Abstract
Understanding on the clinical features and neural mechanisms leading to cognitive impairment and dementia in Parkinson’s disease (PD) has notably increased. At time of diagnosis, nearly all PD patients present some degree of cognitive impairment not enough severe as to significantly affect functional independence. However, even mild cognitive changes have a measurable impact to functional capacity in PD. A clinically practical differentiation is based on the importance of executive deficits in the early phases of cognitive impairment in PD and on the evidence stressing the transitional role of posterior–cortical impairment on the progression of PD-MCI to dementia. However, the pattern of cognitive impairment in PD is variable not just to the extents on which are the affected cognitive domains, but also on which are those domains that became affected first. Specific diagnostic criteria for mild cognitive impairment associated with PD (PD-MCI) and dementia (PDD) and operative guidelines for the cognitive assessment have been developed. In the present review, we will describe general notions regarding the mechanisms and the profile of cognitive deterioration in PD, the diagnostic criteria for PD-MCI, and some of the currently recommended assessment approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) was initially defined as a pure motor disease clinically characterized by the presence of four cardinal clinical features: Bradykinesia, resting tremor, rigidity, and postural instability. However, compelling evidence demonstrated that different non-motor features are inseparable from PD (Obeso et al. 2017; Postuma et al. 2015). Among them, cognitive impairment of different severity and the eventual progression to dementia is known to be a common complication appearing at some point along the course of the disease in a large proportion of PD patients (Aarsland et al. 2010). The high frequency and devastating impact of cognitive deficits in PD has increasingly been recognized in recent years (Obeso et al. 2017). However, the mechanisms leading to cognitive impairment and dementia in PD are only partially understood and there is a lack of effective therapeutic strategies aimed to mitigate or delay cognitive deterioration in PD.
Knowledge on the clinical features and neural mechanisms leading to cognitive impairment and dementia in PD has notably increased in the past 15 years (Barone et al. 2011). More recently, specific diagnostic criteria for mild cognitive deficits associated with PD (PD-MCI) and dementia (PDD) and operative guidelines for the cognitive assessment have been developed. In the present review, we will describe general notions regarding the mechanisms and the profile of cognitive deterioration in PD, the diagnostic criteria for PD-MCI, and some of the currently recommended assessment approaches (Kulisevsky and Pagonabarraga 2009).
Neuropsychological aspects of Parkinson’s disease
Cognitive impairment, and specifically dementia associated with PD, was formally considered a late complication of the disease (Aarsland et al. 2001; Hely et al. 2008). However, compelling evidence proved that cognitive impairment of variable severity can occur in the early stages of the disease (Kulisevsky et al. 2000; Postuma et al. 2015). At time of diagnosis, nearly all PD patients present some degree of cognitive impairment not enough sever as to significantly affect functional independence (Muslimovic et al. 2005; Postuma et al. 2015). However, mild cognitive changes have a measurable impact to functional capacity in PD (Kulisevsky et al. 2013). These early signs of cognitive deterioration are, in many cases, difficult to capture with common screening methods (Aarsland et al. 2009; Muslimovic et al. 2005). It highlights that early mild cognitive deficits associated with PD are, in many cases, not clinically apparent, so formal neuropsychological examination is required (Kulisevsky and Pagonabarraga 2009).
The prototypical neuropsychological pattern of cognitive impairment in PD is predominantly characterized by frontal–executive deficits resembling those observed in patients with prefrontal cortex (PFC) damage (Williams-Gray et al. 2009a). That is, slowed processing speed, attention and working memory, set-shifting, planning, and non-cued facilitated recall often characterize the profile of cognitive impairment in PD (Barone et al. 2011). These difficulties are mostly attributed to the dopamine-mediated dysfunction of the associative circuit of the basal ganglia which maintains reciprocal connections between the dorsal caudate nucleus and the dorsal–lateral prefrontal cortex (DLPFC) (Carbon et al. 2004). On the other hand, some deficits frequently observed in PD patients under dopaminergic replacement and affecting tasks involving the ventro-medial and the orbital PFC are assumed to be a consequence of an excessive dopaminergic stimulation over circuits remaining relatively spared (Cools et al. 2002; Kulisevsky et al. 1996).
