Abstract
Objectives
We aimed to investigate the correlation between an overall cerebral small vessel disease (CSVD) burden and outcomes after endovascular treatment (EVT) for patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO).
Methods
In a multicenter registry study, we enrolled patients with EVT for anterior-circulation LVO-stroke. In 3.0-T MR imaging, we assessed 4 CSVD imaging markers, lacunes, white matter hyperintensities, cerebral microbleeds, and enlarged perivascular spaces, each assigned a score of 0 or 1 and summed up to an overall CSVD burden score of 0–4. We dichotomized the overall CSVD severity as none to mild (score 0–2) and moderate to severe (3–4). Primary outcome was 90-day functional dependence or death (modified Rankin Scale (mRS) 3–6). Secondary outcomes included increase in NIH Stroke Scale ≥ 4 within 24 h (early neurological deterioration (END)) and within 7 days, symptomatic intracranial hemorrhage, 90-day mRS 2–6, and 90-day mortality.
Results
Among 311 patients (63.0% male; mean age 65.1 ± 12.7 years), 260 (83.6%) had none-to-mild and 51 (16.4%) had moderate-to-severe overall CSVD burden. Moderate-to-severe CSVD burden was not significantly associated with the primary outcome (47.1% versus 45.4%; p > 0.05 in univariate and multivariate logistic regression), or the secondary outcomes except for a higher risk of END (11.8% versus 3.1%; p < 0.05 in multivariate analyses). Sensitivity analyses with 0–1 versus 2–4 of the CSVD burden score, and the score as an ordinal variable, showed similar results.
Conclusions
An overall moderate-to-severe CSVD burden was not associated with 90-day functional dependence or death, after EVT for anterior-circulation LVO.
Trial registration
ChiCTR1900022154
Key Points
• Moderate-to-severe cerebral small vessel disease burden on MRI should not be an exclusion indicator in determining the eligibility of an acute ischemic stroke patient for endovascular treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular treatment (EVT) has become a standard emergent treatment for patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO) in the anterior circulation [1]. Compared with conventional treatment, EVT initiated within 6 h of stroke onset almost doubles the chance of achieving functional independence at 3 months [2]. More recent RCTs have further demonstrated the net benefit of EVT in AIS up to 24 h after stroke onset and hence the extended time window for EVT in the latest guidelines [1, 3]. If the eligibility criteria for EVT can be further optimized and more patients who will potentially benefit can be identified, the overall outcomes of LVO-AIS patients will be further improved.
Cerebral small vessel disease (CSVD) is a group of pathological processes affecting arterioles, venules, and capillaries of the brain that can cause various lesions, such as lacunes, white matter hyperintensities (WMH), cerebral microbleeds (CMB), and enlarged perivascular spaces (EPVS) [4]. The associations of some individual CSVD imaging markers with post-EVT outcomes had been investigated in previous studies [5]. Recently, emerging evidence had indicated close inter-correlations among the CSVD markers, and total CSVD burden scores had hence been proposed to capture the overall effect of CSVD on the brain, by considering multiple imaging markers [6]. It provides a more comprehensive estimate of the overall CSVD burden than considering 1 or 2 imaging markers separately. Previous studies had associated severe overall CSVD burden with an increased risk of symptomatic intracranial hemorrhage (sICH) and poor 3-month functional outcome after intravenous thrombolysis (IVT) in AIS [7]. However, data were limited on the associations between overall CSVD burden and post-EVT outcomes, despite the dramatically increasing use of EVT in clinical practice.
In an ongoing registry study, REgiStration study for Critical care of acUte ischemic strokE after REcanalization (RESCUE-RE) [8], we aimed to picture the overall CSVD burden, as well as individual CSVD imaging markers, in LVO-AIS patients undergoing EVT. More importantly, we aim to test the hypothesis that moderate-to-severe overall CSVD burden is associated with unfavorable clinical outcomes of these patients.
