Abstract
Background
Neurocognition is a very important aspect of a brain tumor patient’s quality of life following radiotherapy. The aim of the present study was to assess neurocognitive functions of patients diagnosed with high-grade gliomas undergoing radiotherapy by using the NeuroCogFx® test and to examine relevant dose/volume parameters as well as patient characteristics potentially influencing the neurological baseline status and subsequent outcome.
Methods
The cohort consisted of 44 astrocytoma World Health Organization grade III/IV patients. The NeuroCogFx® test was carried out on patients during (N = 44) and after (N = 21) irradiation. The test examines verbal/figural/short-term/working memory, psychomotorical speed, selective attention and verbal speed. The results were compared with regular patient and treatment data with an emphasis on the dose applied to the hippocampus.
Results
Overall there were only slight changes in the median test results when comparing the baseline to the follow-up tests. In the ‘verbal memory test’ lower percentile ranks were achieved in left-sided tumors compared to right-sided tumors (p = 0.034). Dexamethasone intake during radiotherapy was significantly correlated with the difference between the two test batteries. Concerning figural memory, a correlation was detected between decreased figural recognition and the radiation dose to the left hippocampus (p = 0.045).
Conclusion
We conclude that tumor infiltration of the hippocampus has an impact on neurocognitive function. However, treatment with radiotherapy seems to have less influence on cognitive outcome than expected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In treatment planning, normal tissue sparing is of crucial importance. Lately, advances in modern radiotherapy techniques have continuously improved the quality of the delivered dose distribution—especially in terms of target volume coverage and dose homogeneity, as well as minimizing the dose to the surrounding organs at risk. The appropriate balance between acceptable gross tumor coverage and normal tissue sparing remains a complex clinical and technical challenge, particularly in the treatment of gliomas. While sparing some vital organs at risk (e.g., brainstem, spinal cord) is considered the gold standard, there is still a wide range of other organs at risk in brain tumors which would potentially benefit from dose sparing. The advent of intensity-modulated radiotherapy (IMRT) increased the degree of freedom in treatment planning and allowed doctors to explore the potential of sparing other normal tissue structures, such as the hippocampus. It was recently demonstrated that there might be a beneficial effect for hippocampus tissue sparing [1, 2]. Further investigations are currently under investigation, focusing on the effects of radiation exposure to the hippocampus on neurocognitive functions [2–5]. Hippocampal sparing could have the potential to significantly reduce neurocognitive impairment, including attention and memory deficits, which are known to influence quality of life [2, 6].
In clinical practice, hippocampal sparing has mostly been studied in whole-brain radiotherapy (WBRT) of brain metastases, resulting in favorable outcomes [7–10]. Furthermore, recent studies have analyzed the feasibility of hippocampal sparing using IMRT in primary brain tumors [11–14]. The aim of the present study was to assess neurocognitive functions of patients diagnosed with high-grade gliomas undergoing radiotherapy by using the NeuroCogFx® test. The test results were compared to dose parameters of the hippocampus, in order to draw conclusions regarding the impact of hippocampal sparing on neurocognitive outcome.
Patients and methods
Patient selection and statistical analysis
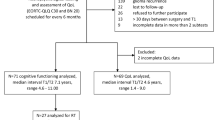
For the present study, 44 eligible patients were identified. All patients were diagnosed with an anaplastic astrocytoma World Health Organization (WHO) grade 3, or a glioblastoma multiforme (GBM, WHO grade 4) and were treated in 2013 or 2014 at the Department of Radiation Oncology, University of Munich, Germany. All patients underwent definitive or postoperative radiotherapy. Patients undergoing radiotherapy for recurrent disease were excluded from the present analysis. To evaluate the radiation dose exposure to the hippocampi, both hippocampi were systematically delineated within all treatment plans. During the 6-week period of radiation treatment, all patients were examined with a baseline NeuroCogFx® test. In 21 patients (48 %) a re-evaluation was performed with repetition of the NeuroCogFx® test on follow-up. Reasons for dropout were poor general health, death or unknown absence to the follow-up examinations. The median interval between the two tests was 155 days (range 81–491 days). Twenty-three patients were lost to follow-up and did not undergo a second testing. The results of the tests were compared to the treatment data and analyzed using IBM SPSS Statistics® Version 22 using non-parametrical tests (Kruskal–Wallis and Mann–Whitney-U test) and the Spearman Rho correlation test. The tests and p values were two-tailed. This prospective study was approved by the ethics committee (votum reference number: 325-11).