In any case, despite frontal–executive difficulties appear as the more characteristically affected cognitive domain in early PD, up to 40% of patients also exhibit deficits in other cognitive domains like visuospatial skills, memory, or language (Muslimovic et al. 2005). In fact, the pattern of cognitive impairment in PD is variable not just to the extents on which are the affected cognitive domains, but also on which are those domains that became affected first.
In terms of progression, despite the occurrence of dementia is not inevitable in all cases, it affects up to 36% of patients after 4 years of follow-up and up to 80% of long-term survival patients above 20 years of disease progression. It means that PD imposes a risk for developing dementia six times greater than the observed in general population (Aarsland et al. 2003, 2005, 2010; Hely et al. 2008).
From the field of AD, we have learnt that early cognitive deficits not severe enough as to significantly impact functionality (mild cognitive impairment or MCI) define a transitional stage between normal cognition and dementia (Petersen et al. 1999). In PD, however, the profile and pattern of progression of PD-MCI is quite heterogeneous between individuals (Mihaescu et al. 2018). Despite it is undeniable that the presence of PD-MCI is strongly associated with an increased risk for the development of dementia, not all patients exhibit the same cognitive profile or the same rate of progression (Aarsland et al. 2004). Longitudinal studies indicate that the presence of deficits in cognitive domains extending beyond executive functions, and involving cortical–instrumental abilities, is more predictable of progression to dementia in PD (Pagonabarraga et al. 2008; Williams-Gray et al. 2009a). This is especially notable in patients exhibiting impaired language and semantic verbal fluency and defective visuospatial/visuoconstructive abilities. It suggests that specific profiles of PD-MCI could associate a different risk and rate of progression to dementia (Martinez-Horta and Kulisevsky 2011). However, the mechanisms sub-serving the relative preservation of cognitive status in some cases or its progression to dementia in others are not fully understood.
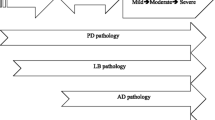
From a neuropsychological perspective, multiple studies showed that difficulties in visuoperceptive and visuoconstructive tasks, language, and episodic memory characterize the cognitive profile of PDD (Emre et al. 2007). Paralleling the progression of cognitive deterioration, neuroimaging studies, illustrated that posterior–cortical cholinergic presynaptic deficits in primary and associative occipital, parietal, and temporal areas increase over the course of PD (Hilker et al. 2005). More recent data also suggest the involvement of dopaminergic denervation on posterior–cortical thinning (Sampedro et al. 2018a). Neuropathological studies performed in patients who developed PDD showed the involvement of widespread cortical and limbic neurodegeneration, and deposition of Lewy bodies and Lewy neurites (Halliday et al. 2014). Co-existing amyloid pathology, cerebrovascular disease, and multiple neurotransmitter system dysfunction are also present in PDD (Hepp et al. 2016; Johar et al. 2017; Vesely and Rektor 2016). It suggests that multiple mechanisms could mediate synergistic processes leading to more aggressive forms of cognitive deteriorations.
Older age, disease duration, worst motor function, comorbid neuropsychiatric features, and worst cognitive performance at baseline are variables associated with increased prevalence of PDD (Emre et al. 2007; Litvan et al. 2011). As said, the mechanisms leading to a worst prognosis in terms of cognitive progression in PD are partially understood, but seem undisputable that the variable expression of the disease also responds to genetic and to environmental mechanisms.
Environmental factors such as social enrichment are known to contribute to better cognitive status in older general population. In PD, social interactions can be limited due to the relation of motor symptoms and/or neuropsychiatric features with social isolation. However, even when proper social participation is present, the variability in cognitive progression still existing. Obesity, hypertension, and other comorbidities such as diabetes also account for a worst cognitive outcome (Smith et al. 2011; van den Berg et al. 2009; Wang et al. 2016).
Some genetic causes of PD like the LRRK2 are not associated with dementia. Conversely, GBA and MAPT mutations are known to contribute to more rapid progression with severe mild cognitive deficits and dementia in PD (Goris et al. 2007; Mata et al. 2014; Morley et al. 2012; Sampedro et al. 2018b; Seto-Salvia et al. 2011). The ApoE4 allele was shown to contribute to amnestic and semantic verbal memory deficits, which, as said, are associated with a cognitive profile of more risk for the development of dementia (Mata et al. 2014; Morley et al. 2012; Williams-Gray et al. 2009b). The role of polymorphism in other genes like the BDNF or the COMT is still conflicting (Guerini et al. 2009; Irwin et al. 2012; Morley et al. 2012). However, a recent study demonstrated that COMT Val/Val homozygotes have a reduced gray matter volume in fronto-temporo-parietal territories and a more severe pattern of cognitive decline (Sampedro et al. 2019). In any case, there is no debate on the multifactorial origin of a cascade of processes that leads to more severe cognitive deterioration in some patients affected by PD.