Methods
Study design and subjects
Subjects were screened and recruited from RESCUE-RE (chictr.org.cn, ChiCTR1900022154) [8], a prospective registry study recruiting LVO-AIS patients receiving EVT at 18 comprehensive stroke centers across China, approved by the medical ethics committees of all participating centers. All patients or their legal representatives provided written informed consent.
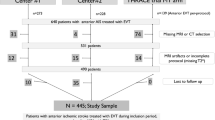
Adult patients with confirmed anterior-circulation LVO-AIS treated with EVT in RESCUE-RE, who had 3.0-T brain MRI exam on admission or within 24 h after EVT and had completed 90-day follow-up, were analyzed in the current study. The occlusion site could be internal carotid artery and anterior or middle cerebral artery. Those with pre-stroke modified Rankin Scale (mRS) > 2 were excluded.
Imaging assessment
Brain MRI exam (3.0 T) included axial T1- and T2-weighted imaging and fluid-attenuated inversion recovery (FLAIR) imaging, diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC) imaging, and T2*-weighted gradient-recalled echo sequence (T2*GRE) or susceptibility-weighted imaging (SWI).
CSVD imaging markers (lacunes, WMHs, CMBs, and EPVSs) were assessed in MRI according to the STRIVE criteria (STandards for ReportIng Vascular changes on nEuroimaging), avoiding acute ischemic lesions (high intensities in DWI and low intensities in ADC) [4]. Lacunes were round or ovoid lesions (3–15 mm diameter) in the basal ganglia, internal capsule, or centrum semiovale of cerebrospinal fluid signal intensity in T2&FLAIR, and usually with a hyperintense rim in FLAIR but without increased signal in DWI [4]. WMHs were bilateral, mostly symmetrical hyperintensities in T2 and isointense/hypointense in T1-weighted images [4], separately graded in deep and periventricular white matter with the Fazekas scale (0 to 3) [9]. Cerebral microbleeds (CMBs) were small (usually < 5 mm), homogeneous, round foci of low signal intensity in T2*GRE/SWI, in basal ganglia, white matter, or cortico-subcortical junction, usually invisible in T1/T2/FLAIR sequences [4]. Enlarged perivascular spaces (EPVSs) were small (usually < 3 mm) punctate (if perpendicular) or linear (if longitudinal to the plane of scan) hyperintensities in T2, and hypointensities in T1&FLAIR images, in basal ganglia, or in centrum semiovale [4, 10]. All images were independently reviewed by 2 trained readers blinded to clinical data, involving a third neuroradiologist upon disagreement.
One point was assigned for each of the following: (1) any lacune; (2) any CMB; (3) (early) confluent deep WMHs (Fazekas score 2 or 3) or irregular periventricular WMHs extending into the deep white matter (Fazekas score 3); and (4) moderate-to-severe (> 10) EPVSs in the basal ganglia. We calculated a total CSVD burden score (ranging from 0 to 4) with the 4 imaging markers in bilateral hemispheres [6, 11], which was dichotomized as none to mild (score of 0–2) and moderate to severe (3–4).
Collateral status was graded as 0 to 4 in pretreatment digital subtraction angiography (DSA), according to the ASITN/SIR Collateral Flow Grading System (grade 0–1 as poor and 2–4 as good collateral status) [8, 12, 13]. Successful recanalization after EVT was defined by Thrombolysis in Cerebral Infarction (TICI) score of 2b or 3 [2].
Outcome measurements
The primary outcome was 90-day functional dependence or death (mRS 3–6). Secondary outcomes included increase in NIH Stroke Scale (NIHSS) ≥ 4 within 24 h (early neurological deterioration (END)) [14] and within 7 days, sICH within 7 days, 90-day mRS 2–6, all-cause 90-day mortality, and an ordinal analysis of the 90-day mRS. sICH was defined according to the ECASSII (European Cooperative Acute Stroke Study II) criteria [15]. The outcomes were obtained through clinic interview or telephone follow-up by trained research coordinators blinded to baseline information.