Contouring of the hippocampi
The hippocampi were contoured following the contouring guide by Chera et al. [15] who recommended delineation on the T1 sequence of the magnetic resonance imaging (MRI). Contouring should start at the caudal part of the hippocampus, which can be found as a hypointense structure next to the amygdala and close to the temporal horn of the lateral ventricle. However, in our experience, it seems more practical to start contouring at the most cranial part of the hippocampus, at the level of the fornix, as many tumors, or their edemas, consume the caudal part of the hippocampus [16]. From there on, the hippocampus can be easily followed next to the lateral ventricle.
Surgery and radiotherapy
Depending on tumor size, anatomical location and expected functional impairment, patients underwent gross total resection (GTR). Subtotal resections were avoided, as there is no clear benefit for this strategy [17]. In the remaining patients, only a biopsy was performed to histologically confirm the diagnosis of glioma. The bioptic material was additionally examined for mutations with prognostic value, with the methylated O6-methylguanine-DNA methyltransferase (MGMT) promoter being the most important. The MGMT promoter is most frequently evaluated using a pyrosequencing technique from formalin-fixed and paraffin-embedded specimens [18].
Patients were treated using three-dimensional conformal radiotherapy to a total dose of 60.0 Gy, in a conventional schedule with 2.0 Gy per fraction. Patients were treated using megavoltage (MV) equipment with a minimal nominal energy of 6 MV and were immobilized with a thermoplastic mask system to ensure accurate setup reproducibility. For treatment planning, computed tomography (CT) with a slice thickness of 2.5 or 3 mm was performed and co-registered with MR images. The CT was fused with the T1-weighted sequence with gadolinium, and the T2-weighted sequence of the MRI. Gross tumor volume (GTV) was delineated based on the contrast-enhancing lesion. The GTV was expanded for the clinical target volume with a 20-mm margin which included the perifocal edema. For planning target volume a 5-mm margin was added additionally. Treatment planning was performed employing the Oncentra® treatment planning system (OTP MasterPlan®; Nucletron, Solingen Germany). Treatment planning and dose calculations were based on reports 50 and 62 of the International Commission on Radiation Units and Measurements.
Patients with a MGMT promoter received concomitant and adjuvant alkylating chemotherapy with temozolomide [19–23]. In patients lacking MGMT promoter methylation, chemotherapy was administered depending on the patient’s clinical condition; elderly patients in particular do not seem to benefit due to toxicity [24].
Equivalent uniform dose concept
The equivalent uniform dose (EUD) concept assumes that different dose distributions are equivalent, if they cause the same radiobiological effect. Therefore, EUD was used as an evaluation tool for non-homogeneous irradiation compared to an idealized homogeneous dose distribution [25]. Since the present study tried to detect neurocognitive effects on the hippocampi, it seemed obvious to use the EUD concept in addition to the regular data of the dose volume histogram. The generalized EUD model was used as described previously [16, 26, 27].
NeuroCogFx®
The NeuroCogFx® test (version 2008) is ‘a computer-based neuropsychological battery of tests developed to investigate neurological patients for cognitive dysfunction after potentially neurotoxic therapy’ [28]. The test examines verbal memory, figural memory, short-term memory, working memory, psychomotorical speed, selective attention and verbal speed. The six subtests are described in detail in Table 1, which is based on the table shown in the original paper by Fliessbach et al. [28]. It was designed as a short test (duration approximately 25 min) that is easily comprehensible for patients with cognitive dysfunction. It has previously been assessed for practicability, reliability and validity [28]. Furthermore, it was standardized on a control group (N = 242; age range 16–75 years) without any known neurological or psychiatric diseases. The test results are presented with the percentile rank (PR) as compared to the standardized test results from the control group.
