Abstract
Aim
The objective of the present review was to systematically characterize the types of cognitive impairment that are found in different non-brain types of cancer as measured by objective and validated tests, and also to further examine depression and cognitive function in cancer patients and explore their available rehabilitation treatments.
Results
A total of 29 articles were reviewed. Most of these studies suggest that chemotherapy as well as the combination of chemotherapy and hormonal therapy can influence cognition in different types of cancer patients. Breast cancer patients appear to be the most affected in neuropsychological function, specifically in terms of cognitive impairment and reduced quality of life, as compared to other non-brain solid tumours. Overall, the most impaired functions were verbal ability, memory, executive function, and motor speed.
Conclusion
Chemotherapy-related cognitive dysfunction remains under-recognized and undertreated. The various studies reported differing and non-homogenous findings with mixed results, obtained by self-reporting and web-assisted assessment, with other confounding factors such as age and depression during both cancer diagnosis and treatment. An objective neuropsychological assessment is fundamental to avoid underestimation of the extent of chemobrain. Self-reported and web-assisted assessment may ultimately result in confusion between the neuropsychological signs of chemobrain versus those of depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is an important cause of death throughout the world and its incidence is dramatically increasing [1]. In the last few decades, screening programmes have improved detection of cancer at earlier stages, leading to greatly increased chances of successful treatment and longer life expectancy. The prolonged lifespan of cancer patients has however resulted in the onset of long-term sequelae, such as cognitive impairment, psychological distress, and reduced quality of life, which are related to both disease evolution and side effects of antineoplastic agents [2, 3]. In fact, cognitive disorders are frequently associated with the effects of chemotherapy [4]. This cognitive impairment, commonly called “chemobrain” or “chemofog”, is typically characterized by deficits in memory, attention, language and visuospatial functions, and occurs in 17–75% of treated patients [5, 6] of which one third may suffer long-term effects. Moreover, recent studies have also found that cognitive impairment may manifest prior to chemotherapy [7, 8]. Many other studies have suggested an association between cognitive impairment and chemotherapy, although other factors associated with the diagnosis and treatment of cancer may contribute [9], such as biological and psychological factors [10]. Furthermore, recent magnetic resonance imaging (MRI) studies investigating cognitive impairment in cancer patients found reduced grey matter density in the frontal and temporal brain areas of these patients [11] and also in the left caudal lateral prefrontal region, which is correlated to the effects of chemotherapy and/or disease severity [12]. In addition, some authors also observed that in pre-treated (prior to chemotherapy) patients, there is a widespread decrease in white matter volume in the bilateral orbital frontal regions. This finding is indicative of subtle frontal hypometabolism and is consistent with the results of neuropsychological testing in particular in the cognitive domains of executive functioning: working memory, and divided attention [13].
Although emerging evidence indicates that cancer and cancer treatments such as chemotherapy may contribute to cognitive impairment, it is still unclear whether chemobrain is related to the disease itself or is an effect of chemotherapy. Numerous reviews have focused attention on self-perceived cognitive deficits or web-based assessments that occur in breast cancer [9, 14]. However, the validity of self-perceived and web-based assessments is highly questionable. As recently demonstrated, patients’ self-perception of mental decline is unrelated to objective cognitive deficits. Cancer patients negatively judge their cognitive performances if they have a negative emotional functioning [15]. Indeed, a previous review highlighted the failure to consistently find an association between subjective and objective measures of cognition [16]. Nevertheless, there are no reviews that have analysed the neuropsychological deficits found in different types of cancer with objective (not self-perceived) instruments. This lack of objective measures encourages reflection and consideration of the best direction and methodologies for this research. Given that both chemotherapy and the pre-treatment disease are associated with cognitive impairment in different types of cancer patients, understanding these factors and their associations with cognitive disorders and depression is a main goal of oncological research [17]. The aim of this paper was to review the existing literature on cognitive impairment focusing specifically on different types of cancer and then examine depression and cognitive function in cancer patients as measured with objective and validated tests.
Methods
Search limits
In order to clarify the status of the evidence for the topic of neuropsychological disorders in patients with cancer, we conducted detailed searches of the published medical literature with a review of the Medline (PubMed and the Cochrane library) databases between January 1995 and December 2016.
For our purposes, we used various combinations of the following keywords: “cognitive disorders”, “chemobrain”, “neuropsychological disorders”, “cancer” and “depression”.
