Abstract
Background and objectives
Cognitive symptoms are common in Parkinson’s disease (PD) and affect patients’ quality of life. Pharmacological interventions often do not improve such deficits that might benefit of cognitive rehabilitation. However, previous meta-analysis on this topic reported inconsistent results. Clarifying the efficacy of cognitive rehabilitation would be pivotal to optimize treatment and reduce care’s costs. This meta-analysis aims at determining whether current literature lays in favor of the effectiveness of cognitive rehabilitation in PD and at understanding whether its effect might change depending on the trained cognitive domain.
Methods
We searched online databases for studies concerning cognitive rehabilitation in PD. Fourteen studies encompassing 767 participants were included. Analyses were conducted for each cognitive domain separately, examining several neuropsychological measures for each function.
Results
We found that rehabilitation improves global cognition, executive functions, and long- and short-term memory.
Conclusion
The current body of research indicates that cognitive rehabilitation improves specific cognitive deficits in PD and that it should be tailored on patients’ specific impairments. These interventions should be employed considering that not all the cognitive domains might benefit of a cognitive training. Finally, the high heterogeneity among studies suggests the need for more controlled clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second-most common neurodegenerative disorder that affects 2–3% of the population ≥ 65 years of age. Although clinical diagnosis relies on the presence of bradykinesia and other motor features, PD is associated with many non-motor features that add to overall disability [1, 2]. Among the non-motor symptoms, cognitive deficits are probably the most relevant, as they affect patients’ autonomy, increase caregiver burden and wield a considerable socio-economic impact [3]. As reported by the Movement Disorder Society, nearly one-third of non-demented patients with PD are affected by mild cognitive impairment (MCI) [4, 5]. The cognitive profile of these patients is characterized by executive deficits, with specific impairments in attention, processing speed, working memory, set-shifting, and planning [6]. Additionally, these patients might exhibit also deficits in other cognitive domains such as visuospatial abilities, memory, or language [7].
Although dopamine replacement medications and deep brain stimulation ameliorate motor symptoms, they are less effective in the treatment of cognitive deficits [8]. Non-pharmacological interventions focused on the neuropsychological aspects could play a pivotal role for patients’ well-being. Among non-pharmacological interventions, cognitive rehabilitation and cognitive trainings include behavioral interventions aimed at reducing cognitive impairment and straighten cognition [9]. In particular, cognitive trainings refer to the teaching of strategies and to the execution of specific tasks targeting cognitive functions [10]. Although the mechanism of action has not been fully clarified yet, it has been suggested that neuroplasticity processes might account for the beneficial effect observed after cognitive trainings [11]. Probably by inducing changes in the pattern of brain activation and in the gray matter volume [12], these treatments have the potential to slow down symptom progressions and help patients to maintain a high level of autonomy and quality of life [13]. Accordingly, a previous meta-analysis reported that cognitive trainings are effective in improving cognitive functioning in PD patients. Namely, in this study, the authors found that cognitive training improves working memory, processing speed, and executive functions [14]. However, the literature has grown since the publication of this study. Conversely, a more recent meta-analysis [15] reviewed the effects of cognitive trainings in patients with PD-MCI and dementia (PD-D). The authors did not find evidence of any important cognitive improvement after cognitive training. However, the inclusion of studies conducted in patients with PD-D makes these two previous meta-analyses not directly comparable. Indeed, there is a crucial difference in the employment of cognitive trainings in MCI and in demented patients. In patients with MCI, cognitive trainings have the aim of slowing the progression into dementia, whereas in patients with dementia, trainings are employed with the aim of stimulating or compensating more severe cognitive decline.
To date, questions remain as to whether cognitive rehabilitation is effective in improving cognitive functions in PD-MCI when compared to other interventions. As 20% of PD patients present cognitive impairments that might turn into dementia [16], clarifying whether cognitive trainings could be used to manage cognitive symptoms in order to slow down the progression into dementia and to maintain cognitive function would be pivotal for both research and clinical purposes. Additionally, the large percentage of people with PD-MCI at diagnosis, and the societal cost, as well as timely access to high-quality neuropsychological care is important to control, maintain, or increase the quality of life. However, to date, conflicting evidence for efficacy is present in literature. Therefore, the aim of the present study was to investigate whether cognitive rehabilitation improves cognition in PD-MCI patients and, if so, which cognitive domains mostly benefit from the rehabilitation. To this end, we have carried out a systematic review and meta-analysis of previous studies conducted on PD-MCI patients and analyzed behavioral outcomes for each cognitive domain separately.