Results
Patient characteristics
The cohort consisted of 44 patients (16 female, 36 %; 28 male, 64 %) undergoing radio(chemo)therapy at the Department of Radiation Oncology, University of Munich, Germany. All but one patient were right-handed (98 %). Nine patients had a bilateral tumor (21 %), 19 a left-sided tumor (43 %) and 16 a right-sided tumor (36 %). 18 patients underwent a gross total resection prior to radiotherapy (41 %), compared to 26 patients who received a biopsy alone (59 %). Histological analysis revealed an astrocytoma WHO grade III in seven pathologic specimens (16 %) and a GBM in 37 cases (84 %). Of these, 24 patients had a methylated MGMT promotor (55 %), 5 a partial methylation (11 %) and 12 patients had a non-methylated MGMT promoter (27 %). In 3 patients the MGMT status was not examined (7 %). Concomitant alkylating chemotherapy with temozolomide was administered in 34 patients (77 %). Concerning hippocampus affection, the hippocampi were affected bilaterally by the tumor in only one case (2 %). In contrast, 20 patients had a unilateral hippocampus affection (46 %), and 23 patients did not have any tumor involvement of the hippocampi (52 %). In this study, hippocampus affection means a radiological infiltration of the hippocampus by tumor volume as seen on MRI. Patient characteristics are summarized in Table 2. The median overall survival was 767 days (standard deviation 132.4 days).
Treatment parameters
Overall, the median brain volume was 1,344.3 ml (range 1,140.7–1,713.4 ml). The median brain mean dose was 34.0 Gy (range 18.2–42.2 Gy), and the median brain EUD was 47.5 Gy (range 33.7–51.4 Gy). Concerning the volume of the hippocampi, the median bilateral hippocampus volume was 3.4 ml (range 1.2–9.1 ml). The median bilateral hippocampus mean dose was 37.57 Gy (range 4.0–56.0 Gy), and the bilateral median hippocampus EUD was 50.8 Gy (range 11.3–58.02 Gy). An overview of the treatment parameters and the exact dose distributions can be found in Table 3.
NeuroCogFx® test results
The results of the NeuroCogFx® test are summarized in Table 4. As expected, a clear performance deficit for the present cohort was found compared to the standardized test results of the standardized control group. To further evaluate these cognitive dysfunctions, the results of the tests were correlated to patient- and therapy-related parameters.
In the ‘verbal memory test’, lower percentile ranks were achieved in left-sided tumors compared to right-sided tumors (median PR: left-sided 3; right-sided 30; bilateral 10)—a correlation which was significant on statistical analysis (p = 0.034, Kruskal–Wallis test). Similarly, the ‘word fluency test’ showed a significant correlation to the affected cerebral hemisphere (median PR: left 1; right 70; both sides 10) (p = 0.006, Kruskal–Wallis test). Moreover, the test results of the ‘verbal memory test’ were significantly influenced by the patient’s educational background (highest degree reached) in the Kruskal–Wallis test (p = 0.007).
Analysis of the ‘figural memory test’ results revealed better test scores if the hippocampus was directly affected by the tumor, with a median percentile rank of 55 versus a median PR of 20 for non-involved hippocampi. This connection was significant on Mann–Whitney U testing (p = 0.011).
A concordance of the NeuroCogFx® test results and gender was detected on the ‘simple reaction time test’ which showed a trend for longer reaction times in women (median PR: 20) compared to men (median PR: 40) (p = 0.011, Mann–Whitney U test).
The operation method (biopsy only or GTR) had a significant influence on the ‘digit span test’ of the baseline tests in nonparametric testing (Mann–Whitney U). In the ‘correct reproduction’ category, the median PR was 50 with GTR and 30 with biopsy only (p = 0.09), and in the ‘maximum digits’ category, the medial PR was 50 with GTR and 40 with biopsy only (p = 0.04). The other subtests did not show any significant difference depending on operation.
