. Neurol Sci 45, 1557–1563 (2024). https://doi.org/10.1007/s10072-023-07166-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07166-0