Abstract
Background and purpose
Alice in Wonderland syndrome (AIWS) is a neurological disorder characterized by erroneous perception of the body schema or surrounding space. Migraine is the primary cause of AIWS in adults. The pathophysiology of AIWS is largely unknown, especially regarding functional abnormalities. In this study, we compared resting-state functional connectivity (FC) of migraine patients experiencing AIWS, migraine patients with typical aura (MA) and healthy controls (HCs).
Methods
Twelve AIWS, 12 MA, and 24 HCs were enrolled and underwent 3 T MRI scanning. Independent component analysis was used to identify RSNs thought to be relevant for AIWS: visual, salience, basal ganglia, default mode, and executive control networks. Dual regression technique was used to detect between-group differences in RSNs. Finally, AIWS-specific FC alterations were correlated with clinical measures.
Results
With respect to HCs, AIWS and MA patients both showed significantly lower (p < 0.05, FDR corrected) FC in lateral and medial visual networks and higher FC in salience and default mode networks. AIWS patients alone showed higher FC in basal ganglia and executive control networks than HCs. When directly compared, AIWS patients showed lower FC in visual networks and higher FC in all other investigated RSNs than MA patients. Lastly, AIWS-specific FC alterations in the executive control network positively correlated with migraine frequency.
Conclusions
AIWS and MA patients showed similar FC alterations in several RSNs, although to a different extent, suggesting common pathophysiological underpinnings. However, AIWS patients showed additional FC alterations, likely due to the complexity of AIWS symptoms involving high-order associative cortical areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alice in Wonderland syndrome (AIWS) is a neurological disorder characterized by erroneous perception of the body schema or surrounding space. Described for the first time by the psychiatrist John Todd in 1955 [1], the syndrome takes its name from Lewis Carroll’s book Alice’s Adventures in Wonderland, in which the protagonist experiences several perception disorders [2].
Core symptoms of AIWS include somesthetic (i.e., macro/microsomatognosia) and visual distortions (such as macro/micropsia or pelopsia/telopsia) [1, 3,4,5]. Other facultative symptoms include derealization, depersonalization, and perception of time slowing [5,6,7]. Patients are generally classified according to their symptoms as type A (somesthetic), type B (visual), or type C (somesthetic and visual) [7,8,9].
In children, AIWS is most frequently due to Epstein-Barr virus infections, while in adults the most common cause is migraine [3, 4, 9]. A recent prospective study showed that the prevalence of AIWS in adult migraineurs referred to a tertiary headache center may be as high as 19%, and that the vast majority of patients with AIWS (95%) received a concomitant diagnosis of migraine with aura (MA), with AIWS symptoms tending to occur within 1 of migraine onset [10]. It is still unclear why some migraineurs experience AIWS, though a common pathophysiological mechanism possibly related to aura has been hypothesized since AIWS symptoms tend to occur close to migraine onset [10]. Indeed, although visual and somatosensory disturbances are the most frequent MA symptoms, MA can also include other symptoms characterized by dysfunctions of associative cortical areas that integrate polymodal sensory information [11].
There is no current agreement on the neural correlates of AIWS. In our previous study, we searched medical literature for AIWS cases following cerebral damage (a less common AIWS etiology). In order to find the anatomical substrate of AIWS, we applied a lesion-mapping approach and found that type B patients showed brain lesions located preferentially, but not exclusively, in the right extrastriate visual cortex and surrounding white matter tracts, while type A and C patients showed lesions located in different areas of the right hemisphere, such as the insula, thalamus, and frontal lobe, possibly suggesting underlying structural/functional disconnections [12]. Single-case functional imaging studies that tried to identify functional abnormalities in AIWS patients showed heterogeneous results [13,14,15].
Resting-state functional magnetic resonance imaging (rs-fMRI), which examines the pattern of synchronous spontaneous fluctuations of the blood oxygen level-dependent (BOLD) signal [16], provides an indirect measurement of brain connectivity. Application of this non-invasive technique has allowed the identification of various resting-state networks (RSNs), or spatially distinct areas of the brain that demonstrate synchronous BOLD fluctuations at rest. Several RSNs showed atypical connectivity in MA patients (mainly visual) [17], some of which involved regions that were frequently associated with AIWS in individual brain-damaged patients and in task-evoked functional MRI (fMRI) and single-photon emission computerized tomography (SPECT) studies, including visual [9, 12, 15, 18], salience and basal ganglia [19,20,21], and default and executive control networks [22, 23].
Studying the neural correlates of the wide array of AIWS symptoms associated with migraine in comparison to more common forms of MA may contribute to a better understanding of whether AIWS phenomena and more common forms of auras share common pathophysiological mechanisms.
The current study aimed to investigate resting-state functional connectivity (FC) in migraine patients with AIWS, in migraine patients with typical visual and somatosensory aura (MA), and in age- and sex-matched healthy controls (HCs) using an automated hypothesis-free approach, i.e., independent component analysis (ICA). This approach has the potential to provide new insights into the pathophysiology of AIWS by clarifying (i) whether AIWS and MA patients are characterized by overlapping or independent FC changes when compared to HCs; (ii) whether AIWS and MA patients differ in terms of FC in specific RSNs, and (iii) whether AIWS-specific FC changes correlate with clinical measures.
