Abstract
The diagnosis of a brain tumor is a life-changing event for patients and their families. Despite numerous treatment advances, malignant brain tumors are universally incurable and long-term survival is limited. Treatment response, prognosis, and survival depend on underlying histopathology and recently defined molecular features. Patients suffer from a disproportionately high symptom burden throughout the disease trajectory and at the end of life. Pronounced neurologic decline and psychological distress significantly impair quality of life (QoL) and impose high supportive care needs relative to other systemic cancers. Palliative interventions addressing brain tumor-specific symptoms, such as seizures, cognitive dysfunction, and headaches, are paramount to maintaining QoL. In the terminal phase of illness, most brain tumor patients lose the ability to communicate and participate in end-of-life decision-making. The benefits of advance care planning and early integration of specialized palliative care are well-established in other systemic cancers and have received wider recognition in neuro-oncology. We review how to approach neurological symptoms in brain tumor patients, as well as address prognosis and advance care planning with the goal of improving QoL for patients and caregivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Brain tumors: an overview
Brain tumors are a diverse group of low-grade and malignant neoplasms arising directly from brain tissue. Primary malignant brain neoplasms comprise 2% of adult cancers and are characterized by high morbidity and mortality [1]. The majority (80–85%) of malignant brain tumors are high-grade gliomas (HGGs), including glioblastoma (GBM), anaplastic astrocytomas, and anaplastic oligodendrogliomas. GBM, a WHO grade IV astrocytoma, is the most common malignant brain tumor in adults and portends an extremely poor prognosis with a median survival of 12–15 months despite aggressive multimodal therapy [2].
Standard oncologic staging paradigms are inapplicable to primary brain tumors as they rarely disseminate beyond the neuroaxis. Instead, brain tumors are graded based on biologic and genetic features per the WHO Classification of Tumors of the Central Nervous System [3]. This grading system provides a framework for prognostication and treatment decision-making. The last several decades have seen a radical shift from histology-based diagnosis towards molecular and genetic categorization of gliomas. These insights represent important progress in our understanding of glioma pathogenesis and are the basis for the future development of targeted therapies. Key prognostic and predictive molecular alterations per the WHO 2021 Classification are delineated in Table 1 [3].
Treatment of gliomas
The current standard of care for HGG includes maximal safe resection and external beam radiotherapy (EBRT) with concurrent temozolomide (TMZ), followed by adjuvant TMZ for 6–12 cycles. Tumor treating field (TTF) is a novel device that is thought to induce tumor cell death by delivering alternating electrical fields through superficial scalp electrodes. TTF is Food & Drug Administration (FDA) approved in the treatment of supratentorial GBM after a statistically significant survival benefit (20.9 vs 16.0 months) was demonstrated in a randomized, un-blinded clinical trial [4]. TMZ is a generally well-tolerated alkylating agent that is orally administered during EBRT and in the adjuvant setting for five sequential days in a 28-day cycle. It induces double-stranded DNA breaks and subsequent tumor cell apoptosis. Commonly encountered adverse effects include dose-dependent myelosuppression (especially thrombocytopenia), fatigue (which may be severe and prolonged after radiation), and GI distress (nausea, vomiting, constipation). Tumor progression is unfortunately inevitable for most patients, but clinical trial enrollment, re-irradiation, second-line chemotherapy, and repeated surgical interventions may improve survival and quality of life (QoL) in an overall fatal disease. Management of low-grade gliomas uses a similar treatment approach; however, decision-making is more complex and depends on molecular profiling. In the case of metastatic brain disease, both primary and secondary tumor characteristics influence treatment course, including surgical resection, whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), and systemic and intrathecal chemotherapy.
Palliative care and symptom management in neuro-oncology
Persons with brain tumors experience progressive neurologic decline resulting in disproportionately high symptom burden throughout the disease trajectory, particularly at the end of life [5]. Caregivers report significant physical burden and high levels of psychological distress [6]. Although many symptoms in central nervous system (CNS) and non-CNS cancers overlap, others are more prevalent in patients experiencing brain tumors, such as seizures, cognitive dysfunction, confusion, headaches, mood disturbances, and fatigue.
The World Health Organization describes palliative care (PC) as a clinical approach that improves the QoL of patients and their families when facing a life-threatening illness, regardless of life expectancy. PC emphasizes the prevention and proactive assessment and treatment of pain and other physical, psychosocial, or spiritual needs. This multidimensional approach has been recommended for chronic and progressive neurological diseases and specifically for patients with brain tumors [7, 8].
Brain tumors produce localization-related focal deficits that may be exacerbated by intrinsic and treatment-induced peritumoral edema. Headaches, seizures, altered mental status, and cognitive dysfunction are prevalent tumor-centric symptoms that are often multifactorial. While radiation plays an integral role in the treatment and palliation of malignant brain neoplasms, its neurotoxic effects, including tumor pseudoprogression and radiation necrosis, often produce significant morbidity. One study involving more than 600 primary brain tumor patients found that 50% of patients reported at least 10 concurrent symptoms, and 40% had at least three symptoms rated as moderate to severe [9]. Symptoms interfered with general activity, ability to work, or enjoyment of life in at least 25% of patients [9].
Prospective literature pertaining to symptomatic management, specifically at the end of life, is sparse. Many neuro-oncologists do not feel adequately equipped to manage the unique and challenging needs of this patient population beyond the scope of commonly encountered neurological symptoms [10]. Early specialized palliative care referral to address more complex symptomatology and decision-making has been recommended [11]. However, the majority of neuro-oncologists in a recent survey reported utilizing PC and hospice late in the disease trajectory, after curative and investigative options have been exhausted [12]. This pattern results in decreased exposure to specialized PC in comparison to other solid tumor populations. Patients with brain cancer require a comprehensive approach with a focus on maintaining QOL beyond prolonging survival.
The goal of this chapter is to provide insight on the unique palliative needs of this patient population, as well as optimization of end-of-life symptom management. It will also delineate the role of PC in the context of a multidisciplinary approach to brain cancer and describe the benefits of earlier involvement of PC and advance care planning. Areas of opportunity for continued research and inquiry will be explored.
Direct tumor effects and treatment-induced neurotoxicity
Mass effect caused by the brain tumor or edema contributes substantially to brain tumor morbidity and mortality. Peritumoral edema can exacerbate focal deficits, headaches, seizures, encephalopathy, and sequelae of increased intracranial pressure. Radiation-induced neurotoxicity is a highly relevant iatrogenic trigger of peritumoral edema and mass effect. “Pseudoprogression” is a common subacute radiotoxic effect that most often presents within the first 90 days of adjuvant radiotherapy and occurs in up to 25% of patients with GBM [13, 14]. Spontaneous recovery over weeks to months is expected, and several studies suggest an improved overall survival of brain tumor patients with pseudoprogression when compared to tumor progression [14].