Neuroimaging data support that posterior–cortical brain changes are characteristically associated with the increased risk for the development of dementia. Accordingly, the addition of posterior–cortical-type deficits in association with widespread cortico-subcortical synuclein pathology and cholinergic changes seems to better characterize the progression of cognitive impairment to PDD than the inherent executive dysfunction present in most patients (Halliday et al. 2014; Williams-Gray et al. 2009a). This notion stressed the idea about defining methods to properly capture cognitive phenotypes of variable risk of cognitive progression to dementia.
The operative definition and assessment of Parkinson’s disease mild cognitive impairment (PD-MCI)
The MDS (Movement Disorder Society) Task Force on MCI in PD has established an operative definition of PD-MCI to homogenize clinical practice and research (Litvan et al. 2011). This task force determined the diagnostic criteria of PD-MCI and the instruments and procedures recommended to properly capture the presence of these criteria (Marras et al. 2014; Skorvanek et al. 2018).
Accordingly, the formal diagnosis of PD-MCI is based on the accomplishment of four key features in the absence of exclusion criteria (see Table 1). This includes evidence of a gradual cognitive decline reported by the patient, relative, or clinician that is objectivized through Level I or Level II testing, and that is not enough severe as to significantly interfere functional independence. Level I testing is based on PD-validated scales of global cognitive performance. Level II testing is based on comprehensive neuropsychological assessment. This latter must be done exploring five cognitive domains (attention, executive function, memory, visuospatial functions, and language) using two single tests for each domain. Using Level I testing, PD-MCI is diagnosed on the basis of either impaired performance on a global cognitive scale validated for being used in PD, or impairment in at least two tests when a limited neuropsychological assessment battery (less than two tests in the five domains) is administered. Level I testing can be assumed as a practical but limited approach to PD-MCI diagnosis, since this method does not allow subtyping patients in different PD-MCI profiles. Using Level II testing, PD-MCI is diagnosed when impairment is captured on at least two tests represented by either two tests in the same cognitive domain, or one test in two different cognitive domains. Thus, impairment must be demonstrated by means of a performance 1.5 SD below normative data or a significant cognitive decline on serial cognitive testing, or a significant decline from estimated premorbid level. Although 1 and 2 SD below normative data have also been used, most authors agree that 1.5 should be recommended. In any case, the formal diagnosis of PD-MCI through Level I or Level II testing requires the use of standardized and validated methods of cognitive testing in PD (Litvan et al. 2011, 2012).
Level I cognitive assessment
Level I assessment consists on abbreviated methods of cognitive assessment through the use of PD-validated global cognitive assessment instruments. Another approach is the use of brief neuropsychological batteries assessing each cognitive domain with one test or not assessing all cognitive domains. It is the case, for example, of batteries focusing on memory and executive functions but not on language or visuospatial skills (Table 2).
Up to date, multiple global cognitive assessment instruments have been used to assess cognition in PD. Some of them were not specifically developed to be used in PD despite they were subjected to validation studies (i.e., MMSE or MoCA) (Kulisevsky and Pagonabarraga 2009; Marras et al. 2014). Others like the Parkinson’s disease—Cognitive Rating Scale (PD-CRS) or the SCOPA-Cog—are PD-specific cognitive assessment instruments (Kulisevsky and Pagonabarraga 2009). The MDS Rating Scales Review Committee recently assessed the clinimetric properties of 12 of commonly used Level I instruments. Among these 12 scales, three were classified as recommended: The PD-CRS, the Montreal Cognitive Assessment (MoCA), and the Mattis Dementia Rating Scale Second Edition (MDRS-2) (Skorvanek et al. 2018). However, of the three instruments, only the PD-CRS was a PD-specific instrument. Despite the acceptable psychometric properties of the MoCA and the MDRS-2 for the screening of PD-MCI, no one of these two instruments were developed specifically focusing on the characteristics of cognitive impairment in PD. It means that the construct validity of these scales may be good capturing global cognitive changes occurring in general forms of cognitive deterioration like amnestic-type MCI or common frontal–subcortical impairment. However, these instruments may lack on measures sensitive to more specific cognitive changes occurring in PD and specially in the transition to more severe forms of cognitive deterioration in this population (Pagonabarraga et al. 2008).