Our approach might provide clinicians and researchers with relevant information. Defining the precise relationship between AIWS and migraine might inform an appropriate therapeutic approach, especially in the presence of frequent or particularly distressing AIWS misperceptions. If AIWS and migraine attacks share, at least in part, some pathophysiological mechanisms, an appropriate migraine preventive therapy might have an effect on AIWS symptoms, as previously suggested in patients with AIWS and vestibular and non-vestibular migraine [10, 24].
Materials and methods
Participants
A consecutive series of 12 right-handed patients with migraine with aura experiencing AIWS (AIWS), 12 patients with migraine with typical visual and somatosensory aura (MA), and 24 age- and sex-matched HCs were included in this study. All subjects were recruited and underwent an MRI scan and clinical testing at Policlinico Umberto I, Sapienza University of Rome, Italy. Patients had to meet the following inclusion criteria to be enrolled in the study: 18–65 years of age with a diagnosis of migraine according to the International Classification of Headache Disorders, 3rd edition (ICHD-3). Patients were screened for AIWS symptoms via an ad hoc questionnaire [25]. After the visit, questionnaire responses were double-checked by two trained physicians (VM, GM) who directly interviewed participants and collected information about AIWS and migraine characteristics (for clinical evaluation details, see [25]). MA patients had an aura characterized by visual symptoms (including phosphenes, photopsia, and visual blurring) or visual and somatosensory symptoms (paresthesia). No patient received a diagnosis of familial or sporadic hemiplegic migraine or vestibular migraine. Due to the high within-subject aura variability [26], we included patients without a preferred side of aura symptoms. All enrolled patients experienced both migraine attacks with and without aura, and none of them took preventive medications. All patients were in the interictal state; they were migraine-free at least 48 h before and after the MRI scan.
The following exclusion criteria were applied to all subjects: medically unstable or with hematological, renal, or hepatic dysfunction; current or past Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnosis of major depression, bipolar disorder, or psychotic disorders; history of moderate-to-severe head injury, stroke, or seizures; alcoholism or drug dependency; for healthy controls specifically, no history of migraine or other types of primary headaches.
Ethics statement
This study was performed in accordance with the ethical code of the ethics committee of Azienda Policlinico Umberto I, Sapienza University of Rome and the Declaration of Helsinki. After approval from the ethics committee, written informed consent was obtained from all subjects.
MRI acquisition
Images were acquired with a 3 Tesla (3 T) scanner (Siemens Magnetom Verio) and a 12-channel head coil designed for parallel imaging (GRAPPA). Participants were advised to avoid consuming psychoactive substances, such as tea or coffee, within 2 h prior to MRI scans.
The following sequences were acquired:
-
BOLD single-shot echo-planar imaging (repetition time (TR) = 3000 ms, echo time (TE) = 30 ms, flip angle = 89°, field of view (FOV) = 192 mm, 64 × 64 matrix, 50 contiguous axial slices 3-mm thick, 140 volumes, voxel size = 3 mm3, acquisition time = 7 min 11 s), with all patients instructed to close their eyes and stay awake during resting-state fMRI acquisitions;
-
High-resolution three-dimensional T1-weighted (T1-3D) magnetization-prepared rapid gradient echo (MP-RAGE) sequence (TR = 1900 ms, TE = 2.93 ms, inversion time (TI) = 900 ms, flip angle = 9°, FOV = 260 mm, matrix = 256 × 256, 176 sagittal slices 1-mm thick, no gap);
-
Dual turbo spin-echo, proton density (PD) and T2-weighted images (TR = 3320 ms, TE1 = 10 ms, TE2 = 103 ms, FOV = 220 mm, matrix = 384 × 384, 25 axial slices 4-mm thick, 30% gap);
-
High-resolution 3D fluid-attenuated inversion recovery (FLAIR) sequence (TR = 6000 ms, TE = 395 ms, TI = 2100 ms, FOV = 256 mm, matrix = 256 × 256, 176 sagittal slices 1-mm thick, no gap).
MRI analysis
Anatomical and functional preprocessing was performed using fMRIPrep 20.2.3 [27, 28], RRID:SCR_016216, which is based on Nipype 1.5.0 [29, 30], RRID:SCR_002502. For a description of anatomical and functional preprocessing, please see the Supplementary information.
Independent component analysis (ICA) of preprocessed functional data was performed using the MELODIC tool (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) [31]. For group-wise ICA, a single four-dimensional (4D) dataset was created by temporally concatenating preprocessed functional data containing 140 timepoints for each subject. The dimensionality of group ICA was performed using different numbers of components (i.e., 20, 25, 30, 35, 40) [32,33,34]. Finally, a dimensionality of 30 was chosen since the explained data variance was sufficient to obtain good estimates of the signals and well-known RSNs were identified [32]. Several RSNs showed atypical connectivity in patients with migraine with aura [17]; of these, we selected those whose regions showed structural (single-case lesion studies) or functional (i.e., task fMRI, SPECT) alterations in patients with AIWS: visual (lateral and medial) [9, 12, 15, 18], salience and basal ganglia [19,20,21], and default and executive control networks [22, 23]. RSNs of interest were identified via spatial correlation coefficients (fslcc tool) using RSNs generated by Smith et al. [32] and Yeo et al. [35] as templates, and then verified by expert visual inspection (CP, NP, PP).
