Abstract
The aim of this study was to determine the function of visual afference in postural control in Parkinson patients. We enrolled 29 patients and 30 healthy controls. The stabilometry test was performed for posture and balance and Romberg ratio coefficients were calculated. In addition, the Berg Balance Scale and the 6-Minute Walking Test were administered to assess balance and functional exercise capacity; the Unified Parkinson’s Disease Rating Scale was used to determine the stage of the disease; and the Short Form (SF)-36 Health Survey was given to collect information on quality of life. Results: significantly longer Center of Pressure (CoP) sway lengths were observed in the parkinson group. The Romberg index for CoP length of sway in parkinson patients was 94.3 ± 19.3%, versus 147.4 ± 120.6% for the control group. (p = 0.025). Conclusion: Parkinson patients use the increase in CoP sway length and ellipse area to stabilize their balance and sight does not facilitate static postural control as in healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to such symptoms as shaking, rigidity, slowness of movement, and difficulty with walking in its early stages, patients with Parkinson’s disease (PD) experience impairments to balance one of the most important elements that must be considered in rehabilitation. As a major axial disorder in PD, postural instability, including imbalance, is the most disabling long-term problem and fails to respond to pharmacological agents, increasing the risk of falls [1, 2].
Balance impairments in PD can be related to disorders that are linked to movement, motor planning, and the integration of various sensorial inputs, such as perception and vision [3].
PD patients have less adaptive capacity in using visual stimuli to effect correct postural control with an “en block” body movement strategy. The frequency, duration, and mean and maximum amplitudes of postural oscillations, reflecting the influence of vision, differ in patients with various neurological diseases compared with healthy subjects [4].
Further, in patients with unilateral PD, balance control has greater reliance on vision input, possibly to compensate for somatosensory system impairments. In the stabilometry test, subjects whose dominant side is affected experience significantly greater variations in center of pressure (CoP) versus the non-dominant side, primarily under conditions in which the eyes are closed [5].
In PD, the visual postural loop apparently becomes hyperactive, and its influence cannot be de-emphasized easily when the visual information is misleading. The participation of basal ganglia in posture is concerned with the reweighting of various sensor-motor loops that control posture during adaptation to novel situations [6]. These patients might have a deficit in reorganizing sensory priorities due to the damage to basal ganglia affecting the integration of sensory information for postural control [7].
Impaired proprioception, visual sense, and a smaller base of support cause postural instability in PD patients, correlating with a quantitative reduction in muscle strength in the spine, hip, and ankle. In particular, several studies support the hypothesis regarding the influence of changes in visual information in triggering balance control disorders in PD [8, 9]. Further, postural sensory deficits that involve specific sensory modalities are strongly associated with the freezing of gait [10]. Certain pharmacological treatments improve muscle strength, gait speed, and the use of ankle strategy but do not worsen proprioceptive sense [8, 11].
Stabilometry is a reliable tool in assessing postural stability in PD [4]. Our study aims to determine whether patients with PD, even in its early stages, have decreased limits of stability and whether changes in visual input impair their postural control by stabilometric analysis.
Material and methods
We conducted a case-control observational study in patients who had had a diagnosis of idiopathic PD for at least 1 year at the Physical Medicine and Rehabilitation Outpatient Clinic, Policlinico Umberto I Sapienza University of Rome, from July 2015 to January 2017. The inclusion criteria were a diagnosis of idiopathic PD with scores of ≤ 3 and > 1 on the Hoehn and Yahr scales, respectively (in the “On” phase medication: 45–90 min after the morning dose of anti-Parkinson drug therapy) [12]; age between 40 and 80 years; Mini-Mental State Examination Score (MMSE) > 27 [13, 14]; visual analog scale (VAS) score < 3 [15]; other disabling diseases that affected movement and gait; and steady pharmacological treatment with anti-Parkinson agents for at least 1 month.