The PD-CRS was designed to capture the full spectrum of cognitive deficits in PD by separately scoring tasks with a major executive (frontal–subcortical subscore) and posterior–cortical (subscore) dependence, and to provide a global total score (Pagonabarraga et al. 2008). This clinically practical differentiation was based on the importance of executive deficits in the early phases of cognitive impairment in PD and also on the evidence stressing the transitional role of posterior–cortical impairment on the progression of PD-MCI to dementia. The psychometric studies showed that, using a cut-off score of PD-CRS total score < 82, this instrument had a high sensitivity (79%) and specificity (80%) for discriminating the cognitive status between patients with normal cognition and PD-MCI (Fernandez de Bobadilla et al. 2013). Other psychometric attributes of this instrument rely on the time of administration (less than 20 min), test–retest reliability, intra-rater reliability, and responsiveness (Fernandez-Bobadilla et al. 2017; Fernandez de Bobadilla et al. 2013; Pagonabarraga et al. 2008).
Another important aspect makes reference to the obvious impact of mild forms of cognitive impairment over functional independence (Marras et al. 2014). Whereas in the field of Alzheimer’s disease, preservation of functional independence is a key feature of amnestic-type MCI, in PD-MCI, it is assumed that, in fact, there is some degree of cognitive-related functional impairment. However, the assessment of functional difficulties related to cognition but not to motor symptoms can be complicated. The Parkinson’s Disease—Cognitive Functional Rating Scale (PD-CFRS)—is presently the unique instrument developed and validated to specifically capture the impact of cognitive changes over functionality in PD (Kulisevsky et al. 2013). This instrument consists of 12 items selected to cover the spectrum of instrumental cognitive changes seen in PD. All 12 questions explore with some examples, whether or not the patient has had trouble in performing an activity (0 = none; 1 = some of the time; 2 = most of the time; 8 = the subject has never done the activity in the past) such as handling money, domestic economy, arranging holidays or meetings, handling personal mail, controlling drug treatment schedule, organizing daily activities, handling home electrical appliances, understanding how to use public transport, solving unforeseen events, explaining things he/she want to say, understanding the things he/she read, and handling the cell phone. The maximum score, obtained by the sum of the ratings, is 24.
In the clinimetric study, the PD-CFRS showed intermediate concurrent validity (ICC = 0.50), high test–retest (ICC = 0.82), inter-rater reliability (ICC = 0.80) and internal consistency (Cronbach’s α = 0.79), and higher coefficient of variation to detect dysfunction in ND-PD patients (PD-CFRS 86.6% vs. OARS-IADL 8.1%). There was a strong relationship between the PD-CFRS and the global cognitive status determined with the PD-Cognitive Rating Scale (r = − 0.72, p < 0.0001). The responsiveness study recruited 63 patients with normal cognition and 57 with mild cognitive impairment (MCI); an increase of two points in the PD-CFRS after 6 months was associated with a clinically significant worsening of the cognitive functional status. According to a discriminant analysis, a PD-CFRS cut-off score of ≥ 3 was found to be optimal for detecting functional impairment in PD-MCI patients (Martinez-Horta and Kulisevsky 2011).
Level II cognitive assessment
Level II assessment consists on the administration of a comprehensive battery of neuropsychological testing (Goldman et al. 2015). By consensus, the assessment of cognitive status in PD may be addressed over five cognitive domains of interest: Attention and working memory, executive function, language, memory, and visuospatial function. The assessment must be done using at least two tests for each cognitive domain (Goldman et al. 2013; Litvan et al. 2012; Marras et al. 2014). To determine the diagnosis of PD-MCI, impairment must be present on at least two tests, either in the same cognitive domain or in different cognitive domains. The definition of impairment must be determined following common standards on neuropsychological assessment. It is by determining a performance of 1–2 SD below the expected range (Goldman et al. 2013). It implies that the selection of tests to compose a comprehensive neuropsychological assessment battery must be done taking into account the need for the existence of normative data allowing the adjustment for age and education. Other approaches to determine impairment are also determining a pattern of significant decline in consecutive serial testing or a significant decline from estimated premorbid level.