Finally, we evaluated the correlation between test results and steroid intake (in mg/d). In the ‘digit span test’ the number of correct reproductions and the maximum length of the sequences correlated significantly with dexamethasone intake (Spearman’s rho: −0.4, p = 0.009; −0.4, p = 0.008, respectively). Patients with a higher intake had worse results. Accordingly, comparable results were obtained in the ‘choice reaction test’ (Spearman’s rho: −0.3, p = 0.046), the ‘verbal memory test’ (Spearman’s rho: −0.3, p = 0.029) and the ‘word fluency test’ (Spearman’s rho: −0.360, p = 0.014).
Differences between baseline and follow-up NeuroCogFx® tests
Overall, there were only slight changes in the median test result when comparing the baseline to the follow-up tests (see Table 5). As mentioned above, in this setting, the dexamethasone intake during radiotherapy was significantly correlated with the difference between the two test batteries. A higher intake negatively influenced the ‘2-back test’ (Spearman’s rho: −0.5, p = 0.042), the ‘choice reaction test’ (Spearman’s rho: −0.5, p = 0.043) and the ‘figural memory test’ (Spearman’s rho: −0.6, p = 0.005). Interestingly, there were significant correlations between the difference for ‘simple reaction tests’ and the right hippocampal mean dose (Spearman’s rho: 0.5, p = 0.016), maximum dose (Spearman’s rho: 0.5, p = 0.033) and EUD (Spearman’s rho: 0.5, p = 0.024). Additionally, the difference for the ‘figural memory test’ was significantly correlated with the maximum dose to the left hippocampus (Spearman’s rho: −0.5, p = 0.045). Operation method also had a significant influence on the ‘figural memory test’ results in non-parametrical testing (Mann–Whitney U). Patients with GTR improved by a median PR of 20, whereas patients with biopsy only had a median decrease of 20 (p = 0.037). Furthermore, age was significantly correlated with the difference for ‘verbal memory tests’ (Spearman’s rho: 0.6, p = 0.008) and ‘word fluency’ (Spearman’s rho: 0.6, p = 0.008). Chemotherapy with temozolomide did not have any significant influence on the test results.
Discussion
It is well known that there are functional differences between the hemispheres of the brain, e.g., the Broca and the Wernike area, which are responsible for language, are located only on the dominant side (for right-handed people usually the left side). The possibility of a functional difference between the sides of the hippocampi has often been discussed but not absolutely verified. It is assumed that the left side is more strongly associated with verbal memory and the right side more strongly associated with spatial memory. The hypothesis could be confirmed for the left side; however, this was not the case for the right side [29, 30]. Overall, the left side might have a slightly more important role than the right side [30]; our data support this as verbal and numeral function is also more strongly affected if the tumor is located on the left side. The test results, however, also show external influences such as gender, education and age. Additionally, the amount of dexamethasone intake during radiotherapy had a negative influence on different subtests. Clearly, there might be a potential for a selection bias, since patients presenting with larger tumors and more perifocal edema usually receive higher doses of dexamethasone. A further limitation of the present study is the small sample size.
The impact of radiotherapy on neurocognitive outcome was analyzed by conducting the NeuroCogFx® test on follow-up and by comparing it to the baseline test. The differences between the two test batteries were less pronounced than expected. The reaction time in the ‘simple reaction’ test was significantly shorter in the follow-up test if the right hippocampus was more strongly exposed. This result could be explained by the fact that, if the right hippocampus was more strongly exposed in these cases, the left hippocampus was less exposed. Concerning figural memory, a correlation was detected between decreased figural recognition and the radiation dose to the left hippocampus (p = 0.045). Overall, tumor localization had a greater impact on neurocognitive function than the impact of radiation exposure on the hippocampi. A tumor reduction by GTR even improved some subtest results, supporting the assumption that the tumor volume has a strong influence on neurocognition. However, it cannot be ruled out that a low-dose exposition of the hippocampus could result in neurocognitive changes on long-term follow-up. In a phase II hippocampal avoidance study for WBRT conducted by Gondi et al. [31], neurocognitive improvement became apparent at only 6 months following radiotherapy. As a result, the timing of the follow-up test in the present study might have been conducted too early or too late to detect a significant impact. For instance it might be possible that the neurocognitive decree is strongest after 3 month but regenerates after 6 months, or that it first becomes apparent much later. Obviously, further studies with larger sample sizes and longer follow-up, including additional NeuroCogFx® tests, are needed to answer these questions.