Radionecrosis refers to the development of a radiation-induced space-occupying necrotic mass lesion. Distinguishing between radionecrosis and disease progression is challenging due to significant clinical and radiographic overlap [15]. Both necrotic tissue and tumor recurrence produce focal symptoms secondary to edema and mass effect. Core MRI features, including intravenous contrast enhancement, mass effect, central necrosis, and vasogenic edema, are shared between the two conditions [15]. Treatment with dexamethasone or bevacizumab (as delineated below) provides short-term symptomatic improvement; however, long-term benefits are uncertain [16].
Corticosteroids are the mainstay of treatment for symptomatic edema in brain tumor patients. Dexamethasone is often preferred due to its low mineralocorticoid activity, long half-life (more than 36 h), ease of administration, and dampened psychiatric effects [17]. Steroids can result in rapid clinical improvement in patients with acute neurologic symptoms and can be given in large intravenous doses in this setting. Despite widespread use, high-quality studies addressing various steroid dosing regimens are lacking, and some data favor a more conservative approach as compared with current practice [17]. While elevated doses are often reflexively prescribed with good intention (i.e., dexamethasone 16 mg daily), resultant side effects can paradoxically lead to negative impacts on QoL. Prolonged high-dose corticosteroid use (weeks to months) is associated with adrenal insufficiency (which may exacerbate fatigue and cognitive changes), diabetes requiring insulin therapy, immune suppression and resultant opportunistic infections (oral thrush), gastritis, peptic ulcers and gastrointestinal bleeding, weakness, and myopathy, as well as neuropsychiatric effects [9]. In addition to deleterious side effects impacting QoL, corticosteroids may negatively influence overall survival and outcomes [18]. Generally, minimum corticosteroid dosing with shortest durations required for symptom control is preferred. Alongside appropriate titration based on symptom control and neuroimaging, 4–8 mg of dexamethasone daily usually provides effective symptom management in clinical practice [16].
Bevacizumab is a monoclonal antibody against vascular endothelial growth factor (VEGF) that exhibits potent anti-angiogenic properties. It is approved as a second-line treatment for HGG in several countries. Bevacizumab is particularly effective in treating refractory peritumoral edema, especially in patients for whom tapering or discontinuing steroids is unfeasible. Notable clinical benefits include improvement of tumor-related neurologic symptoms, decreased steroid requirements, and maintenance of performance status [16]. Radiographic response is often remarkable, with significant “pseudo-resolution” of tumor enhancement. Unfortunately, the anti-tumor effects of bevacizumab treatment are short-lived in most patients, and disease progression ensues after several months of use. Although generally well tolerated, side effects including hypertension, intracranial hemorrhage, thromboembolism, posterior reversible encephalopathy syndrome, and impaired wound healing preclude the routine use of bevacizumab in asymptomatic patients [19].
Seizures
Epileptic seizures are common in patients with brain tumors. Although the exact frequency is unknown, it is suggested that up to 50% of patients experience seizures at some point of their disease [20]. In 30–50% of patients with brain tumors, seizures may be the first clinical sign of an underlying mass [21]. Focal seizures with impaired awareness are the most common semiology and usually localize to the temporal lobes, whereas intact-awareness focal seizures predominate in frontal, parietal, and occipital lesions [22]. Secondary generalization is common, and brain tumor patients are at elevated risk of both convulsive and nonconvulsive status epilepticus (NCSE) [22]. NSCE may masquerade as altered mental status from other causes, such as cognitive dysfunction, fatigue, increased intracranial pressure, hemorrhage, and direct tumor-related effects. Clinical examination alone cannot reliably identify NCSE, and its incidence may therefore be underreported [23]. One study postulates a strongly negative impact of NCSE on survival, making NCSE a potentially critical cause of depressed mental status in brain tumor patients [23]. Currently, it remains unclear how identification and treatment of NCSE may affect outcomes.
Low-grade, IDH1 mutant, WHO grade II, and cortically located tumors (especially in the temporal and parietal lobes) are the most epileptogenic, and up to 100% of patients with low-grade brain tumors develop epilepsy, as compared with GBM (29–49%) and metastatic brain lesions (20–35%) [21]. Perhaps counterintuitively, early-onset seizures are associated with low-grade pathology and might represent more chronic and indolent underlying structural changes. Well-differentiated glioma cells and IDH-1 mutant cells are thought to produce epileptogenic neurotransmitters or modulators that increase seizure propensity [24]. Overall, early-onset seizures are associated with low-grade pathology and may therefore imply favorable survival outcomes [25]. Seizures that are new, reappear, or increase in frequency often signal disease progression and require thorough evaluation.
Seizures are a significant source of direct and indirect morbidity. Patients with brain tumors generally experience more frequent and severe side effects from anti-seizure medications, possibly related to polypharmacy, tumor burden, deficits from prior treatment, and radiation therapy [26]. However, reported adverse effects from anti-seizure medications often pertain to older agents (such as phenytoin, phenobarbital, oxcarbazepine, and carbamazepine), and it is unclear whether a similar degree of morbidity occurs with newer-generation drugs. Seizures are often distressing and impair QoL by evoking fears of tumor recurrence, increasing caregiver burden and anxiety, limiting patient independence, and increasing the frequency of emergency department visits and hospitalizations. Seizure prevalence and refractoriness increase towards death, and a minority (10–15%) of patients with HGG may not develop epilepsy until the terminal phase of illness [27]. One study suggests that seizures may occur in approximately 30% of brain tumor patients at the end of life and have been strongly associated with non-peaceful death [28].
The utility of primary seizure prophylaxis in brain tumor patients, both peri-operatively and otherwise, has been a topic of uncertainty in recent decades. Methodological issues have prevented most studies from rendering conclusive high-level evidence, and it remains unclear whether primary seizure prophylaxis is beneficial [29]. Despite these findings, a recent survey including 144 practicing neurosurgeons found that 63% of respondents reported regularly prescribing empiric postoperative anti-seizure medications in seizure-naïve patients with supratentorial brain tumors [30]. The American Academy of Neurology seizure guidelines were recently updated by the Society of Neuro-Oncology and the European Association of Neuro-Oncology and explicitly do not support the prophylactic use of anti-seizure medications in these settings [29]. This systemic literature review conducted in 2021 identified level A evidence against the use of anti-seizure medications to reduce the risk of seizures in brain tumor patients and concluded that there is overall insufficient evidence to recommend prophylactic peri- or postoperative anti-epileptic drug (AED) treatment [29].
In the event of a single seizure, long-term AED treatment is generally justified and should be considered. No randomized trials have established specific AED superiority. Patient characteristics, seizure type, tolerability, and drug interaction potential should guide AED selection. Generally, lowest effective doses should be given in an effort to minimize toxicity. Cytochrome P450 (CYP450) inducers including phenobarbital, phenytoin, primidone, carbamazepine, and oxcarbazepine may alter the metabolism and efficacy of commonly used chemotherapeutics as well as dexamethasone. Therefore, these older agents are usually avoided unless absolutely necessary. Valproic acid is a known histone deacetylase inhibitor with purported intrinsic antineoplastic properties [26]. Based on the most recent updated seizure guidelines, the use of valproic acid as an antineoplastic agent is not recommended [29].