The exclusion criteria comprised cognitive and visual impairments that could prevent one from understanding and executing the tasks; engagement in another rehabilitative study protocol; participation in a conflicting research study; previous treatment with deep-brain stimulation for symptom management; significant neck, shoulder, or back injuries; uncontrolled hypertension; fall fractures in the past year; lack of sensitivity in the lower limbs; vestibular disorders; symptomatic orthostatic hypotension; and the presence of clinically documented dementia.
The control group included age- and sex-matched healthy subjects who were recruited from a pool of volunteers who did not have acute algic disease or balance deficits; comorbidities, such as diabetes, hypertension, rheumatic, and oncological diseases; vision problems; or endocrinological or neurological disease and did not use antidepressants or anxiolytic drugs.
This study was approved by the ethical committee of Sapienza University of Rome. All participants signed informed consent forms after receiving detailed information about the study’s aims and procedures per the Declaration of Helsinki. Eligible individuals were referred to a physiatrist who was uninvolved in the study and who provided them with details on the experimental protocol. All tests were performed during the on pharmacological phase.
Sociodemographic and clinical data were collected. The following scales were administered to the PD group.
Evaluation scales
The Berg Balance Scale (BBS), for determining the risk of falls, is a widely used instrument that measures static and dynamic balance by assessing one’s performance on functional tasks. It comprises a series of 14 simple tasks, each of which is scored from 0 (lowest level of function) to 4 (highest function). The maximum score is 56 (41–56 = low risk of falls; 21–40 = moderate risk; 0–20 = high risk) [16].
The VAS [15] is a simple and sensitive instrument that enables patients to express their pain intensity as a numerical value. Patients were asked to mark the point that corresponded to their perceived pain intensity on a 10-cm line (0 = absence of pain, 10 = most severe pain).
The UPDRS is the most commonly used scale for monitoring the course of disease in PD patients, consisting of six parts, posing questions on mental state, behavior and mood, ADL, motor functions, complications of advanced disease, stage of disease per the Hoehen and Yahr scale, and everyday life activities per the Schwab and England scale. The UPDRS is a metric scale, ranging from 0 (no disability) to 147 points (severe disability). In this study, we measured scores for UPDRS PART I: Mentation, Behavior, and Mood; UPDRS PART II: Activities in Daily Living; UPDRS PART III: Motor Examination; and UPDRS PART IV: Complications of Therapy and Total [17].
Functional exercise capacity was measured using the 6-minute walk test (6MWT), a practical, simple task that measures the maximum distance that a patient can walk quickly on a flat, hard surface in 6 min [18].
The Short Form (SF)-36 Health Survey was administered to collect information on health status and quality of life. SF-36 is a generic multidimensional health questionnaire that records practical, reliable, and valid information about a patient’s functional health and well-being. It comprises 36 items, divided into 2 general indices that summarize physical and mental health. The physical health category includes 4 domains: physical function (PF), physical role (PR), bodily pain (BP), and general health (GH). Emotional health covers domains on mental health (MH), social function (SF), emotional role (RE), and vitality (VT). Each scale ranges from 0 to 100 (worst and best health state, respectively). The questionnaire has been validated in Italian. [19]. For this observational study, the Strobe guidelines were followed [20].
Assessment of stabilometry—Romberg ratio
Data were collected on a baropodometric platform (Milletrix software-DIASU-Rome) to measure oscillations in terms of the elliptical area that contained 95% of sway points, center of pressure length, and velocities with eyes closed (EC) and open (EO). The stabilometry test was performed, recording the position of the CoP for 51.2 s during quiet standing.
After receiving information about the test procedure, the patients and healthy controls were instructed to stand erect, but not at attention, with their arms along the trunk, their feet open at an angle of approximately 30°, and their heels approximately 3 cm apart. All tests were performed by the same examiner; thus, the participants were supplied with the same instructions prior to each test. Three tests were conducted for each trial condition (EO and EC), for which we have reported the median scores. In the EO condition, subjects fixated on a mark on a wall 1.5 m away at eye level. The test order, EO-EC, was randomized. To minimize external disturbances and cues for the test subjects, the environment was naturally brightly lit and quiet.