The set of spatial maps from the group average analysis was used to generate subject-specific versions of the spatial maps and associated time series using a dual regression technique [36, 37]. For each subject, the group average set of spatial maps was first regressed (as spatial regressors in a multiple regression) into the subject’s 4D space–time dataset, resulting in a set of subject-specific time series, one per group-level spatial map. These time series were then regressed (as temporal regressors in a multiple regression) into the same 4D dataset, resulting in a set of subject-specific spatial maps, one per group-level spatial map.
Statistical analyses
Statistical analyses were performed using SPSS statistics software (version 22.0). Between-group differences in demographic, clinical, and radiological parameters were tested using Mann–Whitney U test and Chi-square test for continuous and dichotomous variables, respectively (p < 0.05 for null hypothesis rejection).
Resting-state functional connectivity
Subject-specific spatial maps obtained from dual regression analysis were entered into group-level voxel-wise analyses. To investigate FC differences between groups, we compared each patient group with the control group and the two patient groups to each other by applying unpaired t-test. Age and sex were entered as nuisance variables in all analyses. Voxel-wise statistical analyses were performed with permutation-based non-parametric statistics using FSL Randomise permutation-based program with 5000 permutations [38]. Results were corrected using false discovery rate (FDR) correction [39] for multiple comparisons (p < 0.05). The Randomise tool (5000 permutations) was also used to examine possible correlations between FC and clinical features, i.e., disease duration and migraine frequency. Correlation analyses were performed inside the masks of significant FC differences between AIWS patients and HCs and resulting statistical maps were thresholded at p < 0.05, FDR corrected. Anatomical localization of significant clusters was established according to the Harvard–Oxford cortical and subcortical structural atlases included in FMRIB’s Software Library (http://www.fmrib.ox.ac.uk/fsl/data/atlas descriptions.html).
Results
Clinical and conventional MRI findings
Descriptive statistics for demographic and clinical parameters in the two patient groups and in HCs are reported in Table 1. Symptoms characterizing AIWS patients included in the present study are reported in the Supplementary Table 1. AIWS, MA, and HC participants did not statistically differ in terms of age or gender distribution. There were no significant differences in disease duration or migraine frequency between AIWS and MA patients. Patients were also comparable according to clinical manifestation: in the AIWS group, 7 patients were classified as type B AIWS (only visual distortions) and 5 as type C (somesthetic and visual distortions), while in the MA group, 9 patients presented with pure visual aura and 3 with visual and somatosensory aura.
Fifteen out of 24 migraine patients (63%) showed at least one millimetric aspecific area of hyperintense T2 and T2 FLAIR signal, and only two patients (1 AIWS and 1 MA) showed more than 10 areas. No difference in the number of hyperintense white matter lesions was detected between MA and AIWS patients (p < 0.05). These findings are consistent with white matter lesions reported in patients with migraine with aura [40, 41], where they did not seem to impact FC [42, 43].
Resting-state functional connectivity
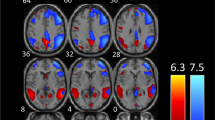
The six selected RSNs of interest were identified as those showing the highest spatial correlation coefficients with RSN templates: the default mode (r = 0.58), executive control (r = 0.63), basal ganglia (r = 0.46), lateral visual (r = 0.49), and medial visual (r = 0.66) networks (Fig. 1). The salience network was instead identified according to previous literature [44, 45].
Resting-state networks (RSNs) identified and used for dual regression analysis. This figure shows sagittal, coronal, and axial slices for the RSNs detected, overlaid onto the MNI152 standard brain. RSNs are shown in FMRIB’s Software Library’s red-yellow color encoding using a 3 < z-score < 10 threshold window
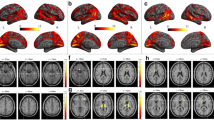
With respect to HCs, AIWS and MA patients showed significantly lower (p < 0.05 FDR corrected) FC in the lateral and medial visual networks and significantly higher FC in the salience and default mode networks (Fig. 2, Tables 2 and 3). Only AIWS patients showed higher FC than HCs in the basal ganglia and executive control networks. When directly compared, AIWS patients showed lower FC in visual networks and higher FC in all other investigated RSNs than MA patients (Fig. 2, Table 4).
RSNs showing significant functional connectivity differences between healthy controls (HCs) and Alice in Wonderland syndrome (AIWS) and migraine with aura (MA) patients (p < 0.05, false discovery rate corrected). Results for each RSN are overlaid onto the corresponding network (green) in the MNI152 standard brain. Red-yellow and blue-light blue color bars represent level of significance
Correlation analysis showed a significant positive correlation (p < 0.05, FDR corrected) between AIWS-specific FC alterations in the executive control network and migraine frequency (Fig. 3, Table 5): the higher the FC, the higher the number of migraine days per month.