Newer-generation anti-seizure medications including levetiracetam, zonisamide, and lacosamide are renally excreted and do not affect CYP450 metabolism. Their superior side effect profiles make them top choices for seizure management in brain tumors. Levetiracetam is frequently used due to its established effectiveness, tolerability, low pricing, and relative ease of dosing [31]. Neuropsychiatric disturbance is an important side effect that may be exacerbated by concomitant steroid use. Brivaracetam is an analogue of levetiracetam that is FDA approved for adjunctive use in focal seizures. In a retrospective study involving 33 patients with brain tumors, brivaracetam induced seizure freedom in 60.6% of patients, with greater than 50% reduction in seizure frequency in 18% [32]. Adverse effects including agitation, anxiety, fatigue, and vertigo occurred in approximately 20% of patients [32]. Brivaracetam is a potentially safe and effective option in brain tumor-related epilepsy pending further trials; however, cost and regional availability may limit widespread use [32]. Perampanel is a non-competitive ionotropic glutamate receptor antagonist that has demonstrated good tolerability and clinically significant seizure reduction in patients with brain tumor-related epilepsy and may also be a viable treatment option in this setting [33].
Brain tumor-related epilepsy is pharmaco-resistant in up to 30% of patients but can improve with tumor-directed therapies including surgical resection, radiotherapy, and chemotherapy [25]. Although high-quality studies are lacking, the prevalence of refractory seizures at the end of life may approach 30% [7]. Patients with a history of tumor-induced epilepsy have the highest risk of developing seizures in the end-of-life stage, which may cause significant distress for caregivers. Dysphagia and altered mental status in this setting may make AED optimization challenging; however, anti-seizure medications should be continued if possible. Other routes, including intranasal, sublingual, buccal, rectal, subcutaneous, or intravenous, can be incorporated. Agents with comparable efficacy include intranasal midazolam, buccal lorazepam, rectal diazepam, and lorazepam oral concentrates [34]. In a small prospective study with 25 glioma patients, prophylactic buccal clonazepam and abortive treatment with intranasal midazolam were found to be feasible, effective, and well received by caregivers in the treatment of brain tumor-related seizures in the home setting [35].
Headaches and pain
Headaches are experienced by 30–70% of patients with brain tumors and are the most common source of pain in this patient group [36]. Only a small minority (2–8%) of patients experience isolated headaches as a first clinical manifestation of a brain tumor, while most headaches occur in conjunction with other neurologic symptoms [37, 38]. Patients with a history of headaches are more likely to experience headaches in the context of brain cancer. In these cases, brain tumor-related headaches are comparable in character but are often more severe than prior headaches [36]. Brain tumor-related headaches are generally described as tension-type and nonspecifically localized, while migrainous headaches are less common [39,40,41]. “Classic” brain tumor-type headaches, described as “worse in the morning,” aggravated by Valsalva-like maneuvers, and associated with nausea or vomiting, occur in a minority (17%) of patients [40]. Ophthalmoscopic evaluation may be of benefit to evaluate for papilledema in this context. The site of pain correlates with tumor location in only 30% of all patients and is therefore of limited diagnostic utility [42]. Generally, supratentorial tumors are associated with vertex and bifrontal pain, whereas occipital pain more reliably accompanies infratentorial tumors [39, 40].
The brain parenchyma itself is devoid of pain receptors. Expanding tumor tissue and peritumoral edema produce pain via traction on richly innervated surrounding structures such as dura, dural, and meningeal vessels, venous sinuses, and cranial periosteum [43]. Direct compression of exiting cranial nerves, such as occipital nerve compression in cranio-medullary junction tumors, has also resulted in similar headaches [38, 43]. In patients with preoperative headaches, neurogenic inflammation and central sensitization may result in headache persistence following surgical debulking [43].
Post-craniotomy headaches (PCHs) occur in over two-thirds of patients and are among the most frequently encountered adverse events after craniotomy [44]. The International Headache Society diagnostic criteria for acute and chronic post-craniotomy headache are delineated in Table 2 [45]. Direct soft tissue trauma, nerve injury, meningeal irritation, neuroma formation, dural muscle adherence, and aberrant nerve regeneration are the leading hypotheses [44]. Longer neurosurgical duration (> 4 h) and suboccipital approach have been reported to increase the risk of post-craniotomy pain [46]. PCHs are typically described to have tension-type character combined with localized surgical site pain [47]. Other manifestations include focal lancinating pain or dysesthesias as well as occipital neuralgiform features [36]. Acute PCH is moderate to severe in up to 80% of patients, and approximately 50% will develop chronic PCH as defined by pain persisting beyond 3 months [48]. Incapacitating pain (22%), negative impact on mood (15%), and interference with daily activities (29–60%) are also reported among post-craniotomy patients [44]. Unfortunately, post-craniotomy pain is often undertreated, and optimal management has not been established. Preoperative diclofenac was associated with decreased headache intensity following infratentorial surgery in one randomized, blinded, single-center trial [49]. Other interventions, including occipital nerve blocks, duloxetine, gabapentin, and tizanidine, are often helpful. Physical therapy, locally applied heat or ice, massage, bio-behavioral interventions, and botulinum toxin are potentially viable non-pharmacologic adjuncts [50]. Secondary causes of PCH, such as cerebrospinal fluid leak, hydrocephalus, hemorrhage, and meningoencephalitis, also need to be considered.
Headache management in patients with brain tumors depends on severity, underlying mechanism, and overall performance status. Tumor- and treatment-induced edema, not tumor size, has been correlated with headache severity and demonstrates excellent steroid responsiveness [36, 51]. If feasible, corticosteroids should be avoided in the late afternoon as they may cause insomnia. Sleep disturbance is common and may exacerbate other QoL-defining symptoms such as fatigue, and mood disorders. Adjunctive bevacizumab can be considered in patients with extensive cerebral edema refractory to corticosteroids [36].
In the absence of increased intracranial pressure, treatment of corticosteroid-refractory headaches follows conventional guidelines, with a few exceptions. Non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are first-line agents for mild headaches. Opioid combinations such as hydrocodone or oxycodone with nonopioid analgesics are often needed to treat moderate headaches, while more severe headaches may warrant higher potency opiates such as morphine or hydromorphone. Tramadol is a weak opioid that is contraindicated in patients with brain tumor-related epilepsy due to its propensity to lower the seizure threshold. Post-marketing surveillance has shown that most seizure events occur with tramadol doses above 200 mg (dosage in clinical practice usually ranges from 50 to 100 mg) and in younger patients (occurring rarely in patients above the age of 59) [52, 53]. Therefore, tramadol may be used with caution in brain tumor patients in the appropriate clinical context. Meperidine also lowers seizure threshold and is strictly contraindicated. Opiate use may decrease QoL due to their addictive potential and adverse effects. Common side effects include constipation, nausea and vomiting, sedation, delirium, and withdrawal symptoms in the event of dependency. In the setting of chronic opioid use, combined long-acting and short-acting opioids exhibit incomplete cross-tolerance resulting in a reduction of total opiate dosage, increased opiate efficacy, and decreased adverse opiate effects [53]. Consultation with a PC team or pain service is encouraged.