Article inclusion criteria were as follows: (1) types of studies: randomized controlled trials (RCTs) were eligible, as were observational studies only if they were published as full paper; (2) types of participants: studies enrolling adult (older than 18 years) patients with a history of non-brain cancer and treated by adjuvant therapy; (3) types of interventions: studies enrolling patients with cancer and treated by adjuvant therapy and/or radiotherapy and/or surgery; (4) types of outcome measures: articles with a specific outcome with regard to cognitive disorders and/or articles with a specific outcome with regard to depression in cancer. Exclusion criteria were as follows: (1) articles not written in English; (2) articles on paediatric cancer; (3) articles with a primary focus on brain tumours due to the direct consequences on cognitive impairment; (4) articles on chemotherapy effects in mouse models; (5) articles regarding cognitive post-cancer impairment assessed with self-perceived instruments.
Selection process
Search terms were used to extract records limited to the subject of cognitive disorders in patients with cancer. An additional search was performed to identify papers specifically focused on chemobrain in elderly patients with cancer. The criterion of “adherence to the keywords” of a paper was defined as the presence of all the above-mentioned keywords in either the text or the abstract. Further studies were sought by means of manual search of secondary sources, including references from primary articles. Conceptually related articles were included as well. Heterogeneous studies on paediatric oncology, brain tumours, articles with scarce methodology and using a self-rating memory scale were excluded. All the articles were initially selected and judged by F.d.I. and L.C as possible candidates for the review because they met the above-mentioned criteria. To find additional publications, we also hand-searched relevant journals and the bibliographies of all the important articles. We found 29 articles suitable for review due to their adherence to the keywords. All other articles cited in the review were conceptually correlated to the topic of neuropsychological disorders in cancer patients.
Neuropsychological disorders in cancer patient
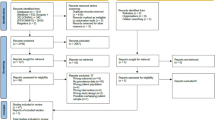
A total of 29 studies evaluating neuropsychological functions in cancer patients were selected (Fig. 1). Among these, one paper investigated neurocognitive functions in long-term survivors of ovarian cancer [18]. Three studies evaluated neurocognitive abilities of small cell lung cancer (SCLC) patients before and after receiving chemoradiation and prophylactic cranial irradiation (PCI) [19,20,21]; four studies were on assessing patients with testicular cancer with neuropsychological tests prior and after adjuvant therapy [22,23,24,25]; the remaining 21 papers were on breast cancer and all the studies assessed patient before chemotherapy, hormonal therapy or radiation [7, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. For the characteristics of the selected studies, see Table 1. Some researchers investigated neuropsychological abilities just after chemotherapy, while others investigated both before and after chemotherapy to explore the manifestation of cognition deficits during treatments. Other studies compared patients with standard-dose versus high-dose chemotherapy and/or after different types of therapy to investigate their effects on cognition. Finally, some articles have compared patients at different stages of cancer (stage 1-2-3). The neuropsychological domain was evaluated using different methods in the different studies reviewed herein (Table 1).
Adjuvant therapy and cognitive impairment: an effect of the disease or the treatment?
Exploration of the relationship between cognitive function, health/disease and treatment-related factors in cancer patients is limited. Patients with cancer may develop cognitive deficits more frequently than in healthy subjects, whether induced by disease-related factors, such as psychological factors (depression, anxiety, fatigue), or related to the effects of chemotherapy.
There is some evidence that patients exhibit cognitive dysfunction before receiving chemotherapy [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35], which suggests that certain pre-treatment factors may play an important role, in particular fatigue, depression, anaemia, genetic variability, tumour biology and the reaction of the immune system to a tumour, which were all associated to cognitive dysfunction [45, 46].
On the other hand, there is also evidence of chemotherapy having a direct effect on neurological function, as imaging studies have identified cerebral atrophy, cortical calcification [47] and decreased metabolic activity in numerous brain regions after chemotherapy [48].
Among the factors associated with cognitive functional impairment in cancer, one of the most well-known is serum haemoglobin levels, which significantly predicted impairment of multiple cognitive measures [36]. It was further found in another study that anaemia may detrimentally affect cognitive performance [49]. Indeed, the loss of cognitive function may be due to anaemia-induced cerebral hypoxia. Systemic hypoxia (evaluated in terms of haemoglobin levels) and pro-neoangiogenic cytokines (evaluated in terms of circulating bFGF-Fibroblast Growth Factor values) were recently well described as the mechanism that cause anaemia in patients with solid cancer [50], underlying that there are mechanisms involving malnutrition, low iron levels and cytokine-mediated factors. The authors argued that there is anaemia with reduced iron availability due to alterations in the levels of hepcidin, the key regulator of iron homeostasis. Increased hepcidin levels block the ferroportin-mediated release of iron from enterocytes and macrophages [51]. One study examined the acute effect of chemotherapy on cytokine levels founding an increased level of IL6, IL8 and IL 10 [52]. Thus, the cytokine mechanism could explain the manifestation of cognitive impairment in cancer patients before undergoing treatment [8, 52]. Inflammatory cytokines inhibit proliferation and differentiation of erythroid progenitor cells and blunt endogenous erythropoietin production in the kidney. In addition, reduced sensitivity to erythropoietin, a reduced life span of erythrocytes, solid tumours or metastases infiltrating the bone marrow, and myelosuppressive effects of chemotherapies can impair normal hematopoiesis [51]. In some cases, a specific paraneoplastic syndrome has been reported that might lead to cancer-related microangiopathic haemolytic anaemia with Coombs-negative haemolytic anaemia with schistocytes and thrombocytopenia [53]. On the other hand, decline of cognitive function is also present after or during chemotherapy. Chemotherapy and chemotherapy-related neurotoxicity is associated with the release of proinflammatory cytokines, substances related to sickness behaviour (e.g. decreased ability to concentrate). Cytokine-induced sickness behaviour is associated with cognitive disturbance, fatigue and depression [54]. Although not extensively studied, there is evidence that standard-dose chemotherapy is associated with increases in cytokine levels.
