Abstract
Background and purpose
Various types of cerebral small vessel diseases (CSVD) are commonly coexisting and the clinical outcome possibly is determined by their combined effect. The present study was designed to explore the possible relationship between the global burden of CSVD and clinical outcomes after recombinant tissue plasminogen activator (rt-PA) treatment of ischemic stroke.
Methods
We enrolled patients with acute ischemic stroke (AIS) after IV rt-PA treatment between August 2016 and July 2018. According to the total burden rating scale of CSVD, we calculated the total CSVD score for white matter hyperintensities, lacunar infarction, cerebral microbleeds, and perivascular spaces. All patients were assessed on the basis of the National Institutes of Health Stroke Scale (NIHSS) score and the modified Rankin Scale (mRS) score at 90 days after stroke. We used multivariate logistic regression analysis to examine the associations between global burden of CSVD and degree of neurological deficit and clinical outcomes. ROC curve analysis was used to determine cut-off values of the total CSVD score in predicting poor outcomes.
Results
The results showed that the total CSVD score was independently associated with moderate to severe stroke (OR 2.187, 95%CI 1.495–3.119, P < 0.001). Initial NIHSS (OR 1.23, 95%CI 1.144–1.330, P < 0.001), OTT (OR 1.007, 95%CI 1.000–1.014, P = 0.037), and CSVD score (OR 3.157, 95%CI 2.120–4.703, P < 0.001) was significantly related to poor functional outcome at 3 months. The total CVSD score cut-off value of 1.5 was determined at best to distinguish between good prognosis and poor outcome (AUC 0.7534 [95%CI 0.6883–0.8185]).
Conclusions
The global burden of CSVD was independently associated with neurological deficit severity and clinical outcomes of AIS after IV rt-PA treatment. The total CVSD score is a reliable predictor for poor outcomes of AIS after IV rt-PA treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second most common cause of death and the leading cause of disability worldwide. Intravenous thrombolysis is regarded as the standard of therapy in acute ischemic stroke [1, 2].
Cerebral small vessel disease (CSVD) is a common finding among patients with acute ischemic stroke, which accounts for about 25% of all ischemic stroke patients, particularly if they are older or have vascular risk factors [3]. From a clinical point of view, CSVD contributes to a risk of cognitive decline, dementia, and stroke, and causes considerable worsening of cognitive function, balance, and gait [4]. The most common type of CSVD is age- and hypertension-related CSVD, which is recognized as a risk factor of ischemic stroke [5]. In general, CSVD has been associated with dysfunction of the blood–brain barrier (BBB), endothelial dysfunction, ischemia, hypoperfusion, amyloid deposition, and genetic factors [6,7,8].
In recent years, the development of neuroimaging has improved the diagnostic rate of CSVD and the number of neuroimaging spectrum ascribable to CSVD has been expanded including leukoaraiosis, microbleeds, lacunar infarcts, perivascular spaces, and brain atrophy. Magnetic resonance (MR) is the gold standard imaging for CSVD, and five closely correlated features are markers on brain MR: white matter hyperintensities (WMHs), lacunar infarction(LI), cerebral microbleeds (CMBs), perivascular spaces (PVS), and brain atrophy.
Previous studies showed that leukoaraiosis severity had been associated with the size of the acute ischemic tissue damage and degree of infarct expansion, as well as initial National Institutes of Health Stroke Scale (NIHSS) deficit severity [9,10,11]. Leukoaraiosis and infarct volume were independently associated with a greater NIHSS deficit in the fully adjusted multivariable model [12]. Leukoaraiosis severity may be associated with poor outcomes after successful recanalization for ischemic stroke with anterior large vessel occlusion [13]. However, these MRI markers of CSVD often occur together in individual people. Recently, efforts have been made to quantitatively assess specific CSVD markers. A lot of current evidence focuses on the effects of single features of CSVD on acute ischemic stroke treated with thrombolysis, and the results indicate that the presence of CSVD may increase the risk of symptomatic intracerebral hemorrhages and may be associated with poor functional outcomes [14, 15]. However, CSVD is a pathological process with heterogeneous etiologies, and whether the combinations of various imaging features of CSVD can better predict deficit severity and relevant clinical outcomes is little known. From the attempts to devise a joint CSVD score representative of the people’s CSVD burden, a major effort has been made by Staals’ Maastricht collaborative group, based on visually rated individual CSVD features which are combined into the “total CSVD burden,” yielding a 0 to 4 total CSVD score [16]. This CSVD score has been reported by multiple research groups to be related to cognitive function [17]. Few studies have combined these features to capture the total CSVD burden and to examine associations with stroke types or cerebrovascular risk factors [18, 19]. The global CSVD burden score might capture the overall effects of CSVD on the cerebral hemisphere better than by considering just one or two individual features separately [20], and provide a more complete estimate of the full impact of CSVD on the brain, in a simple and pragmatic way. This emphasizes the importance of considering total brain damage due to CSVD.