Patients requiring frequent use of abortive medications (more than four times weekly) may benefit from preventive therapy. Commonly used agents include topiramate and tricyclic antidepressants such as nortriptyline and amitriptyline. One study observed that patients on beta-blockers for other indications had lower frequency and intensity of brain tumor-related headaches [39]. It is important to consider potential known side effects of prophylactic headache medications such as neurocognitive impairment and weight loss with topiramate. Treatment with gabapentin or tricyclic antidepressants may result in lethargy, weight gain, and delirium. No studies have concluded superiority of any abortive or preventive agent specifically for this indication, and more research on this topic is needed.
Headaches have both physical and emotional implications on brain tumor patients. One study found that recurrent headaches served as a frequent reminder of life-threatening illness, resulting in anxiety and difficulty maintaining a positive outlook [54]. This demonstrates the complex interplay of commonly experienced symptoms in brain tumor patients, including headaches, mood disorders, sleep disturbances, and fatigue.
Cognitive dysfunction
Most patients with brain tumors exhibit some degree of cognitive impairment throughout their disease course [55]. Due to advanced age or tumor-induced changes, the majority (> 90%) of patients show cognitive deficits prior to treatment [56]. HGG has been associated with a greater degree of impairment regardless of implemented treatments [57]. Frequently affected domains include memory, attention, and executive functioning; however, the severity and pattern of symptoms vary considerably [58]. Given its multifactorial nature, brain tumor-related neurocognitive impairment is often impractical to approach as an isolated clinical syndrome. Mass effect, tumor location, seizures, comorbid psychiatric conditions, fatigue, insomnia, and pharmacologic effects are plausible contributors. The negative impacts caused by neurotoxic effects of local and systemic anti-cancer therapies are gaining more attention in the field of oncology and are important considerations in the brain tumor population.
Radiation-induced neurotoxicity occurs in 50–90% of brain tumor patients and is a frequent source of apprehension and distress [56, 59]. Effects are often debilitating and occur in the acute (during radiation), early-delayed (4–8 weeks), and chronic phase (months to years) of radiation treatment. Acute toxicity is transient and might present with headaches, nausea and vomiting, seizures, fever, somnolence, encephalopathy, and worsening of pre-existing focal deficits [60]. Symptoms are usually briskly responsive to corticosteroids and a full recovery is expected in most patients. Somnolence syndrome is a debilitating subacute toxic encephalopathy characterized by pervasive lethargy and mental clouding. Steroids may hasten recovery or prevent severe presentations. Diffuse cerebral injury is a late radiation-induced neurotoxic sequelae that can occur months to years following brain irradiation. It usually manifests in patients following radiation to low-grade neoplasms due to longer overall survival. Possible clinical features include progressive dementia, gait disturbance, apraxia, and urinary incontinence [13]. While the precise mechanisms causing these symptoms are unknown, direct injury to the hippocampus and to pluripotent neural stem cells (NSCs) have been implicated [61]. Radiation techniques aimed at improving neurocognitive outcomes, such as WBRT with hippocampal sparing, are more relevant in metastatic brain disease and have resulted in better preserved neurocognitive function [62, 63].
There is limited high-quality evidence to guide treatment of cognitive complaints in brain tumor patients [56]. Memantine has been shown to delay and reduce the degree of cognitive dysfunction over time when used preventatively with WBRT or hippocampal-sparing WBRT in brain metastases [62]. Donepezil, a reversible acetylcholinesterase inhibitor, may provide modest improvement in several cognitive domains [64]. Occupational interventions such as cognitive rehabilitation have been used to improve daily functioning by developing compensatory strategies and skills [56]. Although optimal timing has not been established, proactive cognitive training soon after craniotomy is thought to be most effective at preventing adverse treatment-induced cognitive outcomes [56]. Cognitive dysfunction may be irreversible, and even minor deficits can affect health-related QoL and functional independence. In one survey involving 226 patients with terminal illness, 88% of respondents stated that they would rather decline some aspects of treatment if the outcome was prolonged survival but associated with significant cognitive impairment [65]. Impaired cognition threatens individual autonomy by affecting decision-making capacity. Furthermore, rapid cognitive deterioration in the final weeks of life precludes participation in end-of-life decision-making, thus emphasizing the importance of early advance care planning.
The role of palliative care in brain tumor patients
Specialized PC in patients with advanced systemic cancer, particularly early in the disease course during active oncology treatment, has demonstrated positive impacts on QoL and survival. In a single-center, non-blinded randomized trial involving patients with metastatic lung cancer, early and structured PC, parallel to ongoing oncological treatment, resulted in significantly improved QoL and symptom burden of cancer patients [66]. The early involvement of specialized PC during active cancer treatment is now part of the American Society of Clinical Oncology guidelines [67]. Introducing PC early in the disease course serves to build therapeutic relationships and trust between patients, caregivers, and the PC team. Effective PC is navigated by the unique and personal treatment goals of each patient and should involve the development of an early advance care plan that is congruent with the patient’s wishes. These discussions should take place while the patient is able to actively engage in treatment decision-making. Neuropalliative care addresses specific neurological issues in diseases with high symptom burdens, such as ALS, movement disorders, and brain tumors [7, 68]. In an online survey of PC and neurology providers throughout Europe, collaboration between brain tumor services and PC in the form of joint meetings, clinic visits, and telephone encounters were found to positively impact QoL, functional status, complex decision-making, and end-of-life care as compared with other neurology subspecialty services that were lacking such collaboration [69]. Unfortunately, when and how to introduce neuropalliative care is currently not as clear, and this uncertainty likely contributes to the current state of PC underutilization in neuro-oncology [70].
A 2016 survey of neuro-oncology providers in the USA showed that early PC referrals remain rare. In this study, only 14% of patients with HGG were referred to PC at the time of diagnosis, while almost two-thirds of providers referred patients only at the onset of symptoms requiring palliation [10]. In the same survey, only one-third of providers felt comfortable addressing end-of-life issues [10]. Another study demonstrated hospice underutilization in the brain tumor population, with only 63% of patients enrolled in hospice, 20% of whom were enrolled in their last week of life [71]. Delayed hospice enrolment is a disservice to patients and their families as it results in suboptimal utilization of specialized end-of-life care. These findings indicate a need for more specific training in end-of-life management and resources on the value of specialized neuropalliative care for patients and their families.
Advance care planning
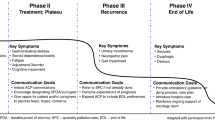
Patients with HGG have a high symptom burden throughout their disease trajectory, especially during the terminal phase of illness [5]. Numerous multifactorial and interrelated symptoms define QoL, including focal weakness, cognitive disturbances, drowsiness, seizures, and the inability to communicate. In contrast with other systemic cancers, brain tumor patients are often referred to PC and hospice later in their disease course, resulting in suboptimal symptom control and prolonged suffering [12, 71].