Some other probable mechanisms for chemotherapy-associated changes in cognitive function have been considered. Firstly, chemotherapy has been associated with DNA damage and telomere shortening, both of which have been implicated in neural degeneration and development of neurodegenerative disorders with cognitive components. There is evidence for oxidative DNA damage in peripheral blood lymphocytes after chemotherapy for breast cancer and increased number of point mutations in mitochondrial DNA in patients with various cancer diagnoses treated with chemotherapy with or without radiation therapy [55]. Secondly, oestrogen and testosterone levels can be reduced following to chemotherapy and as a result of hormonal treatments for cancer such as tamoxifen for breast cancer, and androgen ablation in prostate cancer. Reduction in hormonal levels has been associated with cognitive decline even in cases without chemotherapy [56, 57]. Research also supports the neuroprotective and antioxidant effects of both oestrogen and testosterone and the importance of oestrogen for maintaining telomere length [58, 59]. Even a particular genetic feature has been studied as mechanism induced cognitive changes. In particular, the APOE E4 allele has been associated with worse cognitive performance in cancer survivors, in particular with regard to the visual memory and spatial domains. The reduced ability to repair micro blood vessels was indicated as a possible explanation for the relationship between APOE e4 and the reduced cognitive response to brain damage [60].
Neuropsychological symptoms and depression
Cognitive function could be influenced by the presence of depression. One out of two cancer patients reports psychiatric disorders, especially depression [2]. Despite the high prevalence of depressive disorders in cancer patients, the topic of depression in cancer is still not well explored. Depression can occur in the form of major depressive disorder or minor depressive disorder and depression is equally distributed across the genders [61]. There are, however, forms of depression which may be present in patients with medical comorbidity, but which are “subsyndromal” or “subthreshold”. Subthreshold depression is below the threshold of even a minor depressive disorder diagnosis and thus may be under-recognized. In fact, the average rate of major depressive disorder in cancer is around 25% during the clinical course of the illness and is accompanied by a small number of symptoms. Also, for the diagnostic assessment of mood in cancer patients, it should be considered that many somatic symptoms, such as anorexia, weight loss, low energy and sleep disturbances, are similar to those of cancer itself [62, 63]. Therefore, they may be easily misattributed to cancer and not to depression [64]. For this reason, several authors have proposed exclusion of these somatic symptoms from a depression diagnosis in cancer patients or substitution of them with other non-somatic symptoms [65, 66]. It becomes apparent that depression in cancer patients may have a peculiar phenomenology, and psychological depression is thought to be a predictor of poor survival among cancer patients. Interestingly, anxiety seems to be the symptom that characterizes cancer diagnosis, whereas depression is more common after medical treatment [67].