We analyzed the Romberg ratio for CoP length of sway (i.e., the length of sway with the closed eyes, divided by that measured with the eyes open and expressed as a percentage) [21]. Higher Romberg ratios reflect greater instability with closed eyes, whereas a ratio < 1 indicates higher stability in the EC versus EO condition.
Statistical analysis
The data were reported as mean ± standard deviation. Mixed analysis of variance (ANOVA) was used to compare stabilometric parameters (length of sway path and elliptical area), with the condition open eyes versus closed eyes as the within-subject factor and the group (PD vs. CG) as the between-subject factor. The results of the mixed ANOVA are reported as F, p value, and effect size, based on eta squared (ES). For parameters with a statistically significant difference in the mixed ANOVA, post hoc analysis was performed within groups by paired t test. The Romberg Index, evaluated for the two stabilometric parameters above, was compared between the two groups by unpaired Student’s t test. Other data were compared between subjects with PD and control healthy subjects (CG) by unpaired Student’s t test. The alpha level was set to 0.05, but that of the post hoc comparison was 0.025, based on Bonferroni correction.
Sample size calculation
Manabe et al. [22] showed significant differences in stabilometric parameters between 15 PD patients and 15 healthy subjects. Using the data of Blaszczyk and Orawiec [4] on EC, setting the power of the analysis to 90% (corresponding to a beta level at 10%) and the alpha level to 5%, significant differences between patients and healthy subjects could be detected by enrolling at least 27 subjects per group.
Results
A total of 43 PD patients were screened, 28 of whom were enrolled; 30 subjects formed the healthy group. The demographics and clinical descriptions of the two groups are reported in Table 1. The 28 PD patients were compared with an age-matched healthy Italian population by t test. We observed significant differences in the General Health and Physical Role items on the SF-36 scale [19] (Table 2).
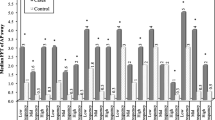
There were no significant differences in age, weight, stature, or body mass index between groups. Figure 1 shows the length of sway path of the center of pressure (CoP) for the two groups. CoP lengths were significantly longer in the PD group [F (1.56) = 14.825, p < 0.001, ES = 0.209]. In contrast, there was no main effect of sight by ANOVA [F (1.56) = 1.227, p = 0.273, ES = 0.021]. Notably, the effect of the interaction between pathology and condition was significant [F (1.56) = 13.474, p = 0.001, ES = 0.194]. In the within-subject post hoc analysis, the length of CoP sway was lower in the CG with open versus closed eyes (p = 0.002). Conversely, in PD subjects, the effect of sight was not significant (p = 0.079).
Mean and standard deviation of center of pressure (CoP) length of sway in the open vs. closed eyes condition for patients with PD (white bars) and the control group (CG), formed of healthy elderly persons (gray bars). PD, group with Parkinson’s disease, CG, control group of healthy elderly, CoP, center of pressure
Similar results were observed for the main factors “group” and “sight” by ANOVA of the elliptical area data; a significant effect was seen for group [F (1.56) = 7.114, p = 0.010, ES = 0.113] but not sight [F (1.56) = 0.352, p = 0.555, ES = 0.006]. In this case, the interaction group × sight approached the threshold of significance for the higher data variability [F (1.56) = 2.822, p = 0.099, ES = 0.048] but trended similarly as length of sway.
The Romberg Index for CoP length of sway (i.e., the length of sway with the eyes closed, expressed as a percentage of that with the eyes open) was 94.3 ± 19.3% in PD patients versus 147.4 ± 120.6% in CG subjects (p = 0.025).
Discussion
The aim of this study was to determine the function of visual afference in postural control in patients with PD.