Statistical analysis
All statistical analyses were performed using SAS software v9.4 (SAS Institute Inc.). Statistical significance was defined by 2-sided p value < 0.05. Interrater reproducibilities of assessing CSVD imaging markers were measured with Cohen’s κ statistic. Continuous and categorical variables were presented as mean ± standard deviation or medians (interquartile range (IQR)), and numbers (percentage). Student’s t or Mann-Whitney U tests were used for comparisons of continuous variables between two groups, and χ2 or Fisher’s exact tests for categorical variables.
We investigated the associations of dichotomized overall CSVD burden score and 4 individual CSVD imaging markers with the outcomes using univariate and multivariate binary Cox (for sICH and 90-day mortality) or logistic (for other outcomes) regression analyses, for ORs or hazard ratios (HR) with 95% confidence interval (CI). Confounders selected based on the clinical significance and/or statistical significance in univariate analyses were adjusted in 4 multivariate regression models. Subgroup analyses were performed to detect potential heterogeneities in the associations between overall CSVD burden and the primary outcome, with the subgrouping factor by the dichotomized overall CSVD burden as an interaction term in multivariate logistic regression analyses. Sensitivity analyses were conducted by dichotomizing total CSVD burden score as 0–1 versus 2–4, and by considering the score as an ordinal variable.
Results
Among 857 adult AIS patients with anterior-circulation LVO receiving EVT in RESCUE-RE (July 2018–May 2019), 311 (63.0% male; mean age 65.1 ± 12.7 years) were included in the current analyses (Supplemental Figure S1). Compared with those (n = 546) excluded from current analyses, fewer of the included patients (n = 311) had history of coronary heart disease or atrial fibrillation, but more had pre-stroke mRS 0–1 and good collateral status at baseline, who in average had lower admission NIHSS (median 14; IQR 11–18) and glucose level, and longer time from stroke onset to groin puncture 360 (median 360 min; IQR 275–515). The proportions of IVT prior to EVT (33.4% versus 30.8%) and successful recanalization (82.6% versus 79.1%) were similar between included and excluded patients (Supplemental Table S1).
Imaging markers and overall burden of CSVD
There were almost perfect interrater (L.Z. and X.T., both with 5-year’s experience of neuroimaging assessment) reproducibilities for presence of individual CSVD imaging markers in 30 cases (Supplemental Table S2). Lacune(s), severe WMHs, CMB(s), and moderate-to-severe EPVSs were found in 132 (42.4%), 200 (64.3%), 90 (28.9%), and 100 (32.2%) patients, respectively. Overall, 260 (83.6%) and 51 (16.4%) patients respectively had none-to-mild and moderate-to-severe overall CSVD burden, while patients with moderate-to-severe CSVD burden were older (means 68.4 versus 64.4 years; p = 0.037; Table 1). The individual CSVD imaging markers and the dichotomized overall CSVD burden were not significantly different between patients with these markers assessed in MRI conducted before (n = 161) or after EVT (n = 150) (Supplemental Table S2).
Associations between overall CSVD burden and clinical outcomes
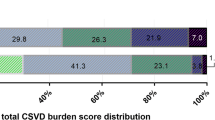
At 3 months, 142 (45.7%) patients were functionally dependent or died (the primary outcome). Age, diabetes mellitus, admission NIHSS, admission glucose level, and collateral status were significantly different between patients with and without a primary outcome (Supplemental Table S3). Moderate-to-severe (versus none-to-mild) CSVD burden was not significantly associated with the primary outcome in univariate (47.1% vs 45.4%; crude OR = 1.07; p = 0.826) or multivariate regression models (Table 2). The distributions of 90-day mRS by the total CSVD burden score, in a range of 0–4 or dichotomized, are presented in Fig. 1. No significant shift was observed towards worse functional outcomes with more severe overall CSVD burden. Supplemental Figure S2 shows CSVD imaging markers in an old patient with moderate-to-severe overall CSVD burden at baseline and functionally independent at 90 days.