Rapid cognitive deterioration several weeks prior to death often precludes effective participation in end-of-life decision-making [72]. Therefore, the concept of advance care planning and hospice should be approached early in the disease trajectory [11]. While the timing of advance care planning in HGG patients has been understudied, it is generally recognized that earlier conversations, while the patient has a higher likelihood of active participation and decision-making, are beneficial [73]. Ideally, the patient should have an opportunity to convey their personal wishes while legal capacity remains intact. Advance care planning should be approached as an ongoing process rather than a one-time conversation. When approaching end-of-life discussions, important topics include the assignment of a healthcare power of attorney and open-ended exploration of values, treatment goals, and end-of-life care [73]. Effective conversations strike a balance between emphasizing the futility of brain cancer while maintaining the hope of healing, comfort, and peace. The focus should be on finding joy in the remaining time rather than focusing on survival.
In addition to preserving patient autonomy and dignity, advance directives alleviate caregiver burden by diminishing sentiments of uncertainty, guilt, or overwhelming responsibility regarding a patient’s end-of-life care. Documented benefits of advance care planning include reduced unwanted and unnecessary treatments, reduced length of stay, reduced number of hospitalizations, and decreased decisional conflict among caregivers in critically ill patients [74]. For many patients, preserving dignity is a crucial goal when considering the end-of-life phase, and families often benefit from hearing explicitly expressed wishes and considerations [75, 76]. During the transition to end-of-life and hospice care, an important motivator for involved caregivers includes the hope that their loved one can experience a dignified death according to his or her wishes [77]. In the case of preserved cognitive and communicative capacity at the end of life, patients who had detailed discussions regarding end-of-life wishes with designated caregivers were perceived to die with dignity more often than those without this opportunity [75]. Advance care planning should therefore include specific conversations about end-of-life care. In a meta-analysis examining the components of effective advance care planning conversations in multiple sclerosis, several factors were found to impact patient perception, participation, and discussion outcomes [78]. Cumulative losses in cognition or functional status throughout the disease resulted in patients redefining themselves as individuals with a terminal illness [78]. Patients thus became more likely to achieve self-acceptance and recognize the value of advance care planning. One other study found that inconsistencies in information, attitudes, and skills among healthcare providers resulted in mistrust, uncertainty regarding prognosis, and impaired PC delivery among patients with chronic neurologic diseases [79]. Further research exploring the applicability and limitations of these findings in brain tumor patients is indicated.
The problem of late referral to PC and hospice is further potentiated by provider discomfort and perceived unpreparedness to lead effective goals-of-care discussions. In a survey involving 552 practicing neuro-oncologists, one-third of respondents were concerned that end-of-life discussions may have negative consequences on their patients’ emotional well-being [10, 80]. However, patient-centered studies show that most patients prefer detailed information about their disease, especially pertaining to anticipated end-of-life symptoms and realistic survival [80, 81]. As such, there remains a significant discrepancy between optimal and current end-of-life transitions for patients with brain tumors.
Psychosocial support
Psychosocial support has been shown to be important for brain tumor patients and their caregivers alike. Providing care to patients with HGG is challenging. Patients and their families face issues beyond those directly related to neurological symptoms. Additional stressors include significant financial burden resulting from the cost of care and an inability to work [82]. Generally, a high level of distress and burnout among brain tumor caregivers is common and often rated higher when compared to other systemic cancers [83]. Neurologic declines, especially in the form of cognitive dysfunction, communication difficulty, mood disturbances, and personality changes, are highly distressing and decrease caregiver well-being [84]. These changes are often present prior to formal diagnosis and have a lasting impact on interpersonal relationships with family and caregivers. The loss of decision-making capacity and ability to participate in care place increasing responsibility on caregivers as the disease progresses [85]. Taking care of the caregiver and providing them with structured support and guidance in clinical practice not only have the potential to improve caregivers’ feelings of mastery and control but also have been shown to be predictive of brain tumor patient survival [86]. Therefore, it is important to provide patients and caregivers with a concrete plan of care [83]. Patients and caregivers often seek detailed information, which should be provided in a stepwise fashion starting at diagnosis, followed by preparing patients and caregivers for future transitions of care as well as end-of-life planning [11]. Hospice enrollment has been shown to increase caregiver satisfaction in the end-of-life phase of brain tumor patients [87]. Several studies show that patients and their caregivers experience reduced anxiety when receiving tailored information about diagnosis, prognosis, treatment options, recurrence, and end-of-life care, all of which collectively diminish the psychosocial impact of the disease [11, 85, 87]. A recently developed framework to address patient and caregiver supportive care needs includes coordination of care, repeated needs assessment, staged information based on symptom and tumor progression, and referrals to behavioral health and PC as needed [11]. Regularly scheduled assessments of patient and family caregiver needs should not only focus on physical symptoms but also include assessments of psychosocial status.
Conclusion