Although, the total CSVD score has been shown to predict cognitive impairment in the elderly population [17] and in patients with hypertension [21]. It remains uncertain whether the score also predicts neurological deficit severity and clinical outcomes after IV recombinant tissue plasminogen activator (rt-PA) treatment in patients with ischemic stroke or transient symptoms with infarction (TSI).
In the present study, we aimed to investigate whether the global burden of CSVD was associated with neurological deficit severity and clinical outcomes after IV rt-PA treatment.
Methods
Study population
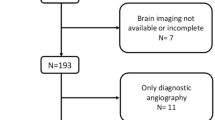
The present study was conducted in the Stroke Center of the First Hospital Affiliated to Soochow University and performed a retrospective analysis of prospectively collected data between August 2016 and July 2018. Two hundred eighteen consecutive patients with acute cerebral infarction were admitted to our study, treated with intravenous (IV) rt-PA within 4.5 h from symptom onset. They underwent admission and finished MR-based imaging. We excluded patients who were treated with combined endovascular thrombectomy and idiopathic ventricular tachycardia (IVT)or had a premorbid modified Rankin scale (mRS) score > 2. All patients gave informed consent to join in and all data were analyzed anonymously. Ethical approval for this study was obtained from the ethics committees of the First Hospital Affiliated to Soochow University (2018104).
Intravenous rt-PA thrombolytic therapy
According to the stroke guidelines, the standard dosage was 0.9 mg/kg and 10% was given as intravenous injection for 1 min. The remaining dosage was continuous intravenous infusion for 60 min, and the maximum thrombolytic dose was no more than 90 mg.
Clinical information
Clinical variates of interest including age, gender, and marital status were collected. Disease history of hypertension, diabetes, stroke, hyperlipidemia, and coronary heart disease were obtained. Onset to treatment time (OTT), admission systolic blood pressure (SBP), admission diastolic blood pressure (DBP), admission glucose level, stroke severity assessed with NIHSS score, Trial of ORG 10172 in Acute Stroke Treatment (TOAST) subtypes, infarct volume, infarct location, and laboratory data were also systematically recorded. mRS score were assessed at 3 month post discharge.
Imaging
The MR examination was performed within 48 h after thrombolysis. All MRIs were assessed blinded to clinical information by two experienced neuroradiologists for the presence, location, and size of the recent symptomatic infarct and any other vascular lesions. Structural MRI included diffusion-weighted imaging (DWI), 3d-TOF-MRA, FLAIR, T2-weighted, T1-weighted, and gradient echo/T2*/susceptibility-weighted sequences.
Lacunes infarctions (LI) were defined as rounded or ovoid lesions, > 3 and < 20-mm diameter, in the basal ganglia, internal capsule, centrum semiovale, or brainstem, of CSF signal intensity on T2 and FLAIR, generally with a hyperintense rim on FLAIR, and no increased signal on DWI. CMBs were defined as small (< 5 mm), homogeneous, round foci of low signal intensity on gradient echo images in cerebellum, brainstem, basal ganglia, white matter, or cortico-subcortical junction, differentiated from vessel flow voids and mineral depositions in the globi pallidi [22]. PVS were defined as small (< 3 mm) round or linear hyperintensities on T2 images in the basal ganglia or centrum semiovale, and they were rated using a five-point ordinal scale as follows: 0 = no EPVS, 1 = 1–10 EPVS, 2 = 11–20 EPVS, 3 = 21–40 EPVS, and 4 = > 40 EPVS [23]. Deep and periventricular WMH were both coded according to the Fazekas scale from 0 to 3 [24].
