Abstract
Balance impairment is a common symptom of Parkinson’s disease (PD), suggesting the reclassification of the PD to a tetrad: rest tremor, rigidity, bradykinesia, and balance impairment. Falling is the most evident symptom of inadequate balance. Falls are expected to be twice as likely in individuals with PD compared to age-matched controls, and PD has a fourfold increased risk of hip fractures compared to age-matched controls. Balance is crucial for walking and, as a result, influences the performance of various daily activities around the home and community. This chapter is designed around the four postural domains to give researchers a framework for assessing balance control in Parkinson’s disease patients: (1) postural transitions; (2) reactive response; (3) quiet and prolonged standing; and (4) dynamic balance during walking.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Parkinson’s disease (PD) is a neurodegenerative and progressive disorder of dopaminergic neurons in the substantia nigra, and the presence of intracellular neural inclusions in the dorsal motor nucleus of the vagus nerve, olfactory bulb, and locus coeruleus, which result in different symptoms, both motor and non-motor (Hawkes et al. 2010; Jellinger 2003). PD is characterized as a prolonged and irreversible pathological process, tending to appear in middle age, and can be divided into six stages according to disease progression (for a review, see Del Tredici and Braak 2016). In the initial phase, PD first affects the peripheral nervous system, the autonomic nervous system, and/or the central nervous system (dorsal motor nucleus of the vagus nerve); in stage 2, it spreads to raphe (serotoninergic) and locus coeruleus (noradrenergic) neurons; in stage 3, it affects the substantia nigra (dopaminergic) and the pedunculopontine nuclei (cholinergic, gabaergic, and glutamatergic), also affecting the spinal cord and amygdala; in stage 4, the thalamus and temporal lobe may be affected; in stage 5, sensory association areas and prefrontal regions are affected; and in the final stage, the primary motor and sensory areas are also affected (Mancini et al. 2019). Another form of classification used in clinical practice divides PD into five stages (V) according to the degree of motor impairment: (I) unilateral disease, usually with minimal or no functional impairment; (II) bilateral disease, without balance impairment; (III) mild-to-moderate bilateral disease, some postural instability, physically independent; (IV) severe disability, still able to stand upright unaided; (V) needs a wheelchair or bed, physically dependent (Hoehn and Yahr 1967).
The clinical diagnosis of PD is made from motor symptoms associated with bradykinesia and non-motor symptoms such as mood disorders, cognitive impairment, olfactory impairment, autonomic function disorders, and/or sleep disorders (Kehagia et al. 2013). The common motor symptoms of PD include bradykinesia and/or akinesia, joint stiffness, resting tremors, postural instability, and gait disorders (Jankovic 2008). In the early stages, motor symptoms tend to be asymmetrical, predominantly affecting one side of the body (Kalia and Lang 2015). However, with the progression of the disease, this impairment becomes bilateral, negatively interfering with the individuals’ daily activities, quality of life, and motor independence (Hoehn and Yahr 1967). Figure 3.1 shows the balance dysfunction in individuals with Parkinson’s disease than in neurologically healthy individuals.
Balance dysfunction in individuals with Parkinson’s disease than in neurologically healthy individuals. The red color indicates worse indices for PD. The up arrow indicates an increase, while the down arrow indicates a decrease. The horizontal black stroke indicates that there is no difference. APA anticipatory postural adjustments
Freezing of gait (FoG) is a common symptom in PD that further affects gait, increasing the risk of falling and worsening the quality of life and functional independence of individuals with this symptom. Often, individuals report the sensation of their feet being stuck to the ground, despite the intention to take the step and continue walking. Usually, FoG is a transient and short-lived episode. It can be triggered in different situations, such as at the beginning of the movement, approaching the final destination of the gait, during the turn, and when passing through narrow places or obstacles. Still, it can also occur for no reason. The FoG can be classified according to its behavioral manifestation associated with the movement of the legs in FoG with short and fast steps; FoG with alternating leg tremor; and akinetic FoG when there is no movement in the legs (Schaafsma et al. 2003; Thompson and Marsden 1995). In addition, individuals with FoG have greater postural instability (Bekkers et al. 2017), more severe motor and cognitive symptoms, longer duration of the disease, and use a higher dose of antiparkinsonian medication (Lord et al. 2020). When analyzing prospective studies that followed individuals with PD from the early stages, a meta-analysis by Gao et al. (2020) showed that gait disorders could predict the development of FoG. In addition, this same study showed that non-motor factors, such as depression and anxiety, cognitive impairment, and lower educational level, can also be considered risk factors for the development of BC, but with limited evidence in the literature.