Voxel-wise positive correlation in AIWS patients between executive control network functional connectivity (z score at cluster local maxima x = 14 y = 60 z = 26) and migraine frequency (days of migraine/month) (p < 0.05, false discovery rate corrected). Results are overlaid onto the executive control network (green) in the MNI152 standard brain. The red-yellow color bar represents level of significance
Discussion
In the present study, we compared patterns of FC in selected RSNs of interest in migraine patients with AIWS, migraine patients with typical aura (MA), and age- and sex-matched HCs. With respect to HCs, AIWS and MA patients both showed FC alterations in lateral and medial visual, salience, and default mode networks, suggesting a similar rearrangement of functional brain connections. However, AIWS patients showed greater and more widespread FC alterations in those RSNs than MA patients, and additional FC alterations in basal ganglia and executive control networks. These findings may reflect the involvement of high-order associative cortical areas related to the complexity of AIWS symptoms [9].
AIWS and MA patients showed common FC abnormalities
In the present study, we demonstrated that AIWS and MA patients were characterized by similar FC alterations in four RSNs when compared to HCs, suggesting common pathophysiological underpinnings. Although this is the first study that investigated FC alterations in migraine patients with AIWS, several studies have looked at FC abnormalities in the interictal phase of migraine patients with typical aura, suggesting the existence of FC alterations in MA patients affecting the visual cortex [43, 46, 47], regions involved in visual processing (including middle frontal regions and the anterior cingulate) [42, 47], and default mode [46, 48] and salience networks [47, 49].
In the present study, both AIWS and MA patients showed lower FC in lateral and medial visual network regions, such as the superior lateral occipital cortices and the lingual gyrus, widely considered the cortical “aura generator” [50]. In patients with MA, Tedeschi and colleagues found higher FC in the right lingual gyrus during the interictal phase [43]; this result was confirmed by the same research group, who also demonstrated that MA patients with complex auras had higher FC of the left lingual gyrus compared to both simple visual MA patients and migraine without aura patients [50]. Increased FC in the visual network was interpreted as due to “a brain lack of habituation or to a hyper-responsiveness”[43]. However, both hyper- and hypo-connectivity can be detected in the same neurological disease, e.g., multiple sclerosis, epilepsy and mild cognitive impairment [51,52,53], likely reflecting different pathophysiological mechanisms related to neural resources [54]. In migraine, FC abnormalities are at least in part related to disease severity [55]. Migraineurs included in the present study had longer disease duration (20 years on average) and a higher number of migraine attacks (more than 4 per month) than those included in the studies of Tedeschi and colleagues (mean disease duration of 10 years and 1–2 attacks per month). Reduced FC within regions of the visual network may reflect habituation or exhaustion of plasticity mechanisms secondary to long-standing migraine aura experience. Finally, it should also be noted that other two studies failed to find significant FC difference between MA patients and controls in visual networks, using respectively a larger sample of patients [56] and a different post-processing method (frequency-spectrum ICA) [46]. Since different results may be due to differences in clinical characteristics of enrolled patients and/or in MRI data acquisition/analysis, future studies are needed to draw definitive conclusions on resting-state FC abnormalities of visual networks in migraineurs with aura.
AIWS and MA patients also showed higher FC in salience and default mode network regions. Only two studies reported FC changes in the salience network, but the authors used different data analysis approaches (seed-based analysis and dynamic FC) [47, 49], so that their findings cannot be directly compared to those of the present work. Our results point to differences located in key nodes of the salience network, such as the insula, which acts as a cortical multisensory hub by receiving, processing, and conveying pain and other sensory inputs to cognitive and emotional control areas [57], thus playing a crucial role in migraines [17, 58]. Since pain is inherently salient, it is highly likely that the intrinsic connectivity in this network may be altered in chronic pain patients such as migraineurs, and may also affect network interactions, as demonstrated by Veréb and colleagues [49].
Concerning the default mode network, between-group differences were found both in anterior and posterior regions of the network, consistent with a previous study showing higher FC of the default mode network in MA patients compared to HCs [48]. The default mode network is highly relevant for self-referential cognitive and affective processing of pain experiences [59]. Increased FC in the default mode network, particularly in the posterior nodes of the network (e.g., precuneus and posterior cingulate cortex) that are engaged in both pain sensitivity and the integration of inputs from different sensory modalities, may suggest a compensatory adaptive mechanism in migraineurs [60].
Distinctive FC alterations in AIWS and MA patients
Although AIWS and MA patients both showed significant FC alterations with respect to HCs in visual, salience, and default networks, a direct comparison of the two patient groups showed that AIWS patients were characterized by significantly greater FC alterations (higher or lower) with respect to MA patients. AIWS patients also showed peculiar FC alterations with respect to HCs, which were not observed in MA patients, including higher FC in basal ganglia and executive control network regions.