Based on the recently described score, we rated the total MRI burden of CSVD on an ordinal scale from 0 to 4, by counting the presence of each of the 4 MRI features of CSVD. A point was awarded for each of the following: One point on the CSVD scale was awarded when one or more LI were present. The presence of one or more CMBs was given one point on the CSVD scale. Presence of PVS was counted if there were moderate to severe (grade 2–4) PVS in the basal ganglia (one point if present). One point was awarded on the CSVD scale when (early) confluent deep WMHs (Fazekas score 2 or 3) and/or irregular periventricular WMHs extending into the deep white matter (Fazekas score 3) were present. We therefore combined the scores of WMHs, CMBs, PVS, and LI [16, 20, 25].
Clinical assessment
All patients were assessed by two neurologists on the basis of the National Institutes of Health Stroke Scale (NIHSS). NIHSS scale was used to assess the degree of neurological deficit. Zero to five scores were mild stroke and > 5 scores were moderate to severe stroke. Short-term functional outcomes at 3 months were determined based on the modified Rankin Scale. Poor outcome was defined as a modified Rankin Scale (mRS) score > 2. The follow-up was conducted by two trained neurological doctors who were blinded to the baseline information.
Statistical analysis
All analyses were done with SPSS version 18 (SPSS, Inc., Chicago, IL, USA).
The normal distribution data were analyzed by independent sample t test, the non-normal distribution data were analyzed by Mann Whitney U test. Fisher’s exact test or the chi-square test was used to compare categorical variables as appropriate. Statistical significance was determined as a bilateral test. Univariate analysis was used to compare the baseline, clinical data, and imaging evaluation between the mild stroke group and the moderate to severe stroke group. Adjusted for age, sex, and variables with p value < 0.1 in univariate analysis (admission DBP, TOAST subtype, infarction location, infarct volume, low-density lipoprotein (LDL), total glyceride (TG), creatinine, c-reactive protein (CRP), atrial fibrillation, coronary artery disease, smoking, antiplatelets/anticoagulation, radiological features, CSVD score) for neurological deficit severity, multivariate logistic regression analysis model was used to determine the relationship between the global burden of CSVD and the degree of neurological impairment in acute ischemic stroke (AIS). Univariate analysis was used to compare the baseline, clinical data, and imaging evaluation between the patients with good prognosis and those with poor outcomes. Then, multivariate logistic regression analysis was applied to identify clinical factors to predict short-term prognosis. Moreover, receiver operator characteristic curves (ROCs) were performed to find out the better clinical indicators and assess the sensitivity and specificity of CSVD score, NIHSS score, and OTT for predict clinical outcome of AIS after rt-PA treatment. P < 0.05 (bilateral) is defined as a statistically significant difference.
Results
Demographic data of the studied patient population
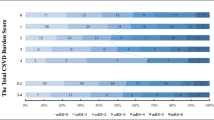
We collected a total of 309 patients and excluded patients who were with incomplete baseline information (n = 28) or had not completed the magnetic resonance examination (n = 63). Finally, we enrolled 218 patients with acute ischemic stroke who received intravenous thrombolysis in the present study. Baseline characteristics of the 218 patients were summarized in Table 1. The mean age was 66 (65.79 ± 11.99) years old and 144(66.1%) were men. One hundred seventy (78%) were accompanied with CSVD. WMHs were found in 25.2% of the subjects (55/218), CMBs in 24.8% (54/218), PVSs in 34.9% (76/218), and LIs in 58.7% (128/218). Total CSVD score assessment showed that 77 cases (35.3%) were 1, 54 cases (24.8%) were 2, 29 cases (13.3%) were 3, and 10 cases(4.6%) were 4.
Association of CSVD burden with severity of neurological deficit
Among the enrolled 218 patients, mild neurological deficit was in 104 (47.71%) patients and moderate to severe was in 114 (52.29%) patients. When comparing patients with mild stroke, those with moderate to severe stroke were older, more frequently with higher admission DBP, CRP, infarct volume, TG and histories of atrial fibrillation, coronary, artery disease, and antiplatelets/anticoagulation. Infarct location, TOAST subtype, radiological features, and the total CSVD score were correlated with the severity of stroke. In univariable analysis, old age, higher DBP, infarct location, more infarct volume, TOAST subtype, TG, CRP, atrial fibrillation, coronary artery disease, and antiplatelets/anticoagulation were associated with moderate to severe stroke (Table 1).
After adjusting for confounders, in multivariable logistic regression analysis, admission DBP, infarct volume (OR = 1.031, 95%CI = 1.011–1.051, P = 0.002), atrial fibrillation, coronary artery disease, and the total CSVD score (OR = 2.187, 95%CI = 1.495–3.119, P = 0.000) were independently associated with moderate to severe stroke (Table 2). Distribution of total CSVD score and comparison of infarct volume in mild and moderate to severe stroke groups were showed in Figs. 1 and 2.