Relationship between neuropsychological disorders and depression
Cognition may be affected by the presence of depression. Indeed, a diminished ability to think or concentrate, or make decisions, is among the depressive symptoms. Thus, due to the overlap between cognitive deficits and depressive symptoms, the former might be misattributed to depression. Although many research groups have assessed depression in cancer patients since the 1960s, studies that have explored the relationship between depression and cognition are sparse. Vearncombe et al. suggested that psychological factors might increase the vulnerability of breast cancer patients to cognitive impairment after chemotherapy [36]; however, several prospective studies have generally found no significant association between psychological distress and objective cognitive performance [40, 43, 68]. In fact, many studies have explored the association between depression and self-reported cognitive function, but in most cases self-impression did not correlate with objective neuropsychological testing [33, 57]. In particular, one study found that the 26% of patients that had distress (due to depression and anxiety) were more likely to be cognitively impaired [39]. Recently, some studies have found that depressive symptomatology was present in patients, but not significantly correlated with any cognitive tests at baseline [38] or in the acute and late intervals between patients with and without decline [40]. In line with this evidence, cognitive test performance was not related to anxiety and depression [32, 37], even when high Beck Depression Interview scores were significantly associated with self-reported cognitive failure [7, 29, 37]. In contrast, a recent study found higher levels of psychological distress were associated with poor cognitive function in breast cancer patients receiving chemotherapy [69]. However, the authors did note that the discrepancy of their results with previous studies could be due to the small number of participants in the study. Even in patients with testicular cancer, mood (depression and anxiety) appears to have a limited impact on decline of cognitive function [22, 24, 25]; one single study found that depression (evaluated with BDI-II) associated with poorer performance for working memory (WAIS-III LN) following chemotherapy [23]. However, according to several researches in the area of SCLC, depressive illness is significantly associated with diagnoses of lung cancer and should not be underestimated [70], and moreover this depression is common and persistent, especially in patients with more severe symptoms or functional limitations [71]. These last studies highlight the need to recognize psychological morbidity in order to improve the quality of life of lung cancer patients, but the question of a possible association between cognition and depression in this type of cancer remains unresolved. There may be a different explanation for these inconsistencies. Firstly, depression symptomatology is assessed with different scales, and often some of the psychological assessments used are not able to diagnose major or minor depressive disorder or subclinical depression. Thus, it is necessary to begin evaluation of depression with instruments able to diagnose clinical mood disorders. Secondly, even if depressive symptoms are actually assessed, in several studies they are mostly analysed and significantly associated with cognitive self-impression [34]. However, no relationship has been found between neuropsychological evaluations and self-reported cognitive difficulties [27, 44, 72]. In fact according to Shilling and Jankins, the proportion of patients receiving chemotherapy reporting problems with cognitive functions after chemotherapy was far greater than those objectively identified to have cognitive impairment [72]. However, it is possible that perceptions of cognitive difficulties are in fact correlated with psychological distress, in cancer patients receiving chemotherapy, and may also occur in depressive disorders in terms of memory and concentration abilities. Such information could help clinicians, neuropsychologists to better understand patients’ cognitive difficulty, as they can then feel and adjust to them more effectively [73, 74]. Finally, certain cancer populations might be more vulnerable to depression than others population: patients with oropharyngeal, pancreatic, breast and lung cancer have higher reported rates of depression than those with colon, gynaecologic or lymphoma cancer [75]; however, it should be noted that these research studies did not all assess depression and neuropsychological functions at the same time point. Studies on the effect of chemotherapy and other therapies on cognitive function and mood are important to better understand their relationship and their impact on the quality of life in cancer survivors. Taken together, these finding seem to indicate that depression is frequent, but under-recognized and undertreated among cancer patients [76], despite its prevalence and the degree of suffering it inflicts on cancer patients. Moreover, depression seems to be associated with minimal cognitive impairment, and as such, cognitive dysfunction in cancer patients cannot be explained exclusively by the presence of depression [77]. Cognitive impairment and depression influence the quality of life of cancer patients as well as the course of their treatment. The prevalence of depression in cancer is now recognized to be high, and is thought to influence both morbidity and the course of cancer treatment in patients [78]. Brain dysfunction in cancer patients, is indeed, complicated by chemotherapy-related depression, and anxiety, which can also contribute to poor cognitive performance [79]. Risk factors that have been described for cognitive impairment after cancer and cancer treatments are as follow: age, lower pre-treatment intelligence quotient and the apolipoprotein E genotype (which is also associated with Alzheimer disease) [60]. For all these reasons, a neuropsychological and depression assessment should be considered before and during the course of cancer treatment as separate conditions that they need to be addressed with a neuropsychological and psycho-diagnostical battery.