Unexpectedly, we found that for patients with PD, the effect of sight was not statistically significant in increasing their stability, whereas it was in the control group. Vision helped healthy subjects maintain their stability, but this effect was not observed in patients with PD, in contrast to previous results [4] in which patients with PD benefited from vision during postural assessment. This difference could be attributed to several factors. For example, the reduced sample size (n = 28 in experimental group) versus that in the previous study (n = 55) could have affected the significance. However, even the trend in stability in our study was opposite to that of the other study, for which we noted increased stability with EC. Another reason could be the differences in the clinical features of the sample: our patients were more severely affected by these symptoms (higher score of UPDRS 36.8 ± 16.1 vs. 23.3 ± 12.1) and developed them in a shorter time (average 4 vs. 5.5 years). Also, in our study, the postural analysis lasted 51.2 s—longer than the 30-s analysis in Blaszczyk and Orawiec [4].
The interactions between sensory and proprioceptive inputs have been studied extensively with regard to motor dysfunction in PD patients [23], but the importance of visual afference in postural control and motor skills remains unknown, especially in the early and mild stages of PD. We observed significantly longer CoP lengths in the PD group [F (1.56) = 14.825, p < 0.001, ES = 0.209] (Fig. 1).
We hypothesize that PD patients cannot use visual afference as a positive reinforcement feedback element for static postural control. Difficulties in using visual afference in PD can be confirmed by their vulnerability to incongruent stimuli of visual information compared with healthy subjects, who are able to adapt to a certain amount of visual incongruity [24]. This pattern is critical, especially in rehabilitation, in which the use of external cues as visual and auditory biofeedback is recommended—for example, to improve fluidity and cadence during walking. In rehabilitation for PD, visual cues appear to increase compensatory steps during gait [25]. Notably, the interaction between pathology and condition was significant in the within-subject analysis, in which the length of CoP sway was lower in the CG under the open versus closed eyes condition, but in PD patients, the effect of sight was not significant (p = 0.079).
Our results contradict the common physiological model in which vision helps stabilize posture [26, 27] and improve walking performance (upper body stability and harmony) in healthy subjects [28] and persons who are affected by subclinical Parkinson, as demonstrated by Panyakaew and colleagues [29]. We hypothesize that visual cues have varying functions, depending on disease severity; for example, attentional shifting is compromised in PD but should be preserved in those with very mild PD and slight motor symptoms, as reported in two studies [4, 29]. Normal body oscillation was only observed in patients without clinical evidence of postural instability (HY stage 2 and UPDRS motor score < 20) [26]. However, this model should be tested in a specific and dedicated experiment. Patients with PD have difficulties organizing and using visual information when receiving visual cues for rehabilitation; thus, they should be contextualized and refer to specific tasks by exploiting attention processes [30].
Preliminary findings also support the hypothesis that cognitive load is an important aspect of postural control: Barbosa et al. demonstrated that balance in a dual task is significantly poorer than balance with the eyes closed [31]. Exteroceptive information is not critical for achieving step length and improving overall gait with visual cues in PD, but it is necessary for the accuracy and precision of foot placement on targets [32]. Also, the presence of visual cues significantly reduces bradykinesia during motor imagery training [33]; thus, it is important to avoid inhibition of overlearned and contextually compatible reactions with visual distracters. Schlick and colleagues found that visual cues, combined with treadmill training, have greater beneficial effects on gait in PD patients [30].
We hypothesize that patients with PD use the increase in CoP sway length and elliptical area to stabilize their postural balance during initial postural bradykinesia: we did not observe any differences in oscillation velocity between groups. The increase in support of the CoP area allows the patient to continue to perform normal activities of daily life without any particular risk of falling.
These results increase our understanding of the function of visual input in static postural control of a mild-grade Parkinson patient. However, one limitation of the study was that we did not stratify patients by severity of disease in evaluating the influence of sight, even in relation to dynamic postural control or cognitive problems, such as difficulty in maintaining attention.