Regarding lateral and medial visual networks, AIWS patients showed lower FC than MA patients in occipital regions almost exclusively located in the left hemisphere (i.e., superior division of the lateral occipital cortex, lingual gyrus, intracalcarine cortex, and occipital pole). In our previous study, we found that brain lesions causing visual AIWS symptoms were mainly located in the right occipital hemisphere, though AIWS cases due to left-sided lesions were also found [61, 62], as well as cases due to bilateral lesions or posterior transcallosal fiber interruption [63,64,65].
AIWS patients also showed higher FC than MA patients in salience network regions such as the right insula, which was previously related to the syndrome in two single-case AIWS studies showing extensive right insular damage [19]. The distortion of the body schema and space experienced by these patients may result from an interruption of the influence of the vestibular system on the spatial representation of the body and space mediated by this region [66].
AIWS patients were also characterized by higher FC in a core region of the default mode network, i.e., the posterior cingulate cortex. FC abnormalities in posterior regions of the default mode network were previously reported in a single-case SPECT study performed in a migraine patient with aura during an AIWS episode caused by acute aripiprazole intake [23].
Lastly, AIWS patients showed higher FC in basal ganglia and executive control networks as compared to both MA patients and HCs. Regarding the basal ganglia network, AIWS patients showed higher FC in the thalamus bilaterally with respect to HCs. Despite the recent evidence of thalamic white matter microstructural alterations [67], no study has investigated intrinsic FC alterations of basal ganglia network (or thalamic FC) in migraineurs with aura in the interictal state. It has previously been reported that thalamic lesions can cause AIWS [20, 21]. In particular, FC alterations in this structure may explain the somesthetic distortions experienced by AIWS patients due to its role in controlling sensory information flow to the cerebral cortices. AIWS patients also showed higher FC in the left putamen and caudate nucleus with respect to MA patients, possibly suggesting a role of these subcortical structures, along with the thalamus, in the pathophysiology of AIWS.
Regarding the executive control network, AIWS patients showed higher FC in several regions, including the bilateral frontal pole, anterior cingulate and paracingulate gyri, and right middle frontal gyrus, compared to both MA patients and HCs. Although the role of the frontal lobe is debated in AIWS, literature regarding this syndrome (case reports) provides some evidence of frontal region involvement in causing AIWS symptoms [14, 22, 68, 69]. It is possible that the intrinsic FC alterations in visual networks observed in both patient groups could predispose these patients to AIWS symptoms when altered FC coexists in basal ganglia and executive networks. A similar mechanism was recently proposed by Silvestro and colleagues, who showed that patients with complex migraine aura were characterized by higher extrastriatal FC and concomitant higher right insular FC with respect to pure visual aura migraineurs [50].
Finally, we found that FC changes in the executive control network correlated with migraine frequency: the higher the FC in the right frontal pole and middle frontal gyrus, the higher the number of headache days per month. This finding is quite interesting since an AIWS-specific alteration was correlated with a marker of headache disability. However, since AIWS symptoms tend to occur close to migraine onset, it is possible that FC rearrangement in this network may be induced by repetitive AIWS episodes, thus representing a secondary effect of AIWS on brain FC related to episode frequency. Since frontal lobes likely play a role in the top-down modulation of the visual pathway [70], we can alternatively speculate that impairment in the inferential process of visual information in the frontal lobe, which seems to be particularly affected by repeat migraine attacks over time [71], may contribute to visual AIWS symptoms [9].
Overall, since AIWS is a distressing condition that aggravates the disability burden of migraine, these results could point towards the possible beneficial use of migraine interventions in controlling also AIWS symptoms.
Study limitations
The first limitation of the present study is the relatively small sample size of AIWS patients due to the rarity of this neurological condition. However, our distribution of the different AIWS types is consistent with previous studies [7, 12, 25], especially with respect to the most common type B form. Another limitation is that we used a group of migraine patients with pure visual and visual-somatosensory aura in order to improve the match with the group of migraineurs with AIWS, that included patients with pure visual symptoms (type B, the most common form) and patients with somesthetic and visual symptoms (type C). However, it has recently been demonstrated that migraine patients with visual-somatosensory aura showed FC differences in visual and somatosensory networks with respect to migraineurs with pure visual aura [50]. Despite this potential methodological limitation, the inclusion of a heterogeneous group of MA patients in the present study has been a trade-off in order to prevent unbalanced group comparison. Future studies with larger sample sizes should include homogenous AIWS and MA populations in order to provide definitive imaging biomarkers for AIWS. Lastly, since migraine attacks with aura are sometimes associated with underlying hereditary or acquired cerebrovascular disorders and might be triggered by hypoperfusion [72], a further limitation is the lack of more direct measures of perfusion (e.g., arterial spin labeling), vascular reactivity (e.g., breath-holding task), and physiological data recording (e.g., pulse oximeter, respiratory bellows, expired gas analyzer).
Conclusion
AIWS and MA patients are characterized by similar intrinsic FC alterations with respect to HCs in visual, default mode, and salience networks, suggesting common pathophysiological underpinnings. However, AIWS patients showed greater and more widespread FC alterations with respect to MA patients, likely due to the complexity of AIWS symptoms involving high-order associative cortical areas. Finally, AIWS-specific FC alterations were found in the thalamus of the basal ganglia network and in several frontal cortical areas of the executive control network, suggesting a role of these regions in the pathophysiology of AIWS.