One of the main drug treatments for PD is the dopaminergic replacement from the administration of levodopa, the immediate dopamine precursor capable of crossing the blood-brain barrier and entering the brain. Once in the brain, levodopa quickly converts to dopamine through simple enzymatic reactions. Dopaminergic replacement therapy is composed of two main components. The first is characterized by short-lived effects (about a few hours), related to the concentration of circulating dopamine. The second is characterized by long-lasting effects (about days to weeks), related to neural plasticity induced by dopaminergic signaling (Albin and Leventhal 2017). However, no evidence demonstrates a decrease in disease progression with conventionally used drug treatment (Tarazi et al. 2014).
Increased levodopa concentration in the body produces acute motor responses, such as reduced bradykinesia, rigidity, resting tremor, and greater movement vigor (Albin and Leventhal 2017). This fluctuating motor response dependent on medication administration is called the ON–OFF phenomenon, characterized by an improvement in the motor pattern in the presence of the medication (ON state) and motor worsening with the decrease in the blood concentration of the medication (OFF state). The motor response to drug treatment occurs due to short-term and long-term effects. However, as the disease progresses, the response to long-term effects decreases, and short-term effects become more important in the treatment (Nutt et al. 1997, 2002). In addition, studies show that continued treatment can induce side effects leading to the development of motor fluctuations, involuntary, dyskinetic movements, and acceleration of dementia (Bastide et al. 2015; Tarazi et al. 2014).
The postural control system must be able to regulate balance in unstable situations and, on the other hand, must be versatile enough to allow rapid movement initiation. Perhaps the postural control system performs the most obvious task of maintaining the bipedal upright posture. Still, this system also acts during walking, for example. As a result, individuals with PD frequently experience balance issues. Because of this, some medical professionals have proposed that the PD triad of rest tremor, stiffness, and bradykinesia be changed to a tetrad, adding balance impairment as a fourth symptom. This observation suggests that decreased balance is a key component of Parkinson’s disease. Nearly all PD individuals will experience balance issues at some point during the disease, and balance control will deteriorate as the disease advances, the doctor may anticipate with confidence.
2 Freezing of Gait
Freezing of gait (FoG) is a unique and disabling clinical phenomenon characterized by “brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk” (Nutt et al. 2011). This definition includes the three subtypes of FoG: a patient who suddenly becomes unable to start walking or to move forward, with a start hesitation; a complete absence of movement (akinesia); or a shuffling gait with small steps (Bloem et al. 2004). Some possible hypotheses on the pathogenesis of FoG are the abnormal coupling of posture with gait (Jacobs et al. 2009a, b), impaired gait rhythmicity and gait cycle coordination (Plotnik and Hausdorff 2008), or impairment in movement automaticity (Hallett 2008; Vandenbossche et al. 2012). FoG is triggered by postural transitions, such as gait initiation, turning, narrow passages, obstacle crossing, or approaching a destination (Nutt et al. 2011). Freezing episodes are often described as “the feeling that the feet are being glued to the floor” while the center of mass continues to move forward, resulting that FoG being one the most common reasons for falls in individuals with PD (Michalowska et al. 2005). The physiological mechanisms that trigger FoG have not yet been fully elucidated, and there are several hypotheses in the literature based on the different FoG phenotypes (Gao et al. 2020; Nieuwboer and Giladi 2013). Two models are worth mentioning: (a) threshold model (Plotnik et al. 2012) and (b) interference model (Lewis and Barker 2009). The first model is based on the gait disturbances presented by individuals with FoG (i.e., decreased stride amplitude, impaired gait coordination, and increased stride time variability). This model assumes that when these gait disturbances accumulate to a certain threshold, a point of motor breakdown triggers the FoG. Thus, according to the authors of the model, by improving the gait of individuals with PD, it is possible to reduce the propensity to FoG (Plotnik et al. 2012). The second model is based on the competitive and complementary relationship between the motor, cognitive, and limbic circuits through the basal ganglia, which is responsible for integrating information from different sensory inputs and coordinating an efficient functional response (Lewis and Barker 2009). The authors of this model suggest that the simultaneous processing of cognitive and/or limbic information during motor tasks would overload a system already affected by the dopaminergic deficit. This overload would result in the inactivation of the pedunculopontine nucleus (PPN), responsible for regulating the functional motor response, thus serving as trigger-to-trigger FoG episodes. Furthermore, the authors add that this overload can be reversed by inducing the focus of attention of the individual with PD to a single external sensory cue (a line on the floor or an obstacle to be overcome), thus reducing the number of sensory inputs and stimulating the PPN again. It is worth mentioning that the models proposed in the literature are not exclusive and the understanding of the possible physiological mechanisms that trigger FoG can help in treatment strategies, pharmacological or not.