Univariable and multivariable analyses of functional outcomes at 3 months
After 3 months from the index stroke, 96 (44%) patients had a mRS of 3–6. Poor functional outcomes at 3 months were more common in higher CSVD score (Fig. 3). In univariable analyses, old age, initial stroke severity, admission DBP, TOAST subtypes, infarct volume, CRP, hypertension, atrial fibrillation, radiological features, and the total CSVD score were associated with short-term outcomes in AIS after rt-PA treatment (Table 3). A number of predictors of poor outcomes were shown in logistic regression model (Table 4). However, after age, sex, and multivariate adjustment, only initial NIHSS (OR = 1.23, 95%CI = 1.144–1.330, P < 0.001), OTT (OR = 1.007, 95%CI = 1.000–1.014, P = 0.037), and CSVD score (OR = 3.157, 95%CI = 2.120–4.703, P < 0.001) were significantly related to poor functional outcomes at 3 months of discharge.
ROC analysis predictive of 90-day functional outcome
In ROC curve analysis, the total CSVD score cut-off value of 1.5 was determined at best to differentiate between favorable prognosis and poor outcome (AUC 0.7534 [95%CI 0.6883–0.8185]). The total CSVD score cut-off value presented 65% sensitivity and 76% specificity. Besides, an NIHSS score cut-off of 6.5 was used as the best cut-off value for evaluating the prognosis (AUC 0.7496 [95%CI 06828–0.8165]). The NIHSS cut-off score presented 68% sensitivity and 75% specificity. However, there was no obvious predictive value of OTT (AUC 0.5564 [95%CI 0.4794–0.6334]) in ROC analysis. (Table 5; Fig. 4).
Discussion
In this study, we demonstrated that (1) CSVD were substantial in older patients, (2) the total CSVD burden and infarct volume were independently associated with moderate to severe neurological deficit in the fully adjusted multivariable model, and (3) initial NIHSS (OR = 1.23, CI = 1.144–1.330, P < 0.001) and CSVD score (OR = 3.157, CI = 2.120–4.703, P < 0.001) were independent predictors of poor functional outcome at 3 months.
CSVD imaging features commonly occur together. A total CSVD measure might better enclose the global effect of CSVD on the brain than the individual MRI feature separately as we described before. Actually, the results on total CSVD burden and the degree of neurological deficit have not been validated. So we tried to quantify the cumulative effect of combined signs and used the total CSVD burden to assess its relationship with clinical outcomes after IV rt-PA treatment. Considerably, our study found signs of two or more severe CSVD features in around about half of our patients. We investigated initial NIHSS and CSVD score in relation to poor functional outcomes at 3 months, especially the predictors of CSVD score. Arba’s results also suggested that CSVD negatively affected stroke outcomes after intravenous thrombolysis and global burden of CSVD quantified with a combined score was associated with disability and functional dependency [26]. Previous studies demonstrated that patients with a higher score were at increasing risk of a recurrent ischemic stroke and ICH and that the total CSVD score predicted both asymptomatic and disabling recurrent ischemic strokes [27].
Our data indicated that patients with increasing total CSVD score were more likely to be with poor outcome at 3 months. The total CSVD score cut-off value of ≥ 2 might indicate poor clinical outcomes after IV rt-PA treatment. The total CSVD score cut-off value presented 65% sensitivity and 76% specificity. Low sensitivity and specificity might attribute to the small sample and retrospective analysis of our study.
Our study also showed that patients with increasing admission NIHSS score were more likely to have poor outcomes, with a suggested NIHSS score ≧ 7. Similar to previous studies, higher NIHSS proved an independent predictor of unfavorable clinical outcome and death [28]. The NIHSS score strongly predicted the likelihood of a patient’s recovery after a stroke; a score of ≤ 6 forecasts a good recovery [29, 30].
A large number of studies suggested that symptomatic intracerebral hemorrhage was an independent risk factor for poor prognosis of thrombolysis [31, 32]. Nevertheless, our study was likely underpowered to demonstrate an independent association between ICH and poor outcomes. The reason might be that it is not specifically classified as symptomatic intracerebral hemorrhage or asymptomatic hemorrhage.