Rehabilitation for cancer- and treatment-related cognitive impairment
Several interventions to reduce cognitive dysfunction related to cancer treatment in patients were recently described: behavioural strategies, physical activity, neuromodulation strategies and pharmacotherapy [80]. Cognitive rehabilitation refers to a clinic-based, therapeutic programme aimed at improving cognitive abilities, functional capacity and real-world skills. Cognitive rehabilitation programmes can be in inpatient or outpatient setting and involve patients meeting individually and/or in groups with a trained clinician (typically a neuropsychologist, psychologist, speech and language pathologist or occupational therapist). Cognitive rehabilitation can also be effective for managing psychological comorbidities, such as anxiety and depression experienced during cancer diagnosis and treatment. Von Ah et al. evaluated the preliminary efficacy and satisfaction/acceptability of group training or improving memory or processing speed as compared to wait list control patients in cohort of 82 breast cancer survivors. Both interventions were associated with improvements in perceived cognitive functioning, symptom distress, quality of life, objective verbal memory and speed of processing [81]. Similarly, Kesler et al. showed that a novel individual cognitive rehabilitation treatment online training programme was effective for improvement of executive function in long-term breast cancer survivors. A total of 41 breast cancer survivors (21 active, 20 wait list controls) completed the 48-session training programme over 12 weeks. The participants were, at an average, of 6 years after cancer therapy. Cognitive computerized training led to significant improvements in cognitive flexibility, verbal fluency and processing speed, with marginally significant downstream improvements in verbal memory as assessed via standardized measures [82]. A qualitative study conducted with nine women concluded that occupational therapy is important to assist women in returning to daily occupations during or following their chemobrain symptoms due to cancer treatment [83]. Physical activity is associated with improved cognitive function in human studies. In healthy adults and also with several pathological conditions, exercise induces the largest and most consistent increases in executive function, which is thought to be due to increased neurogenesis and levels of neurotransmitters and neurotrophins that promote cognitive function, as well as reduction of inflammation [84]. Initial findings have shown that physical fitness activities also increased cognitive health and quality of life in patients that have undergone chemotherapy [85]. Specific multidisciplinary cognitive-based exercise programmes could improve body image related- and overall quality of life in cancer patients [86]. Neurofeedback-based methods provide the best possibility for non-invasive treatment of cognitive disorders among the neuroscience-based interventions. These are methods that involve providing participants with feedback regarding their brain activity, as a means of training them to be able to control the upregulation and downregulation of brain activity using different strategies. Neurofeedback is provided via electroencephalogram (EEG), functional near-infrared spectroscopy (NIRS) or real-time functional magnetic resonance imaging. An example of this application is the Brain Computer Interface, an EEG feedback-based mental practise that was applied in stroke survivors [87]. A recent study was conducted in breast cancer survivors that demonstrated positive effects on cognitive function using EEG neurofeedback, suggesting potential benefits for this and similar neurofeedback techniques [88]. In terms of pharmacotherapy, few psychopharmacologic agents have proven effective in reducing or preventing cognitive impairment in non-CNS cancer patients. Psychostimulants like methylphenidate, dexmethylphenidate and modafinil have produced mixed results [80]. In addition, a recent review showed that pharmacological treatments appeared to have less efficacy as compared to non-pharmacological interventions [89].
Discussion and future directions
In conclusion, most of these studies suggest that chemotherapy alone or in combination with hormonal therapy can influence cognition in patients diagnosed with different types of cancer: breast cancer patients appear to be the most affected in terms of cognitive impairment and reduced quality of life, while testicular and ovarian cancer patients seem to be less impaired. Cognitive impairment has been reported in small cell lung cancer patients, however presently there are few studies confirming these data. Overall, the most impaired functions were verbal ability, memory, executive function and motor speed. Less affected are visuospatial skills, language and abstract reasoning. Some studies have shown that mild cognitive impairment was present before chemotherapy, while others found a decline just after chemotherapy. This decline was decreased in the late post-chemotherapy stage and in standard dose-treated patients. Only a few studies, with methodological limits, have reported no effect from chemotherapy. Considering this breadth of evidence, it appears that cognitive impairment is almost ubiquitous in several different types of cancer, most especially after adjuvant therapy. It must now be clarified whether cognitive impairment is related to the disease itself or is a direct effect of chemotherapy.
This review of the literature regarding cognitive disorders in patients with different type of cancers leads to several considerations and suggestions for future studies.
Firstly, as it is well documented, there is a high incidence of misdiagnosis and variability with this phenomenon [14], further objective guidelines should be developed for clear assessment of cognitive dysfunction in order to avoiding these issues. Further randomized controlled trials must also be done to clarify the effect structured multidisciplinary rehabilitation has on the cognitive performance and quality of life of patients.
The second problem is that chemotherapy can have short-term and sometimes even long-term cognitive effects. Studies have consistently found that a subset of cancer survivors have cognitive declines that persist after cancer treatments. Most of these studies suggest that chemotherapy and even hormonal therapy can influence cognition in different types of cancer patients, although other factors associated with the diagnosis and treatment of cancer may also contribute. Large longitudinal studies with long-term follow-up are required to determine the duration of cognitive impairment after chemotherapy.
The third problem to solve is the evaluation of depressive symptoms in patients with cancer. Depression can influence and be influenced by cancer and cancer treatments. Despite many studies assessing mood in different types of cancer patients, depression is still under-recognized. Thus, it is necessary to refine specific diagnostic criteria for depression in cancer patients and to develop instruments for the assessment of depression severity that can help to distinguish the relative contribution of somatic or psychological factors. Neuropsychological function seems to be minimally associated with the presence of depression, even if few studies assessed this association and very few researchers are able to assess and recognize clinical and subclinical depression in cancer patients. Future studies should also focus on the differential effects of clinical and subclinical depression and assess cognitive function in order to determine their impact on quality of life, medical use and adherence to medical regimens.