Brain tumor patients face an almost invariably futile disease with a limited prognosis and high symptom burden, particularly at the end of life. Identifying and controlling symptoms early in the disease course is paramount to maintaining QoL of patients and their families. Symptoms require frequent assessment and proactive management throughout all stages of illness. Distressing symptoms such as seizures and headaches are common and often multifactorial. Studies pertaining to symptom control in brain tumors are scarce and therapeutic approaches are often based on other cancer types or general neurologic treatment paradigms. Future studies are indicated to address brain tumor-specific issues. Brain tumor patients are at elevated risk of impaired medical decision-making early in the disease course, and they often lose their ability to communicate at the end of life. Advance care planning conversations should be initiated early in the disease process by the treating physician. The benefit of early PC has been clearly established in systemic cancers; however, its role in brain cancer patients remains less defined and continues to be understudied. Physician training, patient factors, and the lack of empiric PC frameworks may contribute to the continued underutilization of specialized PC and hospice services. A holistic and multidisciplinary approach with early involvement of specialized PC may aid in optimizing QoL in this population.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C (2017) Barnholtz-Sloan JS (2020) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol 22:iv1–iv96. https://doi.org/10.1093/neuonc/noaa200
Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM (2020) Longer-term (>/= 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep 10:11622. https://doi.org/10.1038/s41598-020-68011-4
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318:2306–2316. https://doi.org/10.1001/jama.2017.18718
Walbert T, Khan M (2014) End-of-life symptoms and care in patients with primary malignant brain tumors: a systematic literature review. J Neurooncol 117:217–224. https://doi.org/10.1007/s11060-014-1393-6
Au TH, Willis C, Reblin M, Peters KB, Nghiemphu PL, Taylor JW, Colman H, Cohen AL, Ormond DR, Chakravarti A, Willmarth N, Menon J, Ma J, Bauer H, Watanabe AH, Ulrich CM, Singh P, Marshall A, Korytowsky B, Stenehjem D, Brixner D (2021) Caregiver burden by treatment and clinical characteristics of patients with glioblastoma. Support Care Cancer. https://doi.org/10.1007/s00520-021-06514-0
Pace A, Dirven L, Koekkoek JAF, Golla H, Fleming J, Rudà R, Marosi C, Le Rhun E, Grant R, Oliver K, Oberg I, Bulbeck HJ, Rooney AG, Henriksson R, Pasman HRW, Oberndorfer S, Weller M, Taphoorn MJB (2017) European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol 18:e330–e340. https://doi.org/10.1016/s1470-2045(17)30345-5
Oliver DJ, Borasio GD, Caraceni A, de Visser M, Grisold W, Lorenzl S, Veronese S, Voltz R (2016) A consensus review on the development of palliative care for patients with chronic and progressive neurological disease. Eur J Neurol 23:30–38. https://doi.org/10.1111/ene.12889
Armstrong TS, Vera-Bolanos E, Acquaye AA, Gilbert MR, Ladha H, Mendoza T (2016) The symptom burden of primary brain tumors: evidence for a core set of tumor- and treatment-related symptoms. Neuro Oncol 18:252–260. https://doi.org/10.1093/neuonc/nov166
Walbert T, Glantz M, Schultz L, Puduvalli VK (2016) Impact of provider level, training and gender on the utilization of palliative care and hospice in neuro-oncology: a North-American survey. J Neurooncol 126:337–345. https://doi.org/10.1007/s11060-015-1973-0
Philip J, Collins A, Brand C, Sundararajan V, Lethborg C, Gold M, Lau R, Moore G, Murphy M (2018) A proposed framework of supportive and palliative care for people with high-grade glioma. Neuro Oncol 20:391–399. https://doi.org/10.1093/neuonc/nox140
Diamond EL, Russell D, Kryza-Lacombe M, Bowles KH, Applebaum AJ, Dennis J, DeAngelis LM, Prigerson HG (2016) Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol 18:78–86. https://doi.org/10.1093/neuonc/nov156
Dropcho EJ (2010) Neurotoxicity of cancer chemotherapy. Semin Neurol 30:273–286. https://doi.org/10.1055/s-0030-1255217
Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, van Es CA, van den Bent MJ (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410. https://doi.org/10.1002/cncr.23562
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384. https://doi.org/10.1148/radiology.217.2.r00nv36377
Gonzalez J, Kumar AJ, Conrad CA, Levin VA (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67:323–326. https://doi.org/10.1016/j.ijrobp.2006.10.010
Dietrich J, Rao K, Pastorino S, Kesari S (2011) Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 4:233–242. https://doi.org/10.1586/ecp.11.1
Lee EQ, Muzikansky A, Drappatz J, Kesari S, Wong ET, Fadul CE, Reardon DA, Norden AD, Nayak L, Rinne ML, Alexander BM, Arvold ND, Doherty L, Stefanik J, LaFrankie D, Ruland SF, Pulverenti J, Smith KH, Gaffey SC, Hammond S, Wen PY (2016) A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro Oncol 18:849–854. https://doi.org/10.1093/neuonc/now007
Rietjens JA, Korfage IJ, Dunleavy L, Preston NJ, Jabbarian LJ, Christensen CA, de Brito M, Bulli F, Caswell G, Cerv B, van Delden J, Deliens L, Gorini G, Groenvold M, Houttekier D, Ingravallo F, Kars MC, Lunder U, Miccinesi G, Mimic A, Paci E, Payne S, Polinder S, Pollock K, Seymour J, Simonic A, Johnsen AT, Verkissen MN, de Vries E, Wilcock A, Zwakman M, van der Heide PA (2016) Advance care planning–a multi-centre cluster randomised clinical trial: the research protocol of the ACTION study. BMC Cancer 16:264. https://doi.org/10.1186/s12885-016-2298-x
Wasade VS, Viarasilpa T, Balki I, Osman G, Gaddam A, Dharaiya D, Pellumbi N, Snyder J, Walbert T, Spanaki M, Schultz L (2020) Effect of seizure timing on long-term survival in patients with brain tumor. Epilepsy & behav : E&B 111:107307. https://doi.org/10.1016/j.yebeh.2020.107307
van Breemen MS, Wilms EB, Vecht CJ (2007) Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 6:421–430. https://doi.org/10.1016/s1474-4422(07)70103-5
Maschio M, Beghi E, Casazza MML, Colicchio G, Costa C, Banfi P, Quadri S, Aloisi P, Giallonardo AT, Buttinelli C, Pauletto G, Striano S, Salmaggi A, Terenzi R, Daniele O, Crichiutti G, Paladin F, Rossi R, Prato G, Vigevano F, De Simone R, Ricci F, Saladini M, Monti F, Casellato S, Zanoni T, Giannarelli D, Avanzini G, Aguglia U, Group BS (2017) Patterns of care of brain tumor-related epilepsy. A cohort study done in Italian Epilepsy Center. PLoS ONE 12:e0180470. https://doi.org/10.1371/journal.pone.0180470
Marcuse LV, Lancman G, Demopoulos A, Fields M (2014) Nonconvulsive status epilepticus in patients with brain tumors. Seizure 23:542–547. https://doi.org/10.1016/j.seizure.2014.04.003
Ruda R, Bello L, Duffau H, Soffietti R (2012) Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol 14(Suppl 4):iv55-64. https://doi.org/10.1093/neuonc/nos199
Vecht CJ, Kerkhof M, Duran-Pena A (2014) Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist 19:751–759. https://doi.org/10.1634/theoncologist.2014-0060