Although the clinical impact of our findings needs further exploration, a quantitative definition of CSVD could lead to a chart of risk stratification for an acute stroke patient. The results might have significant implications for future research to improve patient selection for acute stroke therapies.
However, this study was subject to some limitations. Firstly, the present study was a retrospective one, which made it impossible to exclude the possibility of bias. Subsequently, our sample size was relatively small, and we recognize that the conclusions need further investigation in larger cohorts. We did not follow up on the recurrence and long-term prognosis of stroke, so it was uncertain whether the patients with a higher burden of CSVDs might suffer from higher stroke recurrence, poor long-term prognosis, and higher mortality rate. Also, a few patients with severe symptomatic intracranial hemorrhage and subarachnoid hemorrhage who could not bear MR imaging were not included in the study, so this might lead to bias in case selection.
In conclusion, this study has shed light on the association of the global burden of CSVD score with neurological deficit severity and clinical outcomes after IV rt-PA treatment in acute ischemic stroke patients. The simple scoring system that combines the various types of CSVD in a single scale is easy to use. Our study suggested that this scoring system could have potential value of risk stratification or early efficacy assessment of CSVD progression and might have a useful role in future clinical practice and research.
Conclusions
The global burden of CSVD was independently associated with neurological deficit severity and clinical outcomes of AIS after IV rt-PA treatment. The total CVSD score is a reliable predictor for poor outcomes of AIS after IV rt-PA treatment with a suggested CSVD score ≥ 2.
References
Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci 38(7):1167–1186. https://doi.org/10.1007/s10072-017-2938-1
Tejada-Meza H, Modrego PJ (2017) Cerebellar vermis: a vulnerable location of remote brain haemorrhages after thrombolysis for ischaemic stroke. Neurol Sci 38(1):185–187. https://doi.org/10.1007/s10072-016-2728-1
Wardlaw JM, Smith C, Dichgans M (2013) Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 12(5):483–497. https://doi.org/10.1016/S1474-4422(13)70060-7
Shi Y, Wardlaw JM (2016) Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol 1(3):83–92. https://doi.org/10.1136/svn-2016-000035
Inzitari D (2003) Leukoaraiosis: an independent risk factor for stroke? Stroke 34(8):2067–2071. https://doi.org/10.1161/01.STR.0000080934.68280.82
Rajani RM, Williams A (2017) Endothelial cell-oligodendrocyte interactions in small vessel disease and aging. Clin Sci 131(5):369–379. https://doi.org/10.1042/CS20160618
Hainsworth AH, Fisher MJ (2017) A dysfunctional blood-brain barrier and cerebral small vessel disease. Neurology 88(5):420–421. https://doi.org/10.1212/WNL.0000000000003561
Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJ, van Buchem MA, Wardlaw JM (2016) Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 36(10):1653–1667. https://doi.org/10.1177/0271678X16662891
Henninger N, Lin E, Haussen DC, Lehman LL, Takhtani D, Selim M, Moonis M (2013) Leukoaraiosis and sex predict the hyperacute ischemic core volume. Stroke 44(1):61–67. https://doi.org/10.1161/STROKEAHA.112.679084
Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, Wu O, Gonzalez RG, Koroshetz WJ, Sorensen AG (2008) Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 39(5):1409–1413. https://doi.org/10.1161/STROKEAHA.107.501932
Henninger N, Khan MA, Zhang J, Moonis M, Goddeau RP Jr (2014) Leukoaraiosis predicts cortical infarct volume after distal middle cerebral artery occlusion. Stroke 45(3):689–695. https://doi.org/10.1161/STROKEAHA.113.002855
Helenius J, Henninger N (2015) Leukoaraiosis burden significantly modulates the association between infarct volume and National Institutes of Health stroke scale in ischemic stroke. Stroke 46(7):1857–1863. https://doi.org/10.1161/STROKEAHA.115.009258
Liu Y, Gong P, Sun H, Zhang S, Zhou J, Zhang Y (2019) Leukoaraiosis is associated with poor outcomes after successful recanalization for large vessel occlusion stroke. Neurol Sci. https://doi.org/10.1007/s10072-018-3698-2
Curtze S, Haapaniemi E, Melkas S, Mustanoja S, Putaala J, Sairanen T, Sibolt G, Tiainen M, Tatlisumak T, Strbian D (2015) White matter lesions double the risk of post-thrombolytic intracerebral hemorrhage. Stroke 46(8):2149–2155. https://doi.org/10.1161/STROKEAHA.115.009318