Methods
Search strategy and study selection
This meta-analysis was conducted following the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) [17] statement and was registered with PROSPERO, number CRD42020210652.
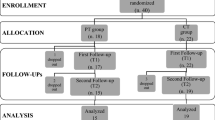
We searched in the following online databases for published articles and registered trials: Cochrane, EMBASE, SCOPUS, and PubMed from 01/2000 to 09/2020. Namely, we used the following keywords: “Parkinson’s disease,” “cognitive rehabilitation,” “cognitive training,” “cognitive treatment,” and “neuropsychological measures.” We also screened the bibliography of previous meta-analyses as well as of all the included studies (Fig. 1).
Flow diagram based on PRISMA Statement [32]
Studies to be included had to investigate changes in cognitive tests in PD-MCI patients before and after a cognitive training compared to a control treatment. In particular, we selected studies that reported individual cognitive trainings counting at least 10 total sessions.
To be included, candidate studies had to meet the following criteria:
-
Being at least single-blind;
-
Sample size ≥ 20 participants;
-
Participants recruited had idiopathic PD diagnosed by using the UK’s Parkinson’s disease society Brain Bank clinical criteria;
-
Participants exhibiting deficits in at least one cognitive domain;
-
Not including patients with dementia;
-
Using cognitive rehabilitation;
-
The effect of the intervention was measured before and after the treatment by using standardized neuropsychological tests;
-
The article was written in English.
Data extraction and risk of bias assessment
Candidate studies were excluded whether they enrolled patients with other neurological or systemic pathologies, whether patients were unblinded or whether means and standard deviations were not reported. If potentially eligible studies did not report means and standard deviations, the corresponding authors were contacted by email and in case of no response in the following two weeks, the study was excluded from the analysis.
Two authors independently screened the article titles and abstracts collected from the database search. Only articles meeting the inclusion criteria were selected. Any doubt or disagreement was discussed and solved among all the authors.
Selected articles were read by one author that extracted relevant information such as participants, interventions, comparisons, outcomes, and study design (PICOS). Additionally, data relative to sample characteristic, methods, and results were extracted. Cognitive outcomes were categorized for the following domains: global cognition, executive function, attention, short term and long-term memory, language, visuospatial processes, and clinical scales. Means and standard deviations were extracted for each outcome before and after the intervention. The Cochrane tool was used to assess the risk of bias [18] (Figure 1 of the supplementary materials). This tool categorizes studies as having low, high, or unclear risk of bias. The specific domains investigated to assess risk of bias are the following: the appropriateness of sequences generation, the allocation concealment, the blindness of participants and experimenters, and the presence of missing or incomplete data.
Data synthesis and analyses
Analyses were performed using R Studio 4.0.2 [19]. The statistical significance was set at p <0.05; for the test relating to publication bias, a p <0.10 was considered significant.
Effects of cognitive training were analyzed separately for each domain considered. Studies using more than one test per cognitive domain were treated as separate studies [20]. Standardized mean differences (SMD) were calculated with Hedges’ g method. Data relative to cognitive tests were adjusted taking into account length and direction of the relative scale in order to allow a comparison among different tests [21].
Heterogeneity among studies was assessed using the total Cochrane Q test (Q) that evaluates if the variability among effect sizes is greater than expected. A significant Q value indicated a lack of homogeneity among studies. Additionally, the I-square inconsistency index (I2) was used to quantify the percentage of true variability in the observed effects (true heterogeneity). I2 values were classified as low (25–50%), moderate (50–75%), or high (75%) [22]. In the case of high heterogeneity, data were pooled using a meta-analytic method based on a random effect model with the Knapp-Hartung adjustment method (HKSJ) [21]. An influence analysis was conducted by using the Baujat Plot and the Leave-one-out method to detect influential cases. A Gosh analysis was performed to individuate and remove outliers among influential cases. Forest plots were generated for each meta-analysis [23]. Publication bias was assessed by performing and inspecting a contour-enhanced funnel plot and by running the Egger’s t-test [24].