Distributions of the 90-day mRS according to the total CSVD burden at baseline. Upper panel: distributions of the 90-day mRS according to the total CSVD burden score from 0 to 4. Lower panel: distributions of the 90-day mRS in patients with none-to-mild (score 0–2) versus moderate-to-severe overall CSVD burden (score 3–4). Abbreviations: CSVD, cerebral small vessel disease; mRS, modified Rankin Scale
Fourteen (4.5%) patients had the secondary outcome of END, 15 (4.8%) had increase in NIHSS ≥ 4 within 7 days, 15 (4.8%) had sICH within 7 days, 189 (60.8%) had mRS 2–6 at 90 days, and 28 (9.0%) died within 90 days. Moderate-to-severe CSVD burden was significantly associated with an increased risk of END in univariate (11.8% vs 3.1%; crude OR = 4.17; p = 0.011) and all 4 multivariate regression models (all p < 0.05; Table 2), but not associated with other secondary outcomes in univariate or multivariate regression.
Associations between individual CSVD imaging markers and clinical outcomes
In univariate regression analyses (Table 3), lacunes, severe WMHs, CMBs, or moderate–severe EPVSs were not significantly associated with functional dependence or death at 90 days or sICH within 7 days. However, severe WMHs significantly increased 90-day mortality (11.5% vs 4.5%; crude HR = 2.59; p = 0.012) in univariate Cox regression, but the statistical significance was borderline after adjusting for confounders in the 4 multivariate regression models (Supplemental Table S4).
Subgroup analyses and sensitivity analyses
Moderate-to-severe overall CSVD burden was not significantly associated with the primary outcome in any subgroup of interest, and there was no between-subgroup heterogeneity in the associations between dichotomized overall CSVD burden and the primary outcome (all p for interaction > 0.05; Supplemental Figure S3).
Sensitivity analyses with total CSVD burden score dichotomized as 2–4 versus 0–1, or as an ordinal variable, showed similar results with the primary analyses, in univariate and multivariate analyses, when total CSVD burden was significantly associated with an increased risk of END, but not the primary and other secondary outcomes (Supplemental Tables S5 and S6).
Discussion
In this multicenter registry study, we systematically assessed the imaging markers and the overall burden of CSVD in 3.0-T brain MRI in LVO-AIS patients receiving EVT in real-world scenarios. Although moderate-to-severe overall CSVD burden was independently associated with an increased risk of END as compared with none-to-mild overall CSVD burden, it was not associated with higher risks of sICH within 7 days, mRS 3–6 or 2–6, or all-cause mortality at 90 days. None of the individual CSVD imaging markers, including lacunes, severe WMHs, CMBs, and moderate-to-severe EPVSs, was significantly, independently associated with these outcomes.
In previous studies, the presence of lacunes or CMB was not associated with poor outcomes after EVT [16, 17] similar to our findings. To date, data were limited regarding the association between EPVSs and post-EVT outcomes. However, severe WMHs were associated with poor 90-day functional outcome after EVT in LVO-AIS in a meta-analysis (20 studies) [5], and increased 90-day mortality in several previous studies [18, 19]. In this study, severe WMHs were borderline associated with an increased risk of 90-day mortality, but not with other outcomes. Discrepancies between the current and previous relevant studies [18,19,20] may be partly attributed to differences in the study populations, e.g., younger age and lower baseline NIHSS of patients in this study, both related to better post-EVT outcomes. Yet, it is also possible that newer generations of EVT devices with a higher recanalization rate and better selected patients in more recent research or clinical practice (like the current study) are associated with a lower risk of sICH and more favorable 90-day outcomes, resembling the differences in outcomes of patients between the recent (after 2015) “positive” [2] and earlier “negative” RCTs [21] on EVT in AIS. Of note, a more recent study based on the MR CLEAN registry (Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) also indicated significant association between WMHs and poor functional outcomes after EVT [22]. However, the proportion of patients receiving IVT before EVT in the MR CLEAN registry was much higher than that in this cohort (77.9 vs 33.4%). Previous studies have associated severe CSVD burden with poor functional outcomes after IVT in AIS patients (discussed below as well) [21], so the different proportions of patients receiving prior IVT in the two cohorts might partly explain the different findings. In fact, in the subgroup analysis in our cohort, moderate-to-severe CSVD burden tended to be associated with poor functional outcome in those receiving IVT before EVT (OR = 1.31), but such association did not exist in those without prior IVT (OR = 0.80), although there was no statistically significant between-subgroup interaction. In addition, the imaging modalities for assessing WMHs were different in the MR CLEAN (mostly CT) and the current registry (3.0-T MRI), when 3.0-T MRI may better differentiate WMHs from acute ischemic lesions in the hyperacute stage of AIS.