Conclusion
Our findings suggest that the relationship between visual integration and postural control is not linear, even in mild-grade PD. Visual integration in a PD patient remains paramount in the management of postural stability—an aspect that should be taken into account in planning the rehabilitation program—necessitating further research into these areas.
References
Rajput AH, Rajput ML, Ferguson LW, Rajput A (2017) Baseline motor findings and Parkinson disease prognostic subtypes. Neurology 89(2):138–143. https://doi.org/10.1212/WNL.0000000000004078
Chou KL, Elm JJ, Wielinski CL et al (2017) Factors associated with falling in early, treated Parkinson’s disease: the NET-PD LS1 cohort. J Neurol Sci 377:137–143. https://doi.org/10.1016/j.jns.2017.04.011
Bonnet CT, Delval A, Defebvre L (2015) Parkinson’s disease-related impairments in body movement, coordination and postural control mechanisms when performing 80° lateral gaze shifts. IEEE Trans Neural Syst Rehabil Eng 23(5):849–856. https://doi.org/10.1109/TNSRE.2014.2369455
Błaszczyk JW, Orawiec R (2011) Assessment of postural control in patients with Parkinson’s disease: sway ratio analysis. Hum Mov Sci 30(2):396–404. https://doi.org/10.1016/j.humov.2010.07.017
Lahr J, Pereira MP, Pelicioni PH et al (2015) Parkinson’s disease patients with dominant hemibody affected by the disease rely more on vision to maintain upright postural control. Percept Mot Skills 121(3):923–934. https://doi.org/10.2466/15.PMS.121c26x0
Bronstein AM, Hood JD, Gresty MA, Panagi C (1990) Visual control of balance in cerebellar and parkinsonian syndromes. Brain 113(Pt 3):767–779
Brown LA, Cooper SA, Doan JB, Dickin DC et al (2006) Parkinsonian deficits in sensory integration for postural control: temporal response to changes in visual input. Parkinsonism Relat Disord 12(6):376–381. https://doi.org/10.1016/j.parkreldis.2006.03.004
Nallegowda M, Singh U, Handa G et al (2004) Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson’s disease: a pilot study. Am J Phys Med Rehabil 83(12):898–908
Suarez H, Geisinger D, Ferreira ED et al (2011) Balance in Parkinson’s disease patients changing the visual input. Braz J Otorhinolaryngol 77(5):651–655
Huh YE, Hwang S, Kim K, Chung WH, Youn J, Cho JW (2016) Postural sensory correlates of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 25:72–77. https://doi.org/10.1016/j.parkreldis.2016.02.004
Pilgram LM, Earhart GM, Pickett KA (2016) Impact of limiting visual input on gait: individuals with Parkinson disease, age-matched controls, and healthy young participants. Somatosens Mot Res 33(1):29–34. https://doi.org/10.3109/08990220.2016.1152237
Hoehn MM, Yahr MD (1967) Parkinsonism: Onset, Progression, and Mortality. Neurology 17:427–442
Folstein MF, Folstein SE, McHugh PR (1975) “Mini Mental State” a practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res 12:189–198
Ferrazzoli D, Ortelli P, Maestri R et al (2016) Does cognitive impairment affect rehabilitation outcome in Parkinson’s disease? Front Aging Neurosci 8:192. https://doi.org/10.3389/fnagi.2016.00192
Huskisson EC (1974) Measurement of pain. Lancet 2:1127–1131
Berg K, Wood-Dauphinee SL, Williams JL (1992) Measuring balance in the elderly: validation of an instrument. Can J Public Health 83(supp 2):S7–S11
Keus SH, Nieuwboer A, Bloem BR et al (2009) Clinimetric analyses of the modified Parkinson activity scale. Parkinsonism Relat Disord 15(4):263–269. https://doi.org/10.1016/j.parkreldis.2008.06.003
Butland RJ, Pang J, Gross ER et al (1982) Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J 284(6329):1607–1608