Data availability
The datasets presented in this article are not readily available because of patient confidentiality and participant privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
References
Todd J (1955) The Syndrome of Alice in Wonderland. Can Med Assoc J 73:701–704
Carroll L (1865) Alice’s adventures in wonderland. MacMillan and Co., London
Blom JD (2016) Alice in Wonderland syndrome: a systematic review. Neurol Clin Pract 6:259–270. https://doi.org/10.1212/CPJ.0000000000000251
O’Toole P, Modestino EJ (2017) Alice in Wonderland Syndrome: a real life version of Lewis Carroll’s novel. Brain Dev 39:470–474. https://doi.org/10.1016/j.braindev.2017.01.004
Blom JD (2020) Alice in Wonderland syndrome. Springer Nature, Cham. https://doi.org/10.1007/978-3-030-18609-8
Podoll K, Ebel H, Robinson D, Nicola U (2002) Obligatory and facultative symptoms of the Alice in wonderland syndrome. Minerva Med 93:287–293
Lanska JR, Lanska DJ (2013) Alice in Wonderland Syndrome: somesthetic vs visual perceptual disturbance. Neurology 80:1262–1264. https://doi.org/10.1212/WNL.0b013e31828970ae
Liu AM, Liu JG, Liu GW, Liu GT (2014) “Alice in wonderland” syndrome: presenting and follow-up characteristics. Pediatr Neurol 51:317–320. https://doi.org/10.1016/j.pediatrneurol.2014.04.007
Mastria G, Mancini V, Viganò A, Di Piero V (2016) Alice in Wonderland Syndrome: a clinical and pathophysiological review. Biomed Res Int 2016:8243145. https://doi.org/10.1155/2016/8243145
Mastria G, Mancini V, Cesare MD et al (2021) Prevalence and characteristics of Alice in Wonderland Syndrome in adult migraineurs: perspectives from a tertiary referral headache unit. Cephalalgia 41:515–524. https://doi.org/10.1177/0333102420968245
Petrusic I, Viana M, Zecca C, Zidverc-Trajkovic J (2020) Dysphasia and other higher cortical dysfunctions during the migraine aura-a systematic review of literature. Curr Pain Headache Rep 24:3. https://doi.org/10.1007/s11916-020-0836-3
Piervincenzi C, Petsas N, Giannì C, Di Piero V, Pantano P (2022) Alice in Wonderland Syndrome: a lesion mapping study. Neurol Sci 43(5):3321–3332. https://doi.org/10.1007/s10072-021-05792-0
Kuo Y-T, Chiu N-C, Shen E-Y et al (1998) Cerebral perfusion in children with Alice in Wonderland syndrome. Pediatr Neurol 19:105–108. https://doi.org/10.1016/S0887-8994(98)00037-X
Gencoglu EA, Alehan F, Erol I et al (2005) Brain SPECT findings in a patient with Alice in Wonderland syndrome. Clin Nucl Med 30:758–759. https://doi.org/10.1097/01.rlu.0000182278.13389.a3
Brumm K, Walenski M, Haist F et al (2010) Functional MRI of a child with Alice in Wonderland syndrome during an episode of micropsia. J AAPOS 14:317–322. https://doi.org/10.1016/j.jaapos.2010.03.007
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541
Chong CD, Schwedt TJ, Hougaard A (2019) Brain functional connectivity in headache disorders: a narrative review of MRI investigations. J Cereb Blood Flow Metab 39:650–669. https://doi.org/10.1177/0271678X17740794
Brooks JBB, Prosdocimi FC, da Rosa PB, Fragoso YD (2019) Alice in Wonderland syndrome: who in the world am I? Arq Neuropsiquiatr 77:672–674
Kumral E, Evyapan D, Sirin T, Alpaydin S (2012) Hyposchematia without spatial neglect in 2 patients with lesions involving the insula. Cogn Behav Neurol 25:149–153. https://doi.org/10.1097/WNN.0b013e31826b70de
Saj A, Wilcke JC, Gschwind M et al (2013) Spatial hyperschematia without spatial neglect after insulo-thalamic disconnection. PLoS ONE 8:e79938. https://doi.org/10.1371/journal.pone.0079938
ElTarhouni AH, Beer L, Mouthon M et al (2020) The right thalamic ventral posterolateral nucleus seems to be determinant for macrosomatognosia: a case report. BMC Neurol 20:393. https://doi.org/10.1186/s12883-020-01970-3
Weijers NR, Rietveld A, Meijer FJA, de Leeuw FE (2013) Macrosomatognosia in frontal lobe infarct-a case report. J Neurol 260:925–926. https://doi.org/10.1007/s00415-012-6827-5
Mancini V, Mastria G, Frantellizzi V et al (2018) Aripiprazole-triggered Alice in Wonderland Syndrome episodes studied with 99mTc-HMPAO brain SPECT. Eur Neurol 79:333–334. https://doi.org/10.1159/000490902
Beh SC, Masrour S, Smith SV, Friedman DI (2018) Clinical characteristics of Alice in Wonderland syndrome in a cohort with vestibular migraine. Neurol Clin Pract 8:389–396. https://doi.org/10.1212/CPJ.0000000000000518
Mastria G, Mancini V, Cesare MD, Puma M, Alessiani M, Petolicchio B, Viganò A, Piero VD (2021) Prevalence and characteristics of Alice in Wonderland Syndrome in adult migraineurs: perspectives from a tertiary referral headache unit. Cephalalgia 41(5):515–524. https://doi.org/10.1177/0333102420968245