The connection between neurocognition and the hippocampus dates back to a 1957 case report by the American neurosurgeon William Beecher Scoville which describes an anterograde amnesia affecting patients with hippocampal lesions after bilateral temporal lobe dissection [32]. Deficits in cognitive function (mostly verbal) could also be connected with hippocampal exposure to radiation [33–35]. The deficits seem to be caused by inhibition of neural proliferation in the subgranular zone. Monje et al. [36] suggest that this could be due to inflammation, as neurogenesis seems to be protected by immunosuppressive medication such as indomethacin. A 2015 study showed that even systemic inflammation due to intestinal bowel disease has an impact on hippocampal neurogenesis, which might cause the loss of cognitive function described for patients with intestinal infections [37]. Thus, hippocampal radiation toxicity seems to be caused by inhibiting neural proliferation either through radiation directly or indirectly by causing inflammation.
To date, hippocampal sparing has mostly been explored in patients diagnosed with brain metastases and treated with WBRT. Obviously, a better functional outcome with decreased neurocognitive impairment was observed in cases of hippocampal avoidance [31]. The risk of an increased occurrence of metastases within the hippocampi has been proven to be negligible [9]. Overall, sparing of the hippocampi seems to be beneficial for patients undergoing WBRT. In Germany a prospective randomized multicenter phase II study is being launched (the HIPPORAD study) to investigate the neurocognitive outcome of patients with metastases receiving WBRT with hippocampal sparing and dose escalation [38]. In patients diagnosed with high-grade gliomas of the central nervous system, sparing of the hippocampus is still under debate. While the technical preservability of the hippocampus in different treatment techniques could already be proved [11, 14], it still remains unclear if hippocampus sparing in high-grade gliomas has an impact on neurocognitive function. Furthermore, the hippocampus contains stem cell niches in the subgranular zone, which could be a reason for the recurrence of gliomas [39–42]. As a consequence, some authors suggest including the ipsilateral hippocampus in the target volume, and to preserve the contralateral hippocampus [2, 12]. Our 2014 study showed that this suggestion is also feasible diametrically [16]. Nevertheless, a real benefit has yet to be revealed in the future.
Conclusion
We conclude that tumor infiltration of the hippocampus has an impact on neurocognitive function. However, treatment with radiotherapy seems to have less influence on cognitive outcome than expected.
The Helsinki declaration of 1975 has been obeyed in all points. The patients provided informed consent. The ethics committee approved this study (votum reference number: 325-11).