Co-existing postural impairments may affect the occurrence and severity of FoG. FoG and postural instability are interconnected (Coelho et al. 2021; Peterson et al. 2020), can affect one another behaviorally, and may share a neural basis (for a review, see Bekkers et al. 2018a). The most prevalent FoG-related postural deficits comprised weight-shifting impairments and insufficient scaling and timing of postural responses, which were especially evident when impending postural alterations when time limitations were present. A negative cycle of coupled and more severe postural impairments will likely exacerbate postural instability in individuals with FoG. Consequently, the deficits in large-scale brain networks associated with FoG may concurrently impact postural stability.
Individuals with FoG (freezers) have worse performance on clinical balance tests (Bekkers et al. 2017), more frequent episodes of falls and near-falls (Gazibara et al. 2017), slower and longer duration of anticipatory postural adjustments (APA) (Schlenstedt et al. 2018), smaller step length in automatic postural responses (APR) (Smulders et al. 2014), poorer flexed postural alignment, smaller voluntary limits of stability that could reflect poorer proprioception (de Souza Fortaleza et al. 2017), and worst variability and coordination of stepping during walking (Weiss et al. 2015) when compared to individuals without FoG (nonfreezers) (Fig. 3.2). A recent review showed that freezers have worse performance on clinical scales that assess domains of balance and gait, such as stability limits, APR, APA, and sensory orientation (Bekkers et al. 2017). Specifically, during quiet stance, Pelykh et al. (2015) showed reduced time-to-boundary and more regular postural sway, as observed by lower sample entropy and lower adaptability of the postural sway in freezers. As a certain amount of dynamic irregularity is considered a highly automatized functioning of postural control systems (Donker et al. 2007), it can be assumed that postural control in quiet standing in freezers needs more attention since it may explain the risk of falling in these patients. When analyzing postural responses after a sudden perturbation, the reactive postural control between freezers and nonfreezers is similar (Bekkers et al. 2018b). Given the similarity of postural responses, the authors hypothesize that reactive postural control seems to affect different mechanisms than those governing FoG. However, this hypothesis remains speculative and has not been directly assessed. Although freezers had smaller mediolateral APA during self-initiated gait without start hesitation, trials with freezing showed larger mediolateral APA when compared to nonfreezers (Schlenstedt et al. 2018). Schlenstedt et al. found a positive correlation between the size of mediolateral APA and the New Freezing of Gait Questionnaire (NFoG-Q), and a start hesitation in FoG is not caused by an inability to weight shifting when preparing gait. Therefore, postural instability is behaviorally exacerbated by FoG and vice versa (Bekkers et al. 2018a).