According to the use of two tests for each cognitive domain and the assessment of five cognitive domains, patients can be classified in different subtypes of PD-MCI. This classification may be important both for research and clinical proposes, since it could allow to explore the different neurobiological substrates associated with those subtypes. The presence of two tests impaired in a single cognitive domain with no evidence of impairment in other domains represents a single-domain pattern of PD-MCI. In the case of impairment in at least one test in two or more cognitive domains, it represents a multiple-domain pattern of PD-MCI. More specific sub-classification may be done based on the impaired domains. For instance, in case of prominent alteration of the frontal executive domain with the absence of impairment in other domains, the sub-classification may be PD-MCI single-domain executive type (Barone et al. 2011; Litvan et al. 2011, 2012).
Regarding the list of tests that can be used to compose a reliable comprehensive neuropsychological assessment battery, current recommendations lists multiple instruments for which appropriate sensitivity and specificity of about 81.3% and 85.7% is found using ten specific tests of this list (Goldman et al. 2015). Although there is no mandatory list, a proposal for the recommended instruments for each cognitive domain is:
-
1.
Attention and working memory: Symbol digit modalities test (SDMT) and Trail Making Test part A.
-
2.
Executive functions: Clock drawing test and Trail Making Test part B.
-
3.
Language: Boston Naming Test and Semantic verbal fluency test.
-
4.
Memory: Free and Cued Selective Reminding Test (FCSRT) and Figural memory.
-
5.
Visuospatial function: Judgment of Line Orientation and Copy of pentagons.
Despite these, ten selected tests showed good psychometric properties in the studied sample of reference; there are many other options that could be considered on the basis of, for example, disposition of normative data. Accordingly, the MDS task force listed a set of tests (Table 3) to be considered.
Conclusion
In PD, cognitive impairment of different severity and profile appears in a vast majority of patients at some point during disease progression and have an enormous impact on health-related quality of life of patients and caregivers (Obeso et al. 2017; Postuma et al. 2015). Despite PD-MCI may be present from the beginning of the disease, it is frequently under recognized in clinical practice (Kulisevsky and Pagonabarraga 2009; Postuma et al. 2015). Subtle signs of cognitive deterioration may be not captured through unspecific global cognitive assessment methods. However, the progression of mild cognitive deficits to dementia occurs in an extremely important proportion of PD patients. Thus, an operative definition of the diagnostic criteria and the assessment procedures to early capture clinically relevant cognitive changes in PD was an unmet need until few years ago. The diagnostic criteria and recommendations developed within the MDS task force on mild cognitive impairment were done according to FDA requirements, aiming to be used in the context of therapeutic development and also to homogenize how researchers deal with the cognitive classification of PD patients (Litvan et al. 2011, 2012).
Level I and Level II assessment approaches are both validated for the diagnosis of PD-MCI, not all testing methods demonstrated the same construct validity in terms of capturing different cognitive phenotypes. In this context, the PD-CRS appears as a recommended Level I instrument thanks to the sensitivity of this scale differentiating frontal–subcortical to posterior–cortical changes (Goldman et al. 2015; Pagonabarraga et al. 2008). In comprehensive neuropsychological assessment, more specific classifications can be done by covering five cognitive domains with two tests per domain, without excluding those initially considered to be mostly spared in PD (i.e.: episodic memory, language, and visuoconstructive abilities).
Acknowledgments
This research was funded by La Marató de TV3 (expedient number 20142910, 2014/U/477); and FIS Grants PI14/02058, PI15/00962 by the Instituto de Salud Carlos III, Spain.