References
Marsh JC, Godbole R, Diaz AZ et al (2011) Sparing of the hippocampus, limbic circuit and neural stem cell compartment during partial brain radiotherapy for glioma: a dosimetric feasibility study. J Med Imag Radiat Oncol 55(4):442–449. doi:10.1111/j.1754-9485.2011.02282.x
Kazda T, Jancalek R, Pospisil P et al (2014) Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol (London, England) 9:139. doi:10.1186/1748-717x-9-139
Gondi V, Tome WA, Mehta MP et al (2010) Why avoid the hippocampus? A comprehensive review. Radiother Oncol J Eur Soc Ther Radiol Oncol 97(3):370–376. doi:10.1016/j.radonc.2010.09.013
Armstrong GT, Jain N, Liu W et al (2010) Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro-Oncology 12(11):1173–1186. doi:10.1093/neuonc/noq104
Welzel G, Fleckenstein K, Mai SK et al (2008) Acute neurocognitive impairment during cranial radiation therapy in patients with intracranial tumors. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 184(12):647–654. doi:10.1007/s00066-008-1830-6
Giovagnoli AR, Meneses RF, Silvani A et al (2014) Quality of life and brain tumors: what beyond the clinical burden? J Neurol. doi:10.1007/s00415-014-7273-3
Awad R, Fogarty G, Hong A et al (2013) Hippocampal avoidance with volumetric modulated arc therapy in melanoma brain metastases—the first Australian experience. Radiat Oncol 8:62. doi:10.1186/1748-717x-8-62
Rong Y, Evans J, Xu-Welliver M et al (2015) Dosimetric evaluation of intensity-modulated radiotherapy, volumetric modulated arc therapy, and helical tomotherapy for hippocampal-avoidance whole brain radiotherapy. PLoS One 10(4):e0126222. doi:10.1371/journal.pone.0126222
Oehlke O, Wucherpfennig D, Fels F et al (2015) Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: Local tumour control and survival. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. doi:10.1007/s00066-014-0808-9
Oskan F, Ganswindt U, Schwarz SB et al (2014) Hippocampus sparing in whole-brain radiotherapy: A review. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. doi:10.1007/s00066-013-0518-8
Canyilmaz E, Uslu GD, Colak F et al (2015) Comparison of dose distributions hippocampus in high grade gliomas irradiation with linac-based imrt and volumetric arc therapy: a dosimetric study. SpringerPlus 4:114. doi:10.1186/s40064-015-0894-x
Pinkham MB, Bertrand KC, Olson S et al (2014) Hippocampal-sparing radiotherapy: the new standard of care for World Health Organization grade II and III gliomas? J Clin Neurosci Off J Neurosurg Soc Australas 21(1):86–90. doi:10.1016/j.jocn.2013.04.005
Marsh JC, Ziel GE, Diaz AZ et al (2013) Integral dose delivered to normal brain with conventional intensity-modulated radiotherapy (IMRT) and helical tomotherapy IMRT during partial brain radiotherapy for high-grade gliomas with and without selective sparing of the hippocampus, limbic circuit and neural stem cell compartment. J Med Imag Radiat Oncol 57(3):378–383. doi:10.1111/1754-9485.12048
Oehler J, Brachwitz T, Wendt TG et al (2013) Neural stem cell sparing by linac based intensity modulated stereotactic radiotherapy in intracranial tumors. Radiat Oncol (London, England) 8:187. doi:10.1186/1748-717x-8-187
Chera BS, Amdur RJ, Patel P et al (2009) A radiation oncologist’s guide to contouring the hippocampus. Am J Clin Oncol 32(1):20–22. doi:10.1097/COC.0b013e318178e4e8
Bodensohn R, Sohn M, Ganswindt U et al (2014) Hippocampal EUD in primarily irradiated glioblastoma patients. Radiat Oncol (London, England) 9:276. doi:10.1186/s13014-014-0276-5
Kreth FW, Thon N, Simon M et al (2013) Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol Off J Eur Soc Med Oncol/ESMO 24(12):3117–3123. doi:10.1093/annonc/mdt388
Bienkowski M, Berghoff AS, Marosi C et al (2015) Clinical Neuropathology practice guide 5-2015: MGMT methylation pyrosequencing in glioblastoma: unresolved issues and open questions. Clin Neuropathol 34(5):250–257
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. doi:10.1016/S1470-2045(09)70025-7
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med 352(10):987–996. doi:10.1056/NEJMoa043330
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl J Med 352(10):997–1003. doi:10.1056/NEJMoa043331