The individual CSVD imaging markers above reflect different aspects of CSVD, with different topology and possibly different underlying pathogenetic mechanisms. They often coexist, while an overall picture of these markers may reflect an overall status of the distal small artery/arteriole bed, hence the overall CSVD burden scores proposed in previous studies [6]. A moderate-to-severe overall CSVD burden, commonly seen in stroke patients (e.g., 16.4% in the current cohort), may represent an overall more vulnerable cerebral microcirculation [23]. Previous studies had associated moderate-to-severe CSVD burden with worse 90-day functional outcomes in AIS patients treated with IVT [7, 23]. This may be explained by the increased permeability of blood-brain barrier (BBB) in the presence of severe CSVD, which may increase the risk of hemorrhage and worsen the functional outcomes [23]. However, to our best knowledge, there were limited data regarding the associations between overall CSVD burden and post-EVT outcomes, which has emerged as first-line treatment for acute stroke in the last few years.
In the current study, we found moderate-to-severe CSVD burden was independently, significantly associated with increased risk of END. In addition to impaired BBB as mentioned above that could aggravate cerebral edema early after AIS, long-term hypoperfusion of the microcirculation in severe CSVD could also accelerate infarct expansion, both of which could lead to deterioration or fluctuations in the neurological deficits in the hyperacute stage (e.g., within 24 h) [24]. However, pretreatment collateral status is another important factor governing post-EVT functional outcome [25], which relies on numerous factors involving hemodynamic, metabolic, and neural mechanisms, but not the microcirculation damage alone [26]. On the other hand, unlike IVT, EVT directly recanalizes the occluded artery and probably reperfuses the ischemic area but had little effect on the overall fibrinolytic activity. So the protective effects of collateral circulation and recanalization/reperfusion in the long term may overwhelm or mitigate the harmful effects of the microcirculation damage in the hyperacute stage, hence the similar risks of sICH and similar 3-month functional outcomes after EVT in those with none-to-mild and moderate-to-severe CSVD burden in this cohort.
Overall, this study implied patients with moderate-to-severe CSVD burden, who constitute a considerable proportion of AIS-LVO patients in real-world scenarios, would benefit from EVT despite possible fluctuations in the neurological deficits in the hyperacute stage. Therefore, moderate-to-severe CSVD burden should not be an exclusion indicator in dominating the clinical decision for EVT, so that more stroke patients can benefit from EVT. However, there may be doubts over the way of dichotomizing the overall CSVD burden score in the primary analyses, with 0–2 and 3–4 respectively representing none-to-mild and moderate-to-severe CSVD burden, as there was no widely accepted cutoff point for the score to grade the severity of CSVD. We therefore conducted sensitivity analyses using 0–1 versus 2–4 to dichotomize the score, and by considering the score as an ordinal variable, which echoed findings with the primary analyses.