Hansen JM, Baca SM, Vanvalkenburgh P, Charles A (2013) Distinctive anatomical and physiological features of migraine aura revealed by 18 years of recording. Brain 136:3589–3595. https://doi.org/10.1093/brain/awt309
Esteban O, Markiewicz CJ, Blair RW et al (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16:111–116. https://doi.org/10.1038/s41592-018-0235-4
Esteban O, Blair R, Markiewicz CJ, Berleant SL, Moodie C, Ma F, Ilkay Isik A et al (2018) FMRIPrep. Software. Zenodo. https://doi.org/10.5281/zenodo.852659
Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh S (2011) Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Front Neuroinform 5:13. https://doi.org/10.3389/fninf.2011.00013
Gorgolewski KJ, Esteban O, Markiewicz CJ, Ziegler E, Ellis DG, Notter MP, Jarecka D et al (2018) Nipype. Software. Zenodo. https://doi.org/10.5281/zenodo.596855
Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005) Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond, B, Biol Sci 360:1001–1013. https://doi.org/10.1098/rstb.2005.1634
Smith SM, Fox PT, Miller KL et al (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.0905267106
Tong Y, Hocke LM, Nickerson LD et al (2013) Evaluating the effects of systemic low frequency oscillations measured in the periphery on the independent component analysis results of resting state networks. Neuroimage. https://doi.org/10.1016/j.neuroimage.2013.03.019
Piervincenzi C, Petsas N, De Giglio L et al (2021) Increased within-network functional connectivity may predict NEDA status in fingolimod-treated MS patients. Front Neurol 12:632917. https://doi.org/10.3389/fneur.2021.632917
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106(3):1125–1165. https://doi.org/10.1152/jn.00338.2011
Filippini N, MacIntosh BJ, Hough MG et al (2009) Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 106:7209–7214. https://doi.org/10.1073/pnas.0811879106
Nickerson LD, Smith SM, Öngür D, Beckmann CF (2017) Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front Neurosci 11:115. https://doi.org/10.3389/fnins.2017.00115
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
Kruit M, van Buchem M, Launer L et al (2010) Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia 30:129–136. https://doi.org/10.1111/j.1468-2982.2009.01904.x
Eikermann-Haerter K, Huang SY (2021) White matter lesions in migraine. Am J Pathol 191:1955–1962. https://doi.org/10.1016/j.ajpath.2021.02.007
Tessitore A, Russo A, Conte F et al (2015) Abnormal connectivity within executive resting-state network in migraine with aura. Headache 55:794–805. https://doi.org/10.1111/head.12587
Tedeschi G, Russo A, Conte F et al (2016) Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia 36:139–147. https://doi.org/10.1177/0333102415584360
Seeley WW, Menon V, Schatzberg AF et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Seeley WW (2019) The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci 39:9878–9882. https://doi.org/10.1523/JNEUROSCI.1138-17.2019
Faragó P, Tuka B, Tóth E et al (2017) Interictal brain activity differs in migraine with and without aura: resting state fMRI study. J Headache Pain 18:8. https://doi.org/10.1186/s10194-016-0716-8
Niddam DM, Lai K-L, Fuh J-L et al (2016) Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia 36:53–66. https://doi.org/10.1177/0333102415583144
Lo Buono V, Bonanno L, Corallo F et al (2017) Functional connectivity and cognitive impairment in migraine with and without aura. J Headache Pain 18:72. https://doi.org/10.1186/s10194-017-0782-6
Veréb D, Szabó N, Tuka B et al (2020) Temporal instability of salience network activity in migraine with aura. Pain 161:856–864. https://doi.org/10.1097/j.pain.0000000000001770
Silvestro M, Tessitore A, Di Nardo F et al (2022) Functional connectivity changes in complex migraine aura: beyond the visual network. Eur J Neurol 29:295–304. https://doi.org/10.1111/ene.15061
Centeno M, Carmichael DW (2014) Network connectivity in epilepsy: resting state fMRI and EEG-fMRI contributions. Front Neurol 5:93. https://doi.org/10.3389/fneur.2014.00093
Lin L, Xing G, Han Y (2018) Advances in resting state neuroimaging of mild cognitive impairment. Front Psychiatry 9:671. https://doi.org/10.3389/fpsyt.2018.00671
Rocca MA, Schoonheim MM, Valsasina P et al (2022) Task- and resting-state fMRI studies in multiple sclerosis: from regions to systems and time-varying analysis Current status and future perspective. Neuroimage Clin 35:103076. https://doi.org/10.1016/j.nicl.2022.103076