Results
Included studies: main characteristics
A total of 14 studies including 767 patients met the inclusion criteria. All studies but one [25] were randomized clinical trials. In the included studies, patients were enrolled if they: i) had an impairment in an objective cognitive test of executive functions but were not demented as assessed with the scales for outcomes in PD cognition scale [26]; ii) had a mini mental state examination (MMSE) score > 24 and absence of diagnosis of dementia [27, 28]; or iii) had a mild to moderate cognitive impairment but not dementia [29, 30]; iv) had a MMSE < 25 but they had not dementia [31,32,33]; v) had a diagnosis of MCI in accordance with Petersen’s criteria [34]. Four studies mentioned as inclusion criteria an overall absence of dementia [25, 35,36,37]. One study included only participants with MCI using established diagnostic criteria [28] and 1 study enrolled participants with single or multiple domain MCI including executive dysfunction [38]. With respect to the treatment, 7 studies compared cognitive trainings with other control conditions [27, 29, 31, 34, 36]. One study used the cognitive training as control condition whereas the experimental group underwent to a virtual reality based training [30]. Two studies used cognitive rehabilitation as control to test whether combining cognitive training with motor exercises induced an improvement in cognition [28, 33]. Another group of studies compared the effect of domain specific cognitive rehabilitation with not specific cognitive trainings [26] or active control conditions [37] (see Table 1). With respect to studies included in the analyses, 5 studies where included for global cognitive functioning [28, 30,31,32, 34, 38], 8 studies contributed to the analysis of attention [25, 26, 28, 30, 31, 34, 35, 38]; 9 studies were included in the meta-analysis for executive functions [25, 26, 28, 30, 34, 35, 37, 38]; 5 studies contributed to the analysis of language [30, 31, 33, 34, 38]; 8 studies were used for the long term memory [25, 30, 31, 33,34,35, 37, 38]; 7 studies contributed to the analysis relative to short term memory [30, 31, 33,34,35, 37, 38]; 5 studies were used for visuospatial abilities [27, 30, 31, 34, 38] and 9 studies contributed to the analysis of the clinical scales [25,26,27,28, 31, 33,34,35, 37] (Table 1 of the supplementary materials).
Global cognition
We pooled data from 5 studies [28, 30, 31, 34, 38] with 314 participants. As studies reporting several cognitive tests for each domain were considered separately, the total number of cases included in the analysis was 12 and the final sample size of the meta-analysis was of 632 participants. Heterogeneity was high (pQ< 0.01; tau2 = 48.02; and I2 = 97.8%). The influence analysis revealed that 9 studies were influential cases. After performing the Gosh analysis, 6 studies were identified as outliers and were removed from the analysis (see Fig. 2a: París a-b, 2011; Maggio a-b, 2018; Reuter a, 2012; Alloni b, 2018). After having removed the outliers, we did not find a significant decrease in heterogeneity (pQ< 0.01; tau2 = 6.33; I2 = 97.5%) but we observed a significant difference between the experimental group and the control group in favor of the experimental group. Although the funnel plot was asymmetrical (Fig. 2b) [35, 40], the Egger’s regression intercept revealed the absence of publication bias (β = −18.09; 95% CI = [−35.81; −0.37]; p = 0.12). This inconsistency is probably due to the numerical scarcity and high heterogeneity of the included studies.
Attention
Data were pooled from 8 studies [25, 26, 28, 30, 31, 34, 35, 38] with 409 participants. As studies reporting several cognitive tests for each domain were considered separately, the total number of cases included in the analysis was 17 and the final sample size of the metanalysis was of 747 participants. Due to the high heterogeneity (pQ < 0.01; tau2 = 12.54; and I2 = 93.6%), we applied a random effect model. The influence analysis revealed that 3 studies had an influential role biasing the results of the analysis. After performing the Gosh analysis, 4 outliers were identified and were removed (see Fig. 3a: Vlagsma a-b, 2020; Petrelli, 2014; Reuter, 2012), leading to a significant reduction in heterogeneity (pQ =1; tau2 = 0.0006; I2 = 0.0%). Although the general effect shows that there is a difference between cognitive training and control treatment in favor of the cognitive training, none of the studies individually reaches significance. The funnel plot was symmetrical (Fig. 3b). The Egger’s regression intercept revealed no publication bias (β = −0.16; 95% CI = [−1.62; 1.3]; p = 0.83).
Executive functions
Data were pooled by 9 studies [25, 26, 28, 30, 31, 34, 35, 37, 38] with 461 participants. As studies reporting several cognitive tests were considered separately, the total number of cases included in the analysis was 27 and the final sample size of the metanalysis was of 1341 participants. Due to the presence of high heterogeneity (pQ< 0.0001; tau2 = 275.58; I2 = 97.9%), a random effect model was used. The influential analysis revealed that 9 studies were influential cases, of which 6 outliers were removed by performing the Gosh analysis (see Fig. 4a: Petrelli, 2014; Reuter a-b-c, 2012; Fellman a-b, 2018). We found that the reduction in heterogeneity was not significant (pQ< 0.01; tau2 = 1.80; I2 = 85.7%) but we did find a significant difference between the cognitive training and the control intervention in favor of the cognitive training. The funnel plot suggests the presence of publication bias (Figure 4B) [2, 14, 25, 33]. The bias was confirmed by the Egger’s regression intercept (β = −8.15; 95% CI= −10.91; −5.39]; p < 0.001).