Balance dysfunction in individuals with Parkinson’s disease with freezing of gait (freezers) than without freezing of gait (nonfreezers). The red color indicates worse indices for the freezers. The up arrow indicates an increase, while the down arrow indicates a decrease. The question mark indicates that the studies are controversial. APA anticipatory postural adjustments
Freezers were shown to have impairments in circuitries involving the frontal cortex (for a review, see Fonoff et al. 2019). Studies have shown that freezers have altered supraspinal locomotor neural networks, including the frontal motor regions (supplementary motor area, presupplementary motor area, and primary motor cortices) and subcortical areas (basal ganglia, pontomedullary reticular formation, and mesencephalic and cerebellar locomotor region) (Butler et al. 2017; Ehgoetz Martens et al. 2018; Fling et al. 2013; Gilat et al. 2015; Peterson et al. 2014; Shine et al. 2013b). Resting-state functional magnetic resonance findings pointed to a decreased activity in the premotor (PM) and orbitofrontal cortex (Gallardo et al. 2018; Matsui et al. 2005) and increased functional connectivity between supplementary motor area (SMA) and areas known to be involved in gait initiation such as cerebellar (CLR) and mesencephalic (MLR) locomotor regions (Fling et al. 2014), which was positively correlated to FoG severity. Such an increase in the SMA and locomotor centers connectivity might indicate a greater influence of cognitive processing on gait initiation. The activity of the frontal cortex, including SMA, becomes higher during motor arrests, compared to PD without FoG (Maidan et al. 2016; Shine et al. 2013a; Vercruysse et al. 2014). Freezers showed decreased structural and functional connectivity between SMA and subthalamic nucleus (STN), the hyperdirect pathway that modulates response inhibition (Fling et al. 2013, 2014). Accordingly, FoG has been associated with cognitive function impairments, especially inhibitory control (Cohen et al. 2014). Decreased connectivity between the dorsolateral prefrontal cortex, a key region involved in the process of response inhibition, with basal ganglia (Shine et al. 2013c) and its correlation with the frequency of FoG supports the notion that there is a decoupling between inhibition control with movement. Furthermore, findings in a resting state functional magnetic resonance imaging demonstrate higher functional connectivity between areas that process sensory information (middle temporal gyrus) and emotion (amygdala) with MLR and basal ganglia, respectively (Gilat et al. 2018; Wang et al. 2016), and increased insula activation during FoG (Shine et al. 2013a). All this evidence shows the influence of cognition, sensory, and emotional processing in the pathophysiology of FoG.
3 Anticipatory Postural Adjustments During Step Initiation
During the step initiation (SI), the nervous system organizes a series of anticipatory postural adjustments (APAs), preparing the body for the disturbances caused by voluntary movement. Postural instability rapidly rises during SI as the base of support shifts and the center of mass (CoM) travels forward and outside the latter. Before foot-off, the initial stage of the SI process can be thought of as a forward fall that needs to be stopped but is essential for further forward body movement. These APAs are important because they release the load on the swing leg, establishing the conditions necessary for the step. Healthy subjects always behave in a rather stereotypical manner before SI. The CoM is properly shifted to the support leg to start a step, releasing the movement leg. Initially, there is a short shift from the center of mass to the moving leg, then the force is directed to the support leg (Breniere et al. 1987). As the first displacement of the CoM is toward the movement leg, inhibitory control mechanisms are required to direct the force–displacement toward the supporting leg. For example, if the objective is to initiate the step with the right leg, initially, the CoM is shifted to the right leg. This movement is inhibited and redirected to the left leg, which will serve as a support, allowing the release of the right leg. This change in body weight causes the center of pressure (CoP) to move backward and toward the swing leg. The CoP is then seen to move first toward the stance leg and subsequently forward. Toe-off of the swing leg happens right before the forward CoP displacement, while the heel-off happens at the beginning of the second phase of the CoP displacement (i.e., with a lateral shift toward the stance leg) (Breniere and Do 1991; Delval et al. 2005). The CoM lies outside the base of support during the single support phase of SI (when the subject lifts the swing leg off the ground), which causes the body to be imbalanced. The CoM fall must be stopped for movement to continue and prevent the body from falling to the ground. Before the swing limb leg makes contact with the ground, healthy adults’ ankle plantar flexors are activated to produce this braking action (Welter et al. 2007).
In individuals with PD (Fig. 3.3), different APA disturbances are observed, causing an abnormal coupling between posture and the motor program of APAs (Cohen et al. 2017; Hass et al. 2005; Mancini et al. 2009; Schlenstedt et al. 2018): bradykinetic and hypokinetic APAs, with the mediolateral and anteroposterior CoP changes are longer and weaker; pure akinesia, extended delays between the commencement of the APA and the onset of the step; and multiple APAs. The onset of these anomalies in PD can be quite early. Untreated early-to-moderate PD (Carpinella et al. 2007; Mancini et al. 2009) has been associated with a lower lateral, but not backward, APA magnitude, suggesting that the pathology of the disease may specifically affect how the legs are loaded and unloaded. Later in the disease, individuals with PD experience bradykinetic lateral and backward APAs. It is still controversial whether APAs are smaller or larger than normally associated with freezing of gait (Schlenstedt et al. 2018). Four out of eight studies reported smaller APA amplitudes and three with a longer duration in freezers than nonfreezers (for a review, see Bekkers et al. 2018a).