There were limitations of this study. First, the sample size was relatively small, but it would have enough power to detect a clinically significant difference in the primary outcome between those with none-to-mild and moderate-to-severe overall CSVD burden (power estimation provided in the supplemental Figure S4) [27]. Second, a 3.0-T MRI exam at baseline was an inclusion criterion in this study, which is not a routine imaging workup for patients receiving EVT treatment. Moreover, patients with more severe stroke or worse general conditions were more likely to be unable to receive brain MRI in the hyperacute stage. This might have caused selection bias, partly reflected by the differences in some baseline characteristics of patients included in and excluded from the current analyses, and we cannot exclude the possibility that patients with poor outcome after EVT are underrepresented in this cohort. Yet, this was inevitable as MRI was needed for the assessment of the CSVD imaging markers, particularly for EPVS and CMBs that cannot be assessed with other imaging modalities (e.g., CT brain) [4]. Third, the CSVD imaging markers were assessed in MRI conducted before or after (within 24 h) EVT in different patients. However, chronic but not acute CSVD imaging markers were assessed in this study, which were not likely to rapidly progress within 24 h of stroke onset. Therefore, the study findings were similar in subgroups of patients with MRI conducted before and after EVT. Fourth, CMBs were assessed in either SWI or T2*GRE sequences in patients from different centers, which may have different sensitivities for assessing CMBs. However, there was no significant between-subgroup heterogeneity by the MRI sequence used for CMB assessment (SWI vs T2*GRE), in the associations between overall CSVD burden and the primary outcome. Fifth, data on some important imaging features possibly associated with post-EVT outcomes, e.g., baseline and final infarct volumes, were not available in this cohort. Last but not least, the generalizability of the current findings in western populations needs further validation.
Conclusions
A considerable proportion of LVO-AIS patients receiving EVT had moderate-to-severe overall CSVD burden. Despite the association with an increased risk of END, moderate-to-severe overall CSVD burden as assessed in MRI was not significantly associated with sICH, or functional dependence or death at 90 days in anterior-circulation LVO-AIS patients receiving EVT. Therefore, moderate-to-severe CSVD burden should not be an exclusion indicator in determining patient eligibility for EVT, which was not clearly indicated in the latest guidelines. The findings and the underlying mechanisms warrant further verification.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AIS:
-
Acute ischemic stroke
- ASITN/SIR:
-
the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology
- ChiCTR:
-
Chinese Clinical Trial Registry
- CI:
-
Confidence interval
- CMB:
-
Cerebral microbleeds
- CSVD:
-
Cerebral small vessel disease
- CT:
-
Computed tomography
- DAWN:
-
DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo
- DWI:
-
Diffusion-weighted imaging
- ECASSII:
-
European Cooperative Acute Stroke Study II
- END:
-
Early neurological deterioration
- EPVS:
-
Enlarged perivascular spaces
- EVT:
-
Endovascular treatment
- FLAIR:
-
Fluid-attenuated inversion recovery
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- IVT:
-
Intravenous thrombolysis
- LVO:
-
Large vessel occlusion
- MR CLEAN:
-
Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands
- MRI:
-
Magnetic resonance imaging
- mRS:
-
Modified Rankin Scale
- NIHSS:
-
National Institutes of Health Stroke Scale
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled trials
- RESCUE-RE:
-
REgiStration study for Critical care of acUte ischemic strokE after REcanalization
- SD:
-
Standard deviation
- sICH:
-
Symptomatic intracranial hemorrhage
- STRIVE:
-
STandards for ReportIng Vascular changes on nEuroimaging
- SWI:
-
Susceptibility-weighted imaging
- T2*GRE:
-
T2*-weighted gradient-recalled echo sequence
- TIA:
-
Transient ischemic attack
- TICI:
-
Thrombolysis in cerebral infarction
- WMH:
-
White matter hyperintensities
References
Powers WJ, Rabinstein AA, Ackerson T et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50:e344–e418
Goyal M, Menon BK, van Zwam WH et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731
Nogueira RG, Jadhav AP, Haussen DC et al (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378:11–21
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Xu T, Wang Y, Yuan J, Chen Y, Luo H (2021) Small vessel disease burden and outcomes of mechanical thrombectomy in ischemic stroke: a systematic review and meta-analysis. Front Neurol 12:602037