Language
Data were pooled by 5 studies [30, 31, 33, 34, 38] with 177 participants. As studies reporting several cognitive tests were considered single studies, the total number of cases included in the analysis was 7 and the final sample size of the meta-analysis was of 336 participants. Due to the high heterogeneity (pQ< 0.01; tau2= 5.33; and I2= 91.7%), a random effect model was applied. The influential analysis revealed that 3 studies had an extreme effect size. After having performed the Gosh analysis, 1 study was identified as outlier and was removed from the analysis (see Figure 5A: Petrelli et al., 2014), leading to a significant decrease in heterogeneity (pQ= 0.02; tau2= 0.338; and I2= 63.8%). We did not find evidence in favor of the experimental group. The funnel plot showed some publication biases (Figure 5B) [33]. However, the Egger’s regression intercept was not significant (β = −2.38; 95% CI= [−10.65; 5.89]; p = 0.60). This inconsistency might be due to the numerical scarcity of the studies and their high heterogeneity.
Forest plot (A) and funnel plot (B) of language. The funnel plot shows the presence of a publication bias [33] not confirmed by the Egger's regression.
Long-term memory
Data were pooled by 8 studies [25, 30, 31, 33,34,35, 37, 38] with a total of 225 participants. As studies reporting several cognitive tests were considered separately, analyses were performed on 19 cases and the final sample size of the metanalysis was of 706 participants. Due to the high heterogeneity (pQ< 0.01; tau2= 56.32; and I2= 93.6%), a random effect model was applied. The influential analysis revealed that 5 studies had an extreme effect size. After having applied the Gosh analysis, the study of Petrelli and colleagues was identified as outlier (see Figure 6A Petrelli et al., 2014). After having removed the outlier, we found a significant reduction in heterogeneity (pQ< 0.01; tau2 = 0.584; I2 = 78.8%) as well as a significant difference between the experimental and control group. The funnel plot was asymmetrical and the Egger’s regression intercept was significant (β = −6.49; 95% CI = [−12.85; −0.14; p = 0.07]). These results indicated the presence of publication bias (Figure 6B) [2, 14, 34].
Short-term memory
Data were pooled from 7 studies [30, 31, 33,34,35, 37, 38] with a total of 219 participants. As studies reporting several cognitive tests were considered separately, analyses were performed on 17 cases and the final sample size of the metanalysis was of 606 participants. Due to the high heterogeneity (pQ< 0.01; tau2 = 2595.13; and I2 = 95.9%), a random effect model was applied. The influential analysis revealed that 6 studies had an extreme effect size. After having applied the Gosh analysis, the study of Petrelli was identified as outlier and was removed from the analysis (see Figure 7A: Petrelli, 2014), leading to a significant reduction in heterogeneity (pQ = <0.01; tau2 =3.25; I2= 91.5%). Overall, the results for the random effects model without outliers showed a significant difference between the experimental and the control group in favor of the experimental group. The funnel plot was asymmetrical and the Egger’s regression intercept was significant (β = −9.02; 95% CI = [−13.44; −4.6]; p =0.0017). These results confirm the presence of publication bias (Figure 7B) [34, 37, 38].
Visuo-spatial abilities
Data were pooled by 5 studies [27, 30, 31, 34, 38] with 197 participants. As studies reporting several cognitive tests were considered separately, analyses were performed on 6 cases and the final sample size of the metanalysis was of 225 participants. Due to the high heterogeneity (pQ< 0.01; tau2 = 2.89; and I2 = 93.1%), we applied the random effect model. The influential analysis revealed that 5 studies were influential cases, and 2 studies were outliers (see Figure 8A: Edwards, 2013; Petrelli, 2014). After having removed the outliers by using the Gosh analysis, we found a significant reduction in heterogeneity (pQ< 0.01; tau2 = 1.42; I2 = 84.8%). The result of the meta-analysis revealed no significant differences between the control and the experimental group. The funnel plot was asymmetrical and suggested the presence of publication biases (Figure 8B) [25, 33]. This bias was confirmed by the significance of the Egger’s regression intercept (β = −9.1; 95% CI = [−13.48; −4.72]; p = 0.06).