Weller M, Stupp R, Wick W (2012) Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol 13:e375-382. https://doi.org/10.1016/s1470-2045(12)70266-8
Gallagher P, Leach JP, Grant R (2014) Time to focus on brain tumor-related epilepsy trials. Neurooncol Pract 1:123–133. https://doi.org/10.1093/nop/npu010
Pace A, Villani V, Di Lorenzo C, Guariglia L, Maschio M, Pompili A, Carapella CM (2013) Epilepsy in the end-of-life phase in patients with high-grade gliomas. J Neurooncol 111:83–86. https://doi.org/10.1007/s11060-012-0993-2
Walbert T, Harrison RA, Schiff D, Avila EK, Chen M, Kandula P, Lee JW, Le Rhun E, Stevens GHJ, Vogelbaum MA, Wick W, Weller M, Wen PY, Gerstner ER (2021) SNO and EANO practice guideline update: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol. https://doi.org/10.1093/neuonc/noab152
Dewan MC, Thompson RC, Kalkanis SN, Barker FG 2nd, Hadjipanayis CG (2017) Prophylactic antiepileptic drug administration following brain tumor resection: results of a recent AANS/CNS Section on Tumors survey. J Neurosurg 126:1772–1778. https://doi.org/10.3171/2016.4.JNS16245
Fonkem E, Bricker P, Mungall D, Aceves J, Ebwe E, Tang W, Kirmani B (2013) The role of levetiracetam in treatment of seizures in brain tumor patients. Front Neurol 4:153. https://doi.org/10.3389/fneur.2013.00153
Maschio M, Maialetti A, Mocellini C, Domina E, Pauletto G, Costa C, Mascia A, Romoli M, Giannarelli D (2020) Effect of brivaracetam on efficacy and tolerability in patients with brain tumor-related epilepsy: a retrospective multicenter study. Front Neurol 11:813. https://doi.org/10.3389/fneur.2020.00813
Vecht C, Duran-Pena A, Houillier C, Durand T, Capelle L, Huberfeld G (2017) Seizure response to perampanel in drug-resistant epilepsy with gliomas: early observations. J Neurooncol 133:603–607. https://doi.org/10.1007/s11060-017-2473-1
Giammalva GR, Iacopino DG, Azzarello G, Gaggiotti C, Graziano F, Guli C, Pino MA, Maugeri R (2018) End-of-life care in high-grade glioma patients. The Palliative and Supportive Perspective. Brain Sci 8(7):125. https://doi.org/10.3390/brainsci8070125
Koekkoek JA, Postma TJ, Heimans JJ, Reijneveld JC, Taphoorn MJ (2016) Antiepileptic drug treatment in the end-of-life phase of glioma patients: a feasibility study. Support Care Cancer 24:1633–1638. https://doi.org/10.1007/s00520-015-2930-3
Loghin M, Levin VA (2006) Headache related to brain tumors. Curr Treat Options Neurol 8:21–32. https://doi.org/10.1007/s11940-996-0021-y
Nelson S, Taylor LP (2014) Headaches in brain tumor patients: primary or secondary? Headache 54:776–785. https://doi.org/10.1111/head.12326
Vázquez-Barquero A, Ibáñez FJ, Herrera S, Izquierdo JM, Berciano J, Pascual J (1994) Isolated headache as the presenting clinical manifestation of intracranial tumors: a prospective study. Cephalalgia 14:270–272. https://doi.org/10.1046/j.1468-2982.1994.1404270.x
Schankin CJ, Ferrari U, Reinisch VM, Birnbaum T, Goldbrunner R, Straube A (2007) Characteristics of brain tumour-associated headache. Cephalalgia 27:904–911. https://doi.org/10.1111/j.1468-2982.2007.01368.x
Forsyth PA, Posner JB (1993) Headaches in patients with brain tumors: a study of 111 patients. Neurology 43:1678–1683. https://doi.org/10.1212/wnl.43.9.1678
Purdy RA, Kirby S (2004) Headaches and brain tumors. Neurol Clin 22:39–53. https://doi.org/10.1016/s0733-8619(03)00099-9
Pfund Z, Szapáry L, Jászberényi O, Nagy F, Czopf J (1999) Headache in intracranial tumors. Cephalalgia 19:787–790; discussion 765. https://doi.org/10.1046/j.1468-2982.1999.1909787.x
Goffaux P, Fortin D (2010) Brain tumor headaches: from bedside to bench. Neurosurgery 67:459–466. https://doi.org/10.1227/01.Neu.0000372092.96124.E6
Lutman B, Bloom J, Nussenblatt B, Romo V (2018) A contemporary perspective on the management of post-craniotomy headache and pain. Curr Pain Headache Rep 22:69. https://doi.org/10.1007/s11916-018-0722-4
The International Classification of Headache Disorders (2013) 3rd edition (beta version). Cephalalgia 33:629–808. https://doi.org/10.1177/0333102413485658
Soumekh B, Levine SC, Haines SJ, Wulf JA (1996) Retrospective study of postcraniotomy headaches in suboccipital approach: diagnosis and management. Am J Otol 17:617–619
Gee JR, Ishaq Y, Vijayan N (2003) Postcraniotomy headache. Headache 43:276–278. https://doi.org/10.1046/j.1526-4610.2003.03053.x
Flexman AM, Ng JL, Gelb AW (2010) Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol 23:551–557. https://doi.org/10.1097/ACO.0b013e32833e15b9
Vegh T (2013) Diclofenac administered before skull operations reduces the severity of headache after the intervention, NCT01907984. https://clinicaltrials.gov/ct2/show/NCT01907984. Accessed September 29 2021
MacKenzie HM, Teasell R, Miller TA, Sequeira K (2015) Peri-incisional botulinum toxin for chronic postcraniotomy headache after traumatic brain injury: a case series. PM R 7:785–788. https://doi.org/10.1016/j.pmrj.2015.02.015
Valentinis L, Tuniz F, Valent F, Mucchiut M, Little D, Skrap M, Bergonzi P, Zanchin G (2010) Headache attributed to intracranial tumours: a prospective cohort study. Cephalalgia 30:389–398. https://doi.org/10.1111/j.1468-2982.2009.01970.x
Kahn LH, Alderfer RJ, Graham DJ (1997) Seizures reported with tramadol Jama 278:1661
Perloff MD (2015) Practical considerations in opioid use for brain neoplasm. Contin (Minneapolis, Minn) 21:480–486. https://doi.org/10.1212/01.CON.0000464183.35322.5f
Bennett SR, Cruickshank G, Lindenmeyer A, Morris SR (2016) Investigating the impact of headaches on the quality of life of patients with glioblastoma multiforme: a qualitative study. BMJ Open 6:e011616. https://doi.org/10.1136/bmjopen-2016-011616
Noll KR, Walbert T, Wefel JS (2020) Impaired neurocognitive function in glioma patients: from pathophysiology to novel intervention strategies. Curr Opin Neurol 33:716–722. https://doi.org/10.1097/WCO.0000000000000865
Coomans MB, van der Linden SD, Gehring K, Taphoorn MJB (2019) Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol 31:540–547. https://doi.org/10.1097/CCO.0000000000000581
Noll KR, Sullaway C, Ziu M, Weinberg JS, Wefel JS (2015) Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol 17:580–587. https://doi.org/10.1093/neuonc/nou233
Wefel JS, Noll KR, Scheurer ME (2016) Neurocognitive functioning and genetic variation in patients with primary brain tumours. Lancet Oncol 17:e97–e108. https://doi.org/10.1016/s1470-2045(15)00380-0
Cordes MC, Scherwath A, Ahmad T, Cole AM, Ernst G, Oppitz K, Lanfermann H, Bremer M, Steinmann D (2014) Distress, anxiety and depression in patients with brain metastases before and after radiotherapy. BMC Cancer 14:731. https://doi.org/10.1186/1471-2407-14-731
McTyre E, Scott J, Chinnaiyan P (2013) Whole brain radiotherapy for brain metastasis. Surg Neurol Int 4:S236-244. https://doi.org/10.4103/2152-7806.111301