Zand R, Tsivgoulis G, Singh M, McCormack M, Goyal N, Ishfaq MF, Shahripour RB, Nearing K, Elijovich L, Alexandrov AW, Liebeskind DS, Alexandrov AV (2017) Cerebral microbleeds and risk of intracerebral hemorrhage post intravenous thrombolysis. J Stroke Cerebrovasc Dis 26(3):538–544. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.11.127
Huijts M, Duits A, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Staals J (2013) Accumulation of MRI markers of cerebral small vessel disease is associated with decreased cognitive function. A study in first-ever lacunar stroke and hypertensive patients. Front Aging Neurosci 5:72. https://doi.org/10.3389/fnagi.2013.00072
Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, Redmond P, Starr JM, Deary IJ, Wardlaw JM (2015) Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 36(10):2806–2811. https://doi.org/10.1016/j.neurobiolaging.2015.06.024
Brenner D, Labreuche J, Pico F, Scheltens P, Poirier O, Cambien F, Amarenco P, Investigators G (2008) The renin-angiotensin-aldosterone system in cerebral small vessel disease. J Neurol 255(7):993–1000. https://doi.org/10.1007/s00415-008-0816-8
Pico F, Labreuche J, Touboul PJ, Leys D, Amarenco P (2005) Intracranial arterial dolichoectasia and small-vessel disease in stroke patients. Ann Neurol 57(4):472–479. https://doi.org/10.1002/ana.20423
Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J (2013) Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke 44(11):2995–2999. https://doi.org/10.1161/STROKEAHA.113.002545
Uiterwijk R, van Oostenbrugge RJ, Huijts M, De Leeuw PW, Kroon AA, Staals J (2016) Total cerebral small vessel disease MRI score is associated with cognitive decline in executive function in patients with hypertension. Front Aging Neurosci 8:301. https://doi.org/10.3389/fnagi.2016.00301
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, nEuroimaging STfRVco (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12(8):822–838. https://doi.org/10.1016/S1474-4422(13)70124-8
Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM (2010) Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41(3):450–454. https://doi.org/10.1161/STROKEAHA.109.564914
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149(2):351–356. https://doi.org/10.2214/ajr.149.2.351
Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM (2014) Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83(14):1228–1234. https://doi.org/10.1212/WNL.0000000000000837
Arba F, Inzitari D, Ali M, Warach SJ, Luby M, Lees KR, Collaboration SVI (2017) Small vessel disease and clinical outcomes after IV rt-PA treatment. Acta Neurol Scand 136(1):72–77. https://doi.org/10.1111/ane.12745
Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, Cheung RTF, Kuker W, Mak HKF, Rothwell PM (2017) Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology 88(24):2260–2267. https://doi.org/10.1212/WNL.0000000000004042
Bandettini di Poggio M, Finocchi C, Brizzo F, Altomonte F, Bovis F, Mavilio N, Serrati C, Malfatto L, Mancardi G, Balestrino M (2019) Management of acute ischemic stroke, thrombolysis rate, and predictors of clinical outcome. Neurol Sci 40(2):319–326. https://doi.org/10.1007/s10072-018-3644-3
Abdul-Rahim AH, Fulton RL, Sucharew H, Kleindorfer D, Khatri P, Broderick JP, Lees KR, Collaborators V (2015) National institutes of health stroke scale item profiles as predictor of patient outcome: external validation on independent trial data. Stroke 46(2):395–400. https://doi.org/10.1161/STROKEAHA.114.006837
Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD (1999) Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53(1):126–131
Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, Kim LJ, Mayer SA, Sheth KN, Schwamm LH, American Heart Association Stroke C, Council on C, Stroke N, Council on Clinical C, Council on Quality of C, Outcomes R (2017) Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48(12):e343–e361. https://doi.org/10.1161/STR.0000000000000152
Yaghi S, Eisenberger A, Willey JZ (2014) Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol 71(9):1181–1185. https://doi.org/10.1001/jamaneurol.2014.1210
Funding
This study was funded by the National Key R&D Program of China (2017YFC0114300) and Science and Technology Department Key Diseases Standardized Diagnosis and Treatment Project of Jiangsu Provincial.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was approved by the ethics committees of the First Hospital Affiliated to Soochow University (No. 2018104) without need for informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Li, T., Diao, S. et al. The global burden of cerebral small vessel disease related to neurological deficit severity and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Neurol Sci 40, 1157–1166 (2019). https://doi.org/10.1007/s10072-019-03790-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03790-x