Clinical scales
Data were pooled by 9 studies [25, 27, 28, 31, 33,34,35, 37] with 549 participants. As studies reporting several cognitive test were considered separately, analyses were performed on 19 cases and the final sample size of the metanalysis was of 867 participants. Due to the high heterogeneity (pQ< 0.01; tau2= 446.82; and I2= 96.5%), a random effect model was applied. The influential analysis revealed that 7 studies had an extreme effect size. The Gosh analysis identified 9 outliers, which were removed from the analysis (see Figure 9A: Barboza; Naismit a; Parìs a-b; Petrelli b; Peña a-c; Reuter; Vlagsma). After having removed the outliers, we found a heterogeneity increase (pQ< 0.01; tau2= 232.44; I2= 98.1%) but no significant differences between the experimental and control group. The asymmetry of the funnel plot shows that there were publication biases (Figure 9B). This result was confirmed by the significance of the Egger’s regression intercept (β= −8.417; 95% CI= [−13.9; −2.93]; p= 0.024) [25, 35, 31].
Discussion
The aim of the present study was to assess the efficacy of cognitive rehabilitation in PD patients. Our results show a selective effect of cognitive trainings in cognition. In particular, we found a beneficial effect of these trainings in global functioning, in executive functions, and in short- and long-term memory. On the other hand, we did not find any beneficial effect in attention, in visuospatial abilities, nor in clinical scales. Overall, our results are in line with previous studies reporting that cognitive rehabilitation could be effective in ameliorating cognition in PD [14]. However, not all the cognitive functions might benefit from the training and the specific cognitive status should be taken into account in order to adapt trainings to the patients’ needs. Indeed, the profile of PD patients with cognitive impairment is variable, both in the affected functions and in the domains impaired first [39].
Previous meta-analyses reported conflicting results [14, 15]. Namely, Leung [14] found that cognitive rehabilitation might have a beneficial impact on cognitive functions, whereas Orgeta [15] did not confirm these findings. Our results insert themselves in this strand of research. Indeed, with respect to the meta-analysis of Leung, our work includes more recent researches whereas, with respect to the metanalysis of Orgeta, we did not include patients with dementia as the severity of the disease might influence the effects of the trainings.
In the present study, we found a significant effect of cognitive trainings in global cognition as measured with several neuropsychological tests. Despite this result should be taken cautiously due to the high heterogeneity of the studies in this domain, it is encouraging. Indeed, improving global cognition is one of the main goals of each cognitive rehabilitation. However, this finding is in contrast with the results reported by two previous meta-analyses conducted by Leung [14] and Orgeta [15], respectively. This discrepancy might be due to differences in studies’ selection. Indeed, we included a larger pool of studies compared to these two previous works. Additionally, studies included in the meta-analysis of Leung mostly assessed global functioning through the MMSE. Similarly, Orgeta and colleagues assessed the effect on global cognition selecting only MMSE scores. On the contrary, in our meta-analysis, global cognition was assessed through several cognitive tests and not limited to the MMSE.
MMSE is not the elective tool for the assessment of cognitive status in PD patients; indeed, it has not enough discriminant validity for the detection of cognitive disorders [40]. The lack of sensitivity of the MMSE might have affected the results of previous studies, reducing the detection of cognitive changes. Indeed, other tests have been recommended for the screening of PD, such as the Montreal Cognitive Assessment and the Parkinson’s disease cognitive rating scale [41]. The inclusion of other scales measuring the global cognitive status in the present meta-analysis probably increased the chance to detect the presence of a cognitive deficit and its relative change after the training.
Global cognition constitutes an indicator of the overall patients’ cognitive status and its assessment often guides clinical practice. Therefore, improving global cognition could greatly affect medical choices with respect to the best practice for the treatment of PD as well as patients’ quality of life.
Besides global cognition, we found a beneficial effect of cognitive training in executive functions. As deficits in this domain are common in patients with PD [42] and play an important role in everyday activities, the evidence that they improved in response to cognitive training is encouraging and suggests the need to further explore this strand of research. The improvement in executive functions is in line with previous studies [14]. Nevertheless, Orgeta and colleagues did not find any evidence of an improvement in executive functions after cognitive rehabilitation. In this last study, executive functions were analyzed by taking into account the performance in two specific executive tests that are the Trail Making Test and the Stocking of Cambridge test. In the present study, other tests have been included such as the Frontal Assessment Battery and the Stroop test. The inclusion of a wider variety of tests might account for our effect in executive functions. Overall, these results highlight that cognitive intervention might be a fundamental treatment to improve patients’ functioning. Due to the relevance that executive functions play in the most common daily life activities, and their role in implementing strategies needed to overcome motor deficits [43], such improvement suggests the need for further studies investigating effects of cognitive training in this pathological population.