Fourthly, as the number of long-term cancer survivors increases, it is necessary to understand the implications of systemic interventions on cognitive function in elderly cancer patients. A diagnosis of cognitive impairment may also alter clinical decision-making in geriatric oncology. Moreover, it would be useful to understand whether cancer survivors are at greater risk for increased age-related brain changes or dementia secondary to cancer treatment, since cancer patients are at increased risk for long-term cognitive dysfunction [90, 91]. Finally, because chemotherapy might accelerate the aging process, studies on long-term survivors might be further necessary.
Conclusions
Despite increasing research in this area, the mechanisms by which chemotherapy-induced cognitive changes occur remains largely unknown. Several possible mechanisms for chemotherapy-induced cognitive changes have been proposed and it is likely that there are several pathways to cognitive decline depending on the treatment and the individual. In conclusion, with all the above-mentioned clinical and biological aspects, future research should aim to provide data on the real prevalence and severity of cognitive dysfunction and mood disorders in elderly patients with cancer, and to improve knowledge regarding the association of cognition, depression or immune function with cancer progression in different types of cancer. In this scenario, objective neuropsychological assessment is fundamental to avoid underestimation of the incidence of chemobrain. In addition, it is important to plan and tailor appropriate cognitive rehabilitation programmes that could specifically target patients’ cognitive function, motor performance and related quality of life in terms of emotional and physical well-being. For all these reasons, further studies are necessary in terms of assessment and multidisciplinary treatment in order to improve the quality of life patients and their caregivers.
References
WHO (2018) The International Agency for Research on Cancer (IARC) is part of the World Health Organization (WHO). Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. https://www.who.int/cancer/en/. Accessed 2 Nov 2018
Akechi T, Okuyama T, Sugawara Y, Nakano T, Shima Y, Uchitomi Y (2004) Major depression, adjustment disorders, and post-traumatic stress disorder in terminally ill cancer patients: associated and predictive factors. J Clin Oncol 22:1957–1965
Breitbart W, Rosenfeld B, Pessin H et al (2000) Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 284:2907–2911
Sioka C, Kyritsis AP (2009) Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol 63:761–767
Ahles TA, Saykin AJ, Furstenberg CT et al (2002) Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 20:485–493
Wieneke MH, Dienst ER (1995) Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psycho-Oncology 4:61–66
Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA (2004) Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26:955–969
Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA (2004) ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer. 101:466–475
Vardy J, Rourke S, Tannock IF (2007) Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol 25:2455–2463
Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB (2008) Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol 19:623–629
McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ (2010) Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat 123:819–828
Kesler SR, Kent JS, O'Hara R (2011) Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol 68:1447–1453
Ponto LL, Menda Y, Magnotta VA, Yamada TH, Denburg NL, Schultz SK (2015) Frontal hypometabolism in elderly breast cancer survivors determined by [(18)F] fluorodeoxyglucose (FDG) positron emission tomography (PET): a pilot study. Int J Geriatr Psychiatry 30:587–594
Selamat MH, Loh SY, Mackenzie L, Vardy J (2014) Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PLoS One 9(9):e108002
Biglia N, Bounous VE, Malabaila A et al (2012) Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur J Cancer Care (Engl) 21:485–492
Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C (2012) Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev 38:926–934
Craig CD, Monk BJ, Farley JH (2014) Chase DM (2014). Cognitive impairment in gynecologic cancers: a systematic review of current approaches to diagnosis and treatment. Support Care Cancer 22:279–287
Correa DD, Zhou Q, Thaler HT, Maziarz M, Hurley K, Hensley ML (2010) Cognitive functions in long-term survivors of ovarian cancer. Gynecol Oncol 119:366–369
Meyers CA, Byrne KS, Komaki R (1995) Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer 12:231–235
Komaki R, Meyers CA, Shin DM et al (1995) Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys 33:179–182
Grosshans DR, Meyers CA, Allen PK, Davenport SD, Komaki R (2008) Neurocognitive function in patients with small cell lung cancer: effect of prophylactic cranial irradiation. Cancer. 112:589–595
Schagen SB, Boogerd W, Muller MJ et al (2008) Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol 47:63–70