Apolone G, Mosconi P (1998) The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol 51:1025–1036
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M (2007) STROBE initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 147(8):W163–94
Tjernström F, Björklund M, Malmström EM (2015) Romberg ratio in quiet stance posturography—test to retest reliability. Gait Posture 42(1):27–31. https://doi.org/10.1016/j.gaitpost.2014.12.007
Manabe Y, Honda E, Shiro Y, Sakai K, Kohira I, Kashihara K et al (2001) Fractal dimension analysis of static stabilometry in Parkinson’s disease and spinocerebellar ataxia. Neurol Res 23(4):397–404. https://doi.org/10.1179/016164101101198613
Adamovich SV, Berkinblit MB, Hening W, Sage J, Poizner H (2001) The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience 104:1027–1041
Van den Heuvel MR, Daffertshofer A, Beek PJ, Kwakkel G, van Wegen EE (2016) The effects of visual feedback during a rhythmic weight-shifting task in patients with Parkinson’s disease. Gait Posture 48:140–145. https://doi.org/10.1016/j.gaitpost.2016.03.020
Jacobs JV, Horak FB (2006) Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience 141(2):999–1009. https://doi.org/10.1016/j.neuroscience.2006.04.014
Frenklach A, Louie S, Koop MM, Bronte-Stewart H (2009) Excessive postural sway and the risk of falls at different stages of Parkinson’s disease. Mov Disord 24(3):377–385. https://doi.org/10.1002/mds.22358
Louie S, Koop MM, Frenklach A, Bronte-Stewart H (2009) Quantitative lateralized measures of bradykinesia at different stages of Parkinson’s disease: the role of the less affected side. Mov Disord 24(13):1991–1997. https://doi.org/10.1002/mds.22741
Iosa M, Fusco A, Morone G, Paolucci S (2012) Effects of visual deprivation on gait dynamic stability. ScientificWorldJournal 2012:1–7. https://doi.org/10.1100/2012/974560
Panyakaew P, Anan C, Bhidayasiri R (2015) Visual deprivation elicits subclinical postural inflexibilities in early Parkinson’s disease. J Neurol Sci 349(1-2):214–219. https://doi.org/10.1016/j.jns.2015.01.022
Schlick C, Ernst A, Bötzel K, Plate A, Pelykh O, Ilmberger J (2016) Visual cues combined with treadmill training to improve gait performance in Parkinson’s disease: a pilot randomized controlled trial. Clin Rehabil 30(5):463–471. https://doi.org/10.1177/0269215515588836
Barbosa AF, Souza Cde O, Chen J, Francato DV, Caromano FA, Chien HF et al (2015) The competition with a concurrent cognitive task affects posturographic measures in patients with Parkinson disease. Arq Neuropsiquiatr 73(11):906–912. https://doi.org/10.1590/0004-282X20150153
Rocha PA, Porfírio GM, Ferraz HB, Trevisani VF (2014) Effects of external cues on gait parameters of Parkinson’s disease patients: a systematic review. Clin Neurol Neurosurg 124:127–134. https://doi.org/10.1016/j.clineuro.2014.06.026
Heremans E, Nieuwboer A, Feys P, Vercruysse S, Vandenberghe W, Sharma N, Helsen WF (2012) External cueing improves motor imagery quality in patients with Parkinson disease. Neurorehabil Neural Repair 26(1):27–35. https://doi.org/10.1177/1545968311411055
Acknowledgments
We would like to thank the Physiotherapy School of S. Filippo Neri Hospital “Sapienza” University of Rome. The authors would like to thank all patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Paolucci, T., Iosa, M., Morone, G. et al. Romberg ratio coefficient in quiet stance and postural control in Parkinson’s disease. Neurol Sci 39, 1355–1360 (2018). https://doi.org/10.1007/s10072-018-3423-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3423-1