We found a beneficial effect of cognitive training in short- and long-term memory. This result is not in line with previous meta-analyses, where no changes in memory were found [14, 15]. In these previous studies, memory domain counted both short- and long-term memory tests. Additionally, in the study of Orgeta, only the verbal component of memory was analyzed. These differences might account for the mismatch in the results. On the other hand, also the different pool and number of studies included in the meta-analyses (9 in our study vs. 5 in Leung’s and Orgeta) might account for the discrepancy in the results. Finally, another possible explanation is that in the present meta-analysis, we included studies focusing on specific memory trainings [25, 28]. Such inclusion might have strengthened the effects in memory domain. In line with these results, cognitive trainings have been found to be overall effective in the treatment of memory impairment in many pathological populations [44].
In line with previous works, we did not find evidence in favor of cognitive treatments in attention. Indeed, although the general effect was in favor of cognitive trainings, none of the studies individually reached significance, probably due to their limited sample sizes. This trend is encouraging and should be further explored in future studies. Additionally, we did not find evidence in favor of cognitive treatments in visuospatial abilities nor in clinical scales. This might be probably due to the lack of training focusing on these aspects.
Due to the lack of efficacy of pharmacological therapies in PD cognitive symptoms, it is important to develop new treatments targeting these common deficits. Cognitive trainings are non-invasive, safe, and low cost; therefore, more studies aimed at improving the quality and the efficacy of cognitive trainings would be needed. For instance, cognitive trainings might be combined with motor and physical activity to reach more stable results and improve quality of life [45]. Additionally, the combination of cognitive intervention in PD patients and psycho-education with caregivers has shown promising results [46].
An important issue that we have to acknowledge is that we observed a high variability among included studies with respect to both the neuropsychological tests used for the diagnosis of MCI and to the criteria used to rule out the presence of dementia. To reduce differences among published studies, more standardized and shared procedures to assess cognitive impairment in PD patients would be needed.
A limitation of the present meta-analysis was that, despite the included studies were conducted on patients with MCI, two of them did not explicitly state the exclusion of patients with dementia. Thus, we cannot exclude that in these studies some of the enrolled participants had dementia.
The number of studies is still low and in turn the small sample size and the high heterogeneity limit the power of the analysis. More studies should be conducted to straighten the power of meta-analysis focusing on this topic.
Conclusion
This meta-analysis suggests that cognitive training overall improves cognitive performance in patients with PD. In particular, global cognition, memory, and executive functions that are generally impaired in these patients benefit from the treatment. These results are encouraging and highlight the pivotal role that cognitive rehabilitation might play in PD. However, to enhance the overall effect and to better understand mechanisms underlying the improvement, future studies employing larger samples, combining rehabilitation with imaging techniques as well as testing novel trainings, are required.
Data availability
Not applicable.
Change history
18 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10072-022-05943-x
References
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Prim. https://doi.org/10.1038/nrdp.2017.13
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.26424
Hiseman JP, Fackrell R (2017) Caregiver Burden and the Nonmotor Symptoms of Parkinson’s Disease. Int Rev Neurobiol. https://doi.org/10.1016/bs.irn.2017.05.035
Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Tröster AI, Weintraub D (2011) MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord 26:1814–1824. https://doi.org/10.1002/mds.23823
Baiano C, Barone P, Trojano L, Santangelo G (2020) Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov Disord. https://doi.org/10.1002/mds.27902
Barone P, Aarsland D, Burn D, Emre M, Kulisevsky J, Weintraub D (2011) Cognitive impairment in nondemented Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.23919
Muslimović D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. https://doi.org/10.1212/01.wnl.0000180516.69442.95
Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, Hametner EM, Poewe W, Rascol O, Goetz CG, Sampaio C (2011) The movement disorder society evidence-based medicine review update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.23884
Bahar-Fuchs A, Martyr A, Goh AMY, Sabates J, Clare L (2019) Cognitive training for people with mild to moderate dementia. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD013069.pub2
Mowszowski L, Batchelor J, Naismith SL (2010) Early intervention for cognitive decline: Can cognitive training be used as a selective prevention technique? Int Psychogeriatrics. https://doi.org/10.1017/S1041610209991748
Chapman SB, Aslan S, Spence JS, Hart JJ, Bartz EK, Didehbani N, Keebler MW, Gardner CM, Strain JF, Defina LF, Lu H (2015) Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex. https://doi.org/10.1093/cercor/bht234
Nombela C, Bustillo PJ, Castell PF, Sanchez L, Medina V, Herrero MT (2011) Cognitive rehabilitation in Parkinson’s disease: Evidence from neuroimaging. Front Neurol. https://doi.org/10.3389/fneur.2011.00082
Calleo J, Burrows C, Levin H, Marsh L, Lai E, York MK (2012) Cognitive rehabilitation for executive dysfunction in Parkinson’s disease: Application and current directions. Parkinsons Dis. https://doi.org/10.1155/2012/512892
Leung IHK, Walton CC, Hallock H, Lewis SJG, Valenzuela M, Lampit A (2015) Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology 85:1843–1851. https://doi.org/10.1212/WNL.0000000000002145
Orgeta V, McDonald KR, Poliakoff E, Hindle JV, Clare L, Leroi I (2020) Cognitive training interventions for dementia and mild cognitive impairment in Parkinson’s disease. Cochrane Database Syst, Rev
Saredakis D, Collins-Praino LE, Gutteridge DS, Stephan BCM, Keage HAD (2019) Conversion to MCI and dementia in Parkinson’s disease: a systematic review and meta-analysis. Park Relat Disord. https://doi.org/10.1016/j.parkreldis.2019.04.020
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160. https://doi.org/10.1136/bmj.n160
Corbett MS, Higgins JPT, Woolacott NF (2014) Assessing baseline imbalance in randomised trials: Implications for the Cochrane risk of bias tool. Res Synth Methods. https://doi.org/10.1002/jrsm.1090
>R Core Team (2020), R: A language and environment for statistical computing., R A Lang. Environ. Stat. Comput. R Found. Stat. Comput. Vienna, Austria. (2020)
Shim SR, Kim SJ (2019) Intervention meta-analysis: application and practice using R software. Epidemiol Health. https://doi.org/10.4178/epih.e2019008
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019). Cochrane handbook for systematic reviews of interventions. https://doi.org/10.1002/9781119536604
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J. https://doi.org/10.1136/bmj.327.7414.557
Lewis S, Clarke M (2001) Forest plots: Trying to see the wood and the trees. Br Med J. https://doi.org/10.1136/bmj.322.7300.1479
Egger M, Smith GD, Phillips AN (1997) Meta-analysis: Principles and procedures. Br Med J. https://doi.org/10.1136/bmj.315.7121.1533
Naismith SL, Mowszowski L, Diamond K, Lewis SJG (2013) Improving memory in Parkinson’s disease: A healthy brain ageing cognitive training program. Mov Disord. https://doi.org/10.1002/mds.25457
Vlagsma TT, Duits AA, Dijkstra HT, van Laar T, Spikman JM (2020) Effectiveness of ReSET; a strategic executive treatment for executive dysfunctioning in patients with Parkinson’s disease. Neuropsychol Rehabil 30:67–84. https://doi.org/10.1080/09602011.2018.1452761
Edwards JD, Hauser RA, O’Connor ML, Valdés EG, Zesiewicz TA, Uc EY (2013) Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology. https://doi.org/10.1212/WNL.0b013e3182a823ba
Reuter I, Mehnert S, Sammer G, Oechsner M, Engelhardt M (2012) Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in parkinsons disease. J Aging Res. https://doi.org/10.1155/2012/235765
De Luca R, Latella D, Maggio MG, Di Lorenzo G, Maresca G, Sciarrone F, Militi D, Bramanti P, Calabrò RS (2019) Computer assisted cognitive rehabilitation improves visuospatial and executive functions in Parkinson’s disease: Preliminary results. NeuroRehabilitation 45:285–290. https://doi.org/10.3233/NRE-192789
Maggio MG, De Cola MC, Latella D, Maresca G, Finocchiaro C, La Rosa G, Cimino V, Sorbera C, Bramanti P, De Luca R, Calabrò RS (2018) What About the Role of Virtual Reality in Parkinson Disease’s Cognitive Rehabilitation? Preliminary Findings From a Randomized Clinical Trial. J Geriatr Psychiatry Neurol 31:312–318. https://doi.org/10.1177/0891988718807973
Petrelli A, Kaesberg S, Barbe MT, Timmermann L, Fink GR, Kessler J, Kalbe E (2014) Effects of cognitive training in Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat Disord 20:1196–1202. https://doi.org/10.1016/j.parkreldis.2014.08.023