Pedersen AD, Rossen P, Mehlsen MY, Pedersen CG, Zachariae R, von der Maase H (2009) Long-term cognitive function following chemotherapy in patients with testicular cancer. J Int Neuropsychol Soc 15:296–301
Skaali T, Fossa SD, Andersson S et al (2011) A prospective study of neuropsychological functioning in testicular cancer patients. Ann Oncol 22:1062–1070
Wefel JS, Vidrine DJ, Veramonti TL et al (2011) Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer. 117:190–196
van Dam FS, Schagen SB, Muller MJ et al (1998) Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210–218
Brezden CB, Phillips KA et al (2000) Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695–2701
Tchen N, Juffs HG, Downie FP et al (2003) Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol 21:4175–4183
Shilling V, Jenkins V, Fallowfield L, Howell T (2003) The effects of hormone therapy on cognition in breast cancer. J Steroid Biochem Mol Biol 86:405–412
Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D (2005) The effects of adjuvant chemotherapy on cognition in women with breast cancer—preliminary results of an observational longitudinal study. Breast. 14:142–150
Scherwath A, Mehnert A, Schleimer B et al (2006) Neuropsychological function in high-risk breast cancer survivors after stem-cell supported high-dose therapy versus standard-dose chemotherapy: evaluation of long-term treatment effects. Ann Oncol 17:415–423
Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J (2008) Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 17:1189–1195
Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA (2010) A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer 19:1647–1656
Ahles TA, Saykin AJ, McDonald BC et al (2010) Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 28:4434–4440
Ahles TA, Saykin AJ, McDonald BC et al (2008) Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat 110:143–152
Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle GJ (2009) Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc 15:951–962
Schilder CM, Eggens PC, Seynaeve C et al (2009) Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol 48:76–85
Quesnel C, Savard J, Ivers H (2009) Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat 116:113–123
Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA (2004) The cognitive sequeale of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer 100:2292–2299
Wefel JS, Saleeba AK, Buzdar AU, Meyers CA (2010) Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 116:3348–3356
Schagen SB, Muller MJ, Boogerd W et al (2002) Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol 13:1387–1397
Donovan KA, Small BJ, Andrykowski MA, Schmitt FA, Munster P, Jacobsen PB (2005) Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 104:2499–2507
Hermelink K, Untch M, Lux MP et al (2007) Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 109:1905–1913
Debess J, Riis JO, Engebjerg MC, Ewertz M (2010) Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res Treat 121:91–100
Cheung YT, Ng T, Shwe M et al (2015) Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol 26:1446–1451
Ahles TA, Saykin AJ (2007) Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 7:192–201
Verstappen CC, Heimans JJ, Hoekman K, Postma TJ (2003) Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 63:1549–1563
Silverman DH, Dy CJ, Castellon SA et al (2007) Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat 103:303–311
Jacobsen PB, Garland LL, Booth-Jones M et al (2004) Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. J Pain Symptom Manag 28:7–18
Porta C, Imarisio I, De Amici M (2005) Pro-neoangiogenic cytokines (VEGF and bFGF) and anemia in solid tumor patients. Oncol Rep 13:689–695
Aapro M, Osterborg A, Gascón P, Ludwig H, Beguin Y (2012) Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol 23:1954–1962
Tsavaris N, Kosmas C et al (2002) Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer 87(1):21–27
Lechner K, Obermeier HL (2012) Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore) 91:195–205
Kelley KW, Bluthe RM et al (2003) Cytokine-induced sickness behavior. Brain Behav Immun 17(Suppl 1):S112–S118
Nadin SB, Vargas-Roig LM, Drago G, Ibarra J, Ciocca DR (2006) DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: a pilot study on the implications in the clinical response to chemotherapy. Cancer Lett 239:84–97
Bender CM, Paraska KK et al (2001) Cognitive function and reproductive hormones in adjuvant therapy for breast cancer: a critical review. J Pain Symptom Manag 21(5):407–424
Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL (2005) Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int 96:48–53
Unfer TC, Conterato GM, da Silva JC, Duarte MM, Emanuelli T (2006) Influence of hormone replacement therapy on blood antioxidant enzymes in menopausal women. Clin Chim Acta 369:73–77
Lee SA, Ross RK, Pike MC (2005) An overview of menopausal oestrogen-progestin hormone therapy and breast cancer risk. Br J Cancer 92:2049–2058
Ahles TA, Saykin AJ, Noll WW et al (2003) The relationship of APOE genotype to neuropsychological performances in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 12:612–619