Anticipatory postural adjustment (APA) before step initiation. (a) Three phases of step initiation: quiet stance, weight transfer to the contralateral leg, and step. The red line shows mediolateral force amplitude during the APA, and the dashed line shows the forward displacement of the reflective marker attached to the ankle during the step. The shaded area indicates the APA. (b) Result of the average lateral force of the freezers, nonfreezers, and healthy
The motor program for SI must change when the motor task’s parameters change. APA is generated according to the context and inhibited if the support is directed to an inappropriate place (Cohen et al. 2011, 2017). Patients with PD seem to have difficulty releasing the step after it has been previously inhibited, which is further evidence of changes in the inhibitory control of step initiation present in patients with PD. Recent research has shown that SI of the healthy (Stins and Beek 2011) and PD (Lagravinese et al. 2018; Naugle et al. 2012) individuals are influenced by the valence of emotional inputs, suggesting that the limbic system may be involved in SI.
APA ranges from the participation of cortical and subcortical structures (Jacobs and Horak 2007; Yiou et al. 2017) to afferent signals that also emerge in the spinal cord, where they are modulated. Some important regions of the brain have been described as essential to initiate gait, such as mesencephalic, subthalamic, and cerebellar locomotor regions (Takakusaki 2017) in the subcortical level and premotor (PM) and supplementary motor area (SMA) (de Lima-Pardini et al. 2020; Jacobs et al. 2009a, b; Takakusaki 2017) in the cortical level. These cortical areas act in a feedforward control, projecting to the corticospinal tract (CST) (He et al. 1995) and having substantial connections with subcortical locomotor regions through cortico-reticular tracts. PM and SMA modulate step initiation by sending information on the required muscle tone to prepare the body for stepping according to the context via cortico-reticular and reticular spinal tracts (Keizer and Kuypers 1989). In parallel, SMA and PM contribute to the output from CST that elicit voluntary motor commands. Therefore, there are two important systems involved in gait initiation: one, comprising cortical and subcortical levels, that prepares the body by adjusting the muscle tone properly, and the other, that cortically modulates the voluntary movement of the leg. Given the challenge of shifting from an upright stance with both feet on the ground to a more unstable condition of moving the body forward, these two systems must be coupled to elicit a correct muscle tone background during the forward movement of the leg. When these systems work properly, gait initiation is characterized by an initial backward movement toward the moving leg, then to the support leg. The numerous connections between the basal ganglia and the supplementary motor area, and the premotor area, both of which are implicated in movement preparation (Massion 1992; Nakano et al. 2000; Schlenstedt et al. 2017), or with the peduncular pontine nucleus in the brainstem, which is implicated in locomotion initiation, may explain why APAs are impaired in PD (Pahapill and Lozano 2000). Changes in the basal ganglia, which slow down the sequential execution of the preparation and stepping task’s subcomponents, may also cause gait beginning issues in PD patients. Additionally, spinal control mechanisms are involved in SI and are important in executing motor commands (Fling et al. 2013; Honeine et al. 2016). Lira et al. (2020) show that loss of presynaptic inhibition (i.e., facilitation) during deficient APAs in the freezers may be due to FoG. Their results support the notion that lack of central inhibition of stance posture to allow stepping to commence is reflected in a loss of presynaptic inhibition in freezers, which is associated with more FoG severity. During the APA, only freezers showed facilitation rather than inhibition. This conclusion may be explained by three different aberrant changes in the processes underpinning the FoG in Parkinson’s disease: abnormal proprioceptive inputs, abnormal supraspinal motor commands, or a mix of both abnormalities (i.e., deficits in sensorimotor integration).
4 Reactive Response
Smaller than normal strength postural responses are the most evident abnormality of postural responses to external perturbations in PD. PD is linked to less stability in response to perturbations in all directions, but the difference is greatest in the backward direction (Carpenter et al. 2004). Postural response latencies are normal in individuals with PD despite the slowness to produce force and lower peak forces of postural responses, especially as the disease advances with increased bradykinesia and more falls. These findings also show that bradykinesia and akinesia significantly impact the rate of development and maximal muscular torque exerted by postural reactions (Horak et