Petrelli A, Kaesberg S, Barbe MT, Timmermann L, Rosen JB, Fink GR, Kessler J, Kalbe E (2015) Cognitive training in Parkinson’s disease reduces cognitive decline in the long term. Eur J Neurol 22:640–647. https://doi.org/10.1111/ene.12621
Barboza NM, Terra MB, Bueno MEB, Christofoletti G, Smaili SM (2019) Physiotherapy Versus Physiotherapy Plus Cognitive Training on Cognition and Quality of Life in Parkinson Disease: Randomized Clinical Trial. Am J Phys Med Rehabil 98:460–468. https://doi.org/10.1097/PHM.0000000000001128
París AP, Saleta HG, de la Cruz Crespo M, Maraver E, Silvestre MG, Freixa CP, Torrellas SA, Pont MF, Nadal SA, Garcia MVP, Bartolomé VL, Fernández A.R. Bayés (2011) Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Mov Disord 26:1251–1258. https://doi.org/10.1002/mds.23688
Peña J, Ibarretxe-Bilbao N, García-Gorostiaga I, Gomez-Beldarrain MA, Díez-Cirarda M, Ojeda N (2014) Improving functional disability and cognition in Parkinson disease: randomized controlled trial. Neurology 83:2167–2174. https://doi.org/10.1212/WNL.0000000000001043
Zimmermann R, Gschwandtner U, Benz N, Hatz F, Schindler C, Taub E, Fuhr P (2014) Cognitive training in Parkinson disease: Cognition-specific vs nonspecific computer training. Neurology. https://doi.org/10.1212/WNL.0000000000000287
Fellman D, Salmi J, Ritakallio L, Ellfolk U, Rinne JO, Laine M (2020) Training working memory updating in Parkinson’s disease: A randomised controlled trial. Neuropsychol Rehabil. https://doi.org/10.1080/09602011.2018.1489860
Alloni A, Quaglini S, Panzarasa S, Sinforiani E, Bernini S (2018) Evaluation of an ontology-based system for computerized cognitive rehabilitation. Int J Med Inform. https://doi.org/10.1016/j.ijmedinf.2018.04.005
Martinez-Horta S, Kulisevsky J (2019) Mild cognitive impairment in Parkinson’s disease. J Neural Transm. https://doi.org/10.1007/s00702-019-02003-1
Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D (2009) Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. https://doi.org/10.1212/WNL.0b013e3181c34b47
Skorvanek M, Goldman JG, Jahanshahi M, Marras C, Rektorova I, Schmand B, van Duijn E, Goetz CG, Weintraub D, Stebbins GT, Martinez-Martin P (2018) Global scales for cognitive screening in Parkinson’s disease: Critique and recommendations. Mov Disord. https://doi.org/10.1002/mds.27233
Kudlicka A, Clare L, Hindle JV (2011) Executive functions in Parkinson’s disease: Systematic review and meta-analysis. Mov Disord. https://doi.org/10.1002/mds.23868
Ferrazzoli D, Ortelli P, Zivi I, Cian V, Urso E, Ghilardi MF, Maestri R, Frazzitta G (2018) Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: A randomised controlled study. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2017-316437
Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M (2011) Cognitive and memory training in adults at risk of dementia: A systematic review. BMC Geriatr. https://doi.org/10.1186/1471-2318-11-55
Biundo R, Weis L, Abbruzzese G, Calandra-Buonaura G, Cortelli P, Jori MC, Lopiano L, Marconi R, Matinella A, Morgante F, Nicoletti A, Tamburini T, Tinazzi M, Zappia M, Vorovenci RJ, Antonini A (2017) Impulse control disorders in advanced Parkinson’s disease with dyskinesia: The ALTHEA study. Mov Disord. https://doi.org/10.1002/mds.27181
A’Campo LEI, Wekking EM, Spliethoff-Kamminga NGA, Le Cessie S, Roos RAC (2010) The benefits of a standardized patient education program for patients with Parkinson’s disease and their caregivers. Park Relat Disord. https://doi.org/10.1016/j.parkreldis.2009.07.009
Acknowledgements
This work was supported by “Progetto giovani ricercatori: FINAGE” (GR-2018-12367927) from the Ministry of Health to F. B.
Author information
Authors and Affiliations
Contributions
Andreina Giustiniani had the idea for the article, performed the literature search, and drafted the work; Lorenza Maistrello performed the data analysis; Laura Danesin and Elena Rigon performed the literature search and contributed in drafting the manuscript; Francesca Burgio performed the literature search and critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original published online version contains an error. An entire paragraph is missing in the manuscript, that is, "Included studies: main characteristics" in the Results section. The references citation is also missing, thus, list of the references are now updated.
Supplementary Information
ESM 1
(DOCX 117 kb)
Rights and permissions
About this article
Cite this article
Giustiniani, A., Maistrello, L., Danesin, L. et al. Effects of cognitive rehabilitation in Parkinson disease: a meta-analysis. Neurol Sci 43, 2323–2337 (2022). https://doi.org/10.1007/s10072-021-05772-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05772-4