Miller S, Lo C, Gagliese L et al (2011) Patterns of depression in cancer patients: an indirect test of gender-specific vulnerabilities to depression. Soc Psychiatry Psychiatr Epidemiol 46:767–774
Angelino AF, Treisman GJ (2001) Major depression and demoralization in cancer patients: diagnostic and treatment considerations. Support Care Cancer 9:344–349
Ciaramella A1, Poli P (2001) Assessment of depression among cancer patients: the role of pain, cancer type and treatment. Psychooncology. 10:156–165
McDaniel JS, Musselman DL, Porter MR, Reed DA, Nemeroff CB (1995) Depression in patients with cancer. Diagnosis, biology, and treatment. Arch Gen Psychiatry 52:89–99
Endicott J (1984) Measurement of depression in patients with cancer. Cancer 53(10 Suppl):2243–2249
Bukberg J, Penman D, Holland JC (1984) Depression in hospitalized cancer patients. Psychosom Med 46:199–212
Gil F, Costa G, Hilker I, Benito L (2012) First anxiety, afterwards depression: psychological distress in cancer patients at diagnosis and after medical treatment. Stress Health 28:362–367
Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C (2008) The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 17:122–130
Ando-Tanabe N, Iwamitsu Y, Kuranami M et al (2014) Cognitive function in women with breast cancer receiving adjuvant chemotherapy and healthy controls. Breast Cancer 21:453–462
Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR (1998) Anxiety and depression in patients with lung cancer before and after diagnosis: findings from a population in Glasgow, Scotland. J Epidemiol Community Health 52:203–204
Hopwood P, Stephens RJ (2000) Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol 8:893–903
Shilling V, Jenkins V (2007) Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs 11:6–15
Boykoff N, Moieni M, Subramanian SK (2009) Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv 3:223–232
Munir F, Burrows J, Yarker J, Kalawsky K, Bains M (2010) Women’s perceptions of chemotherapy-induced cognitive side effects on work ability: a focus group study. J Clin Nurs 19:1362–1370
Massie MJ (2004) Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr 2004:57–71
Hotopf M, Chidgey J, Addington-Hall J, Ly KL (2002) Depression in advanced disease: a systematic review. Part 1. Prevalence and case finding. Palliat Med 16:81–97
Poppelreuter M, Weis J, Kulz AK, Tucha O, Lange KW, Bartsch HH (2004) Cognitive dysfunction and subjective complaints of cancer patients. A cross-sectional study in a cancer rehabilitation centre. Eur J Cancer 40:43–49
Croyle RT, Rowland JH (2003) Mood disorders and cancer: a National Cancer Institute perspective. Biol Psychiatry 54:191–194
Ganz PA (2012) Doctor, will the treatment you are recommending cause chemobrain? J Clin Oncol 30:229–231
Wefel JS, Kesler SR, Noll KR, Schagen SB (2015) Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin 65:123–138
Von Ah D, Carpenter JS, Saykin A et al (2012) Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 135(3):799–809
Kesler S, Hadi Hosseini SM, Heckler C et al (2013) Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer 13:299–306
Player L, Mackenzie L, Willis K, Loh SY (2014) Women’s experiences of cognitive changes or ‘chemobrain’ following treatment for breast cancer: a role for occupational therapy? Aust Occup Ther J 61:230–240
Hotting K, Roder B (2013) Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev 37:2243–2257
Fitzpatrick TR, Edgar L, Holcroft C (2012) Assessing the relationship between physical fitness activities, cognitive health, and quality of life among older cancer survivors. J Psychosoc Oncol 30:556–572
Morone G, Iosa M, Fusco A et al (2014) Effects of a multidisciplinary educational rehabilitative intervention in breast cancer survivors: the role of body image on quality of life outcomes. Sci World J 2014:451935
Morone G, Pisotta I, Pichiorri F et al (2015) Proof of principle of a brain-computer interface approach to support poststroke arm rehabilitation in hospitalized patients: design, acceptability, and usability. Arch Phys Med Rehabil 96(3 Suppl):S71–S78
Alvarez J, Meyer FL, Granoff DL, Lundy A (2013) The effect of EEG biofeedback on reducing postcancer cognitive impairment. Integr Cancer Ther 12:475–487
Borghgraef C, Libert Y, Etienne AM, Delvaux N, Reynaert C, Razavi D (2014) Treatment of cognitive impairments in oncology: a review of longitudinal controlled studies. Bull Cancer 101:866–875
Heflin LH, Meyerowitz BE et al (2005) Cancer as a risk factor for long-term cognitive deficits and dementia. J Natl Cancer Inst 97:854–856
Kurita K, Meyerowitz BE et al (2011) Long-term cognitive impairment in older adult twins discordant for gynecologic cancer treatment. J Gerontol A Biol Sci Med Sci 66:1343–1349
Funding
This review has been financed by the Line of Research “Cognitive and Motor Neurorehabilitation” of Santa Lucia Foundation, Rome, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Iulio, F., Cravello, L., Shofany, J. et al. Neuropsychological disorders in non-central nervous system cancer: a review of objective cognitive impairment, depression, and related rehabilitation options. Neurol Sci 40, 1759–1774 (2019). https://doi.org/10.1007/s10072-019-03898-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03898-0