Staals J, Makin SDJ, Doubal FN, Dennis MS, Wardlaw JM (2014) Stroke subtype, vascular risk factors, and total MRI brain small vessel disease burden. Neurology 83:1228–1234
Liu X, Li T, Diao S et al (2018) The global burden of cerebral small vessel disease related to neurological deficit severity and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Neurol Sci 40:1157–1166
Wei Y, Pu Y, Pan Y et al (2020) Cortical microinfarcts associated with worse outcomes in patients with acute ischemic stroke receiving endovascular treatment. Stroke 51:2742–2751
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Doubal FN, MacLullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM (2010) Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41:450–454
Klarenbeek P, van Oostenbrugge RJ, Rouhl RPW, Knottnerus ILH, Staals J (2013) Ambulatory blood pressure in patients with lacunar stroke. Stroke 44:2995–2999
Higashida RT, Furlan AJ, Roberts H et al (2003) Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34:e109–e137
Ding Y, Leng X, Nie X et al (2021) Intraluminal thrombus and outcomes of patients with acute large vessel occlusive stroke undergoing endovascular treatment. Stroke 52:1473–1477
Girot J-B, Richard S, Gariel F et al (2020) Predictors of unexplained early neurological deterioration after endovascular treatment for acute ischemic stroke. Stroke 51:2943–2950
Berger C, Fiorelli M, Steiner T et al (2001) Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke 32:1330–1335
Sillanpää N, Pienimäki J-P, Protto S, Seppänen J, Numminen H, Rusanen H (2018) Chronic infarcts predict poor clinical outcome in mechanical thrombectomy of sexagenarian and older patients. J Stroke Cerebrovasc Dis 27:1789–1795
Gratz PP, El-Koussy M, Hsieh K et al (2014) Preexisting cerebral microbleeds on susceptibility-weighted magnetic resonance imaging and post-thrombolysis bleeding risk in 392 patients. Stroke 45:1684–1688
Hooper D, Nisar T, McCane D et al (2021) Severe cerebral small vessel disease burden is associated with poor outcomes after endovascular thrombectomy in acute ischemic stroke with large vessel occlusion. Cureus 13:e13122
Mistry EA, Mistry AM, Mehta T et al (2020) White matter disease and outcomes of mechanical thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol 41:639–644
Guo Y, Zi W, Wan Y et al (2019) Leukoaraiosis severity and outcomes after mechanical thrombectomy with stent-retriever devices in acute ischemic stroke. J NeuroInterv Surg 11:137–140
Broderick JP, Palesch YY, Demchuk AM et al (2013) Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 368:893–903
Uniken Venema SM, Postma AA, van den Wijngaard IR et al (2021) White matter lesions and outcomes after endovascular treatment for acute ischemic stroke: MR CLEAN registry results. Stroke 52:2849–2857
Arba F, Palumbo V, Boulanger J-M et al (2015) Leukoaraiosis and lacunes are associated with poor clinical outcomes in ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke 11:62–67
Guo Y, Zhang S, Li M et al (2020) Leukoaraiosis and earlier neurological outcome after mechanical thrombectomy in acute ischemic stroke. J Neuroradiol 47:428–432
Leng X, Fang H, Leung TWH et al (2016) Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 87:537–544
Mechtouff L, Nighoghossian N, Amaz C et al (2020) White matter burden does not influence the outcome of mechanical thrombectomy. J Neurol 267:618–624
Ryu W-S, Jeong S-W, Kim D-E (2020) Total small vessel disease burden and functional outcome in patients with ischemic stroke. PLoS One 15:e0242319
Acknowledgements
We thank the study quality coordinators’ meticulous work for data quality control. The authors thank all the participants and investigators who took part in the RESCUE-RE study.
Funding
This study has received funding by the National Key R&D program of China (2016YFC1307301, 2018YFC1312402) and the National Natural Science Foundation of China (81820108012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Liping Liu.
Conflict of interest
The authors declare no competing interests.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all patients or their legal representatives in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• case-control study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 3944 kb)
Rights and permissions
About this article
Cite this article
Zheng, L., Leng, X., Nie, X. et al. Small vessel disease burden may not portend unfavorable outcome after thrombectomy for acute large vessel occlusion. Eur Radiol 32, 7824–7832 (2022). https://doi.org/10.1007/s00330-022-08795-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08795-3