References
Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P (2001) Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 56:730–736
Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P (2003) Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 60:387–392
Aarsland D, Andersen K, Larsen JP, Perry R, Wentzel-Larsen T, Lolk A, Kragh-Sorensen P (2004) The rate of cognitive decline in Parkinson disease. Arch Neurol 61:1906–1911. https://doi.org/10.1001/archneur.61.12.1906
Aarsland D, Zaccai J, Brayne C (2005) A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord 20:1255–1263. https://doi.org/10.1002/mds.20527
Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study G (2009) Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 72:1121–1126. https://doi.org/10.1212/01.wnl.0000338632.00552.cb
Aarsland D et al (2010) Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 75:1062–1069. https://doi.org/10.1212/WNL.0b013e3181f39d0e
Barone P, Aarsland D, Burn D, Emre M, Kulisevsky J, Weintraub D (2011) Cognitive impairment in nondemented Parkinson’s disease. Mov Disord 26:2483–2495. https://doi.org/10.1002/mds.23919
Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D (2004) Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. NeuroImage 21:1497–1507. https://doi.org/10.1016/j.neuroimage.2003.12.014
Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM (2002) Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain 125:584–594
Emre M et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707. https://doi.org/10.1002/mds.21507 (quiz 1837)
Fernandez de Bobadilla R, Pagonabarraga J, Martinez-Horta S, Pascual-Sedano B, Campolongo A, Kulisevsky J (2013) Parkinson’s disease-cognitive rating scale: psychometrics for mild cognitive impairment. Mov Disord 28:1376–1383. https://doi.org/10.1002/mds.25568
Fernandez-Bobadilla R, Martinez-Horta S, Marin-Lahoz J, Horta-Barba A, Pagonabarraga J, Kulisevsky J (2017) Development and validation of an alternative version of the Parkinson’s Disease-Cognitive Rating Scale (PD-CRS). Parkinsonism Relat Disord 43:73–77. https://doi.org/10.1016/j.parkreldis.2017.07.015
Goldman JG, Holden S, Bernard B, Ouyang B, Goetz CG, Stebbins GT (2013) Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson’s disease. Mov Disord 28:1972–1979. https://doi.org/10.1002/mds.25655
Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT (2015) Diagnosing PD-MCI by MDS Task Force criteria: how many and which neuropsychological tests? Mov Disord 30:402–406. https://doi.org/10.1002/mds.26084
Goris A et al (2007) Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson’s disease. Ann Neurol 62:145–153. https://doi.org/10.1002/ana.21192
Guerini FR et al (2009) BDNF Val66Met polymorphism is associated with cognitive impairment in Italian patients with Parkinson’s disease. Eur J Neurol 16:1240–1245. https://doi.org/10.1111/j.1468-1331.2009.02706.x
Halliday GM, Leverenz JB, Schneider JS, Adler CH (2014) The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov Disord 29:634–650. https://doi.org/10.1002/mds.25857
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23:837–844. https://doi.org/10.1002/mds.21956
Hepp DH et al (2016) Distribution and load of amyloid-beta pathology in Parkinson disease and dementia with lewy bodies. J Neuropathol Exp Neurol 75:936–945. https://doi.org/10.1093/jnen/nlw070
Hilker R et al (2005) Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 65:1716–1722. https://doi.org/10.1212/01.wnl.0000191154.78131.f6
Irwin DJ et al (2012) Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 72:587–598. https://doi.org/10.1002/ana.23659
Johar I, Mollenhauer B, Aarsland D (2017) Cerebrospinal fluid biomarkers of cognitive decline in Parkinson’s disease. Int Rev Neurobiol 132:275–294. https://doi.org/10.1016/bs.irn.2016.12.001
Kulisevsky J, Pagonabarraga J (2009) Cognitive impairment in Parkinson’s disease: tools for diagnosis and assessment. Mov Disord 24:1103–1110. https://doi.org/10.1002/mds.22506
Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A (1996) Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain 119(Pt 6):2121–2132
Kulisevsky J, Garcia-Sanchez C, Berthier ML, Barbanoj M, Pascual-Sedano B, Gironell A, Estevez-Gonzalez A (2000) Chronic effects of dopaminergic replacement on cognitive function in Parkinson’s disease: a two-year follow-up study of previously untreated patients. Mov Disord 15:613–626
Kulisevsky J et al (2013) Measuring functional impact of cognitive impairment: validation of the Parkinson’s disease cognitive functional rating scale. Parkinsonism Relat Disord 19:812–817. https://doi.org/10.1016/j.parkreldis.2013.05.007