Balducci M, Fiorentino A, De Bonis P et al (2013) Concurrent and adjuvant temozolomide-based chemoradiotherapy schedules for glioblastoma. Hypotheses based on two prospective phase II trials. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 189(11):926–931. doi:10.1007/s00066-013-0410-6
Gerstein J, Franz K, Steinbach JP et al (2011) Radiochemotherapy with temozolomide for patients with glioblastoma. Prognostic factors and long-term outcome of unselected patients from a single institution. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 187(11):722–728. doi:10.1007/s00066-011-2230-x
Niyazi M, Schwarz SB et al (2012) Radiotherapy with and without temozolomide in elderly patients with glioblastoma. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 188(2):154–159. doi:10.1007/s00066-011-0026-7
Niemierko A (1997) Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys 24(1):103–110
Niyazi M, Sohn M, Schwarz SB et al (2012) Radiation treatment parameters for re-irradiation of malignant glioma. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 188(4):328–333. doi:10.1007/s00066-011-0055-2
Niyazi M, Karin I, Sohn M et al (2013) Analysis of equivalent uniform dose (EUD) and conventional radiation treatment parameters after primary and re-irradiation of malignant glioma. Radiat Oncol (London, England) 8:287. doi:10.1186/1748-717x-8-287
Fliessbach K, Hoppe C, Schlegel U et al (2006) NeuroCogFX–a computer-based neuropsychological assessment battery for the follow-up examination of neurological patients. Fortschr Neurol Psychiatr 74(11):643–650. doi:10.1055/s-2006-932162
Braun M, Weinrich C, Finke C et al (2011) Lesions affecting the right hippocampal formation differentially impair short-term memory of spatial and nonspatial associations. Hippocampus 21(3):309–318. doi:10.1002/hipo.20752
Witt JA, Coras R, Schramm J et al (2014) The overall pathological status of the left hippocampus determines preoperative verbal memory performance in left mesial temporal lobe epilepsy. Hippocampus 24(4):446–454. doi:10.1002/hipo.22238
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP (2014) Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol Off J Am Soc Clin Oncol 32(34):3810–3816. doi:10.1200/jco.2014.57.2909
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20(1):11–21
Raber J, Rola R, LeFevour A et al (2004) Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 162(1):39–47
Pereira Dias G, Hollywood R, Bevilaqua MC et al (2014) Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms. Neuro-oncology. doi:10.1093/neuonc/not321
Tallet AV, Azria D, Barlesi F et al (2012) Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiat Oncol (London, England) 7:77. doi:10.1186/1748-717x-7-77
Monje ML, Toda H, Palmer TD et al (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science (New York, NY) 302(5651):1760–1765. doi:10.1126/science.1088417
Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS, Chesnokova V (2015) Chronic intestinal inflammation alters hippocampal neurogenesis. J Neuroinflammation 12(1):65. doi:10.1186/s12974-015-0281-0
http://www.neuroonkologie.de/studien. Accessed on 26.07.2015
Evers P, Lee PP, DeMarco J et al (2010) Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer 10:384. doi:10.1186/1471-2407-10-384
Chen L, Guerrero-Cazares H, Ye X et al (2013) Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys 86(4):616–622. doi:10.1016/j.ijrobp.2013.02.014
Gibbs IC, Haas-Kogan D, Terezakis S et al (2013) The subventricular zone neural progenitor cell hypothesis in glioblastoma: epiphany, Trojan Horse, or Cheshire fact? Int J Radiat Oncol Biol Phys 86(4):606–608. doi:10.1016/j.ijrobp.2013.03.002
Lee P, Eppinga W, Lagerwaard F et al (2013) Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys 86(4):609–615. doi:10.1016/j.ijrobp.2013.01.009
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
O. Schnell is an advisor for Novocure, Roche; C. Belka receives research funding from the Merck-group Darmstadt, Elekta; R. Bodensohn, S. Corradini, U. Ganswindt, J. Hofmaier and M. Niyazi declare that they have no conflict of interest.
About this article
Cite this article
Bodensohn, R., Corradini, S., Ganswindt, U. et al. A prospective study on neurocognitive effects after primary radiotherapy in high-grade glioma patients . Int J Clin Oncol 21, 642–650 (2016). https://doi.org/10.1007/s10147-015-0941-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0941-1