Marsh JC, Godbole R, Diaz AZ, Gielda BT, Turian JV (2011) Sparing of the hippocampus, limbic circuit and neural stem cell compartment during partial brain radiotherapy for glioma: a dosimetric feasibility study. J Med Imaging Radiat Oncol 55:442–449. https://doi.org/10.1111/j.1754-9485.2011.02282.x
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D, Radiation Therapy Oncology G (2013) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 15:1429–1437. https://doi.org/10.1093/neuonc/not114
Rodríguez de Dios N, Couñago F, Murcia-Mejía M, Rico-Oses M, Calvo-Crespo P, Samper P, Vallejo C, Luna J, Trueba I, Sotoca A, Cigarral C, Farré N, Manero RM, Durán X, Gispert JD, Sánchez-Benavides G, Rognoni T, Torrente M, Capellades J, Jiménez M, Cabada T, Blanco M, Alonso A, Martínez-San Millán J, Escribano J, González B, López-Guerra JL (2021) Randomized phase III trial of prophylactic cranial irradiation with or without hippocampal avoidance for small-cell lung cancer (PREMER): a GICOR-GOECP-SEOR study. J Clin Oncol 39:3118–3127. https://doi.org/10.1200/jco.21.00639
Rapp SR, Case LD, Peiffer A, Naughton MM, Chan MD, Stieber VW, Moore DF Jr, Falchuk SC, Piephoff JV, Edenfield WJ, Giguere JK, Loghin ME, Shaw EG (2015) Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol 33:1653–1659. https://doi.org/10.1200/JCO.2014.58.4508
Fried TR, Bradley EH, Towle VR, Allore H (2002) Understanding the treatment preferences of seriously ill patients. N Engl J Med 346:1061–1066. https://doi.org/10.1056/NEJMsa012528
Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733–742. https://doi.org/10.1056/NEJMoa1000678
Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, Firn JI, Paice JA, Peppercorn JM, Phillips T, Stovall EL, Zimmermann C, Smith TJ (2017) Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:96–112. https://doi.org/10.1200/JCO.2016.70.1474
The Lancet N (2021) Editorial: New hope for advancing neuropalliative care. The Lancet Neurology 20(6):409. https://doi.org/10.1016/s1474-4422(21)00142-3
Oliver D, Borasio GD, Veronese S, Voltz R, Lorenzl S, Hepgul N (2020) Current collaboration between palliative care and neurology: a survey of clinicians in Europe. BMJ Support Palliat Care. https://doi.org/10.1136/bmjspcare-2020-002322
Creutzfeldt CJ, Kluger B, Kelly AG, Lemmon M, Hwang DY, Galifianakis NB, Carver A, Katz M, Curtis JR, Holloway RG (2018) Neuropalliative care: priorities to move the field forward. Neurology 91:217–226. https://doi.org/10.1212/WNL.0000000000005916
Forst D, Adams E, Nipp R, Martin A, El-Jawahri A, Aizer A, Jordan JT (2018) Hospice utilization in patients with malignant gliomas. Neuro Oncol 20:538–545. https://doi.org/10.1093/neuonc/nox196
Pace A, Koekkoek JAF, van den Bent MJ, Bulbeck HJ, Fleming J, Grant R, Golla H, Henriksson R, Kerrigan S, Marosi C, Oberg I, Oberndorfer S, Oliver K, Pasman HRW, Le Rhun E, Rooney AG, Ruda R, Veronese S, Walbert T, Weller M, Wick W, Taphoorn MJB, Dirven L (2020) Determining medical decision-making capacity in brain tumor patients: why and how? Neurooncol Pract 7:599–612. https://doi.org/10.1093/nop/npaa040
Fritz L, Dirven L, Reijneveld JC, Koekkoek JA, Stiggelbout AM, Pasman HR, Taphoorn MJ (2016) Advance care planning in glioblastoma patients. Cancers (Basel) 8https://doi.org/10.3390/cancers8110102
Chiarchiaro J, Buddadhumaruk P, Arnold RM, White DB (2015) Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc 12:1528–1533. https://doi.org/10.1513/AnnalsATS.201504-253OC
Sizoo EM, Pasman HR, Dirven L, Marosi C, Grisold W, Stockhammer G, Egeter J, Grant R, Chang S, Heimans JJ, Deliens L, Reijneveld JC, Taphoorn MJ (2014) The end-of-life phase of high-grade glioma patients: a systematic review. Support Care Cancer 22:847–857. https://doi.org/10.1007/s00520-013-2088-9
Chochinov HM (2006) Dying, dignity, and new horizons in palliative end-of-life care. CA Cancer J Clin 56:84–103; quiz 104–105. https://doi.org/10.3322/canjclin.56.2.84
Goswami P (2021) Advance care planning and end-of-life communications: practical tips for oncology advanced practitioners. J Adv Pract Oncol 12:89–95. https://doi.org/10.6004/jadpro.2021.12.1.7
Cottrell L, Economos G, Evans C, Silber E, Burman R, Nicholas R, Farsides B, Ashford S, Koffman JS (2020) A realist review of advance care planning for people with multiple sclerosis and their families. PLoS ONE 15:e0240815. https://doi.org/10.1371/journal.pone.0240815
Gofton TE, Chum M, Schulz V, Gofton BT, Sarpal A, Watling C (2018) Challenges facing palliative neurology practice: a qualitative analysis. J Neurol Sci 385:225–231. https://doi.org/10.1016/j.jns.2017.12.008
Walbert T, Puduvalli VK, Taphoorn MJB, Taylor AR, Jalali R (2015) International patterns of palliative care in neuro-oncology: a survey of physician members of the Asian Society for Neuro-Oncology, the European Association of Neuro-Oncology, and the Society for Neuro-Oncology. Neurooncol Pract 2:62–69. https://doi.org/10.1093/nop/npu037
Parker G (2007) Patients’ perceptions of palliative care: a pilot study. Palliat Med 21:59–60. https://doi.org/10.1177/0269216306073132
Haider SA, Asmaro K, Kalkanis SN, Lee IY, Bazydlo M, Nerenz DR, Salloum RG, Snyder J, Walbert T (2020) The economic impact of glioma survivorship: the cost of care from a patient perspective. Neurology 95:e1575–e1581. https://doi.org/10.1212/wnl.0000000000010263
Page MS, Chang SM (2017) Creating a caregiver program in neuro-oncology. Neurooncol Pract 4:116–122. https://doi.org/10.1093/nop/npw019
Applebaum AJ, Kryza-Lacombe M, Buthorn J, DeRosa A, Corner G, Diamond EL (2016) Existential distress among caregivers of patients with brain tumors: a review of the literature. Neurooncol Pract 3:232–244. https://doi.org/10.1093/nop/npv060
Ford E, Catt S, Chalmers A, Fallowfield L (2012) Systematic review of supportive care needs in patients with primary malignant brain tumors. Neuro Oncol 14:392–404. https://doi.org/10.1093/neuonc/nor229
Boele FW, Given CW, Given BA, Donovan HS, Schulz R, Weimer JM, Drappatz J, Lieberman FS, Sherwood PR (2017) Family caregivers’ level of mastery predicts survival of patients with glioblastoma: a preliminary report. Cancer 123:832–840. https://doi.org/10.1002/cncr.30428
Fortunato JT, Van Harn M, Haider SA, Phillips J, Walbert T (2021) Caregiver perceptions of end-of-life care in patients with high-grade glioma. Neurooncol Pract 8:171–178. https://doi.org/10.1093/nop/npaa077
Funding
This study was supported by the Department of Neurosurgery and the Hermelin Brain Tumor Center, Henry Ford Health System.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
None required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stec, N.E., Walbert, T. Neuro-oncology and supportive care: the role of the neurologist. Neurol Sci 43, 939–950 (2022). https://doi.org/10.1007/s10072-021-05862-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05862-3