Litvan I et al (2011) MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord 26:1814–1824. https://doi.org/10.1002/mds.23823
Litvan I et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27:349–356. https://doi.org/10.1002/mds.24893
Marras C, Troster AI, Kulisevsky J, Stebbins GT (2014) The tools of the trade: a state of the art “How to Assess Cognition” in the patient with Parkinson’s disease. Mov Disord 29:584–596. https://doi.org/10.1002/mds.25874
Martinez-Horta S, Kulisevsky J (2011) Is all cognitive impairment in Parkinson’s disease “mild cognitive impairment”? J Neural Transm 118:1185–1190. https://doi.org/10.1007/s00702-011-0675-9
Mata IF et al (2014) APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol 71:1405–1412. https://doi.org/10.1001/jamaneurol.2014.1455
Mihaescu AS et al (2018) Brain degeneration in Parkinson’s disease patients with cognitive decline: a coordinate-based meta-analysis. Brain Imaging Behav. https://doi.org/10.1007/s11682-018-9922-0
Morley JF et al (2012) Genetic influences on cognitive decline in Parkinson’s disease. Mov Disord 27:512–518. https://doi.org/10.1002/mds.24946
Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65:1239–1245. https://doi.org/10.1212/01.wnl.0000180516.69442.95
Obeso JA et al (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 32:1264–1310. https://doi.org/10.1002/mds.27115
Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A (2008) Parkinson’s disease-cognitive rating scale: a new cognitive scale specific for Parkinson’s disease. Mov Disord 23:998–1005. https://doi.org/10.1002/mds.22007
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Postuma RB et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Sampedro F, Marin-Lahoz J, Martinez-Horta S, Pagonabarraga J, Kulisevsky J (2018a) Dopaminergic degeneration induces early posterior cortical thinning in Parkinson’s disease. Neurobiol Dis 124:29–35. https://doi.org/10.1016/j.nbd.2018.11.001
Sampedro F, Marin-Lahoz J, Martinez-Horta S, Pagonabarraga J, Kulisevsky J (2018b) Early gray matter volume loss in MAPT H1H1 de novo PD patients: a possible association with cognitive decline. Front Neurol 9:394. https://doi.org/10.3389/fneur.2018.00394
Sampedro F, Marin-Lahoz J, Martinez-Horta S, Pagonabarraga J, Kulisevsky J (2019) Reduced gray matter volume in cognitively preserved COMT (158)Val/Val Parkinson’s disease patients and its association with cognitive decline. Brain Imaging Behav. https://doi.org/10.1007/s11682-018-0022-y
Seto-Salvia N et al (2011) Dementia risk in Parkinson disease: disentangling the role of MAPT haplotypes. Arch Neurol 68:359–364. https://doi.org/10.1001/archneurol.2011.17
Skorvanek M et al (2018) Global scales for cognitive screening in Parkinson’s disease: Critique and recommendations. Mov Disord 33:208–218. https://doi.org/10.1002/mds.27233
Smith E, Hay P, Campbell L, Trollor JN (2011) A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev 12:740–755. https://doi.org/10.1111/j.1467-789X.2011.00920.x
van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ (2009) Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: a systematic comparison of their impact on cognition. Biochimica et biophysica acta 1792:470–481. https://doi.org/10.1016/j.bbadis.2008.09.004
Vesely B, Rektor I (2016) The contribution of white matter lesions (WML) to Parkinson’s disease cognitive impairment symptoms: a critical review of the literature. Parkinsonism Relat Disord 22(Suppl 1):S166–S170. https://doi.org/10.1016/j.parkreldis.2015.09.019
Wang M, Norman JE, Srinivasan VJ, Rutledge JC (2016) Metabolic, inflammatory, and microvascular determinants of white matter disease and cognitive decline. Am J Neurodegener Dis 5:171–177
Williams-Gray CH et al (2009a) The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain 132:2958–2969. https://doi.org/10.1093/brain/awp245
Williams-Gray CH, Goris A, Saiki M, Foltynie T, Compston DA, Sawcer SJ, Barker RA (2009b) Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol 256:493–498. https://doi.org/10.1007/s00415-009-0119-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JK has received consultancy or lecture fees from Zambon, UCB, Sanofi, Bial, Teva, Lundbeck, and research grants from CIBERNED, Instituto de Salud Carlos III, Fundació La Marató de TV3, Abbvie and Zambon. SMH declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martinez-Horta, S., Kulisevsky, J. Mild cognitive impairment in Parkinson’s disease. J Neural Transm 126, 897–904 (2019). https://doi.org/10.1007/s00702-019-02003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02003-1