Hillary FG, Roman CA, Venkatesan U et al (2015) Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology 29:59–75. https://doi.org/10.1037/neu0000110
Coppola G, Di Renzo A, Petolicchio B et al (2019) Aberrant interactions of cortical networks in chronic migraine: a resting-state fMRI study. Neurology 92:e2550–e2558. https://doi.org/10.1212/WNL.0000000000007577
Hougaard A, Amin FM, Magon S et al (2015) No abnormalities of intrinsic brain connectivity in the interictal phase of migraine with aura. Eur J Neurol 22:702-e46. https://doi.org/10.1111/ene.12636
Benarroch EE (2019) Insular cortex: functional complexity and clinical correlations. Neurology 93:932–938. https://doi.org/10.1212/WNL.0000000000008525
Borsook D, Veggeberg R, Erpelding N et al (2016) The insula: a “hub of activity” in migraine. Neuroscientist 22:632–652. https://doi.org/10.1177/1073858415601369
de Tommaso M, Vecchio E, Quitadamo SG et al (2021) Pain-related brain connectivity changes in migraine: a narrative review and proof of concept about possible novel treatments interference. Brain Sci 11:234. https://doi.org/10.3390/brainsci11020234
Zhang J, Su J, Wang M et al (2016) Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. J Headache Pain 17:98. https://doi.org/10.1186/s10194-016-0692-z
Mastria G, Mancini V, Viganò A et al (2018) Temporal-occipital glioblastoma presenting with Alice in Wonderland Syndrome in a patient with a long-time history of migraine without aura. Neurocase 24:242–244. https://doi.org/10.1080/13554794.2018.1562079
Tokida H, Takeshima S, Takeshita J et al (2018) A case of various illusion, and hallucination caused by occipital lobe infarction. Rinsho Shinkeigaku 58:556–559. https://doi.org/10.5692/clinicalneurol.cn-001081
Cohen L, Gray F, Meyrignac C et al (1994) Selective deficit of visual size perception: two cases of hemimicropsia. J Neurol Neurosurg Psychiatry 57:73–78
Hong YH, Lim T-S, Yong SW, Moon SY (2010) Ipsilateral visual illusion after unilateral posterior cerebral artery infarction: a report of two cases. J Neurol Sci 295:114–116. https://doi.org/10.1016/j.jns.2010.05.015
Coven I, Horasanlı B, Sönmez E et al (2013) The Alice in Wonderland syndrome: an unusual in acute disseminated encephalomyelitis. Am J Emerg Med 31:638.e1–3. https://doi.org/10.1016/j.ajem.2012.10.029
Rode G, Vallar G, Revol P et al (2012) Facial macrosomatognosia and pain in a case of Wallenberg’s syndrome: selective effects of vestibular and transcutaneous stimulations. Neuropsychologia 50:245–253. https://doi.org/10.1016/j.neuropsychologia.2011.11.018
Coppola G, Di Renzo A, Tinelli E et al (2021) Thalamo-cortical networks in subtypes of migraine with aura patients. J Headache Pain 22:58. https://doi.org/10.1186/s10194-021-01272-0
Zwijnenburg PJG, Wennink JMB, Laman DM, Linssen WHJP (2002) Alice in Wonderland syndrome: a clinical presentation of frontal lobe epilepsy. Neuropediatrics 33:53–55. https://doi.org/10.1055/s-2002-23599
Morland D, Wolff V, Dietemann J-L et al (2013) Robin Hood caught in Wonderland: brain SPECT findings. Clin Nucl Med 38:979–981. https://doi.org/10.1097/RLU.0000000000000230
Urbanski M, Thiebaut de Schotten M, Rodrigo S et al (2008) Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry 79:598–601. https://doi.org/10.1136/jnnp.2007.126276
Schwedt TJ, Dodick DW (2009) Advanced neuroimaging of migraine. Lancet Neurol 8:560–568. https://doi.org/10.1016/S1474-4422(09)70107-3
Dalkara T, Nozari A, Moskowitz MA (2010) Migraine aura pathophysiology: the role of blood vessels and microembolisation. Lancet Neurol 9:309–317. https://doi.org/10.1016/S1474-4422(09)70358-8
Author information
Authors and Affiliations
Contributions
Claudia Piervincenzi contributed to method definition, data analysis, statistics, and manuscript editing. Nikolaos Petsas contributed to study design, experimental settings, subject recruitment, MRI acquisition, and manuscript editing. Costanza Giannì contributed to MRI acquisition, data interpretation, and manuscript editing. Alessandro Viganò, Valentina Mancini, Giulio Mastria and Marta Puma contributed to study design, recruitment, and data interpretation. Vittorio Di Piero contributed to study design and recruitment and, together with Patrizia Pantano, supervised the study and manuscript editing.
Corresponding author
Ethics declarations
Ethical approval
None.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Piervincenzi, C., Petsas, N., Viganò, A. et al. Functional connectivity alterations in migraineurs with Alice in Wonderland syndrome. Neurol Sci 44, 305–317 (2023). https://doi.org/10.1007/s10072-022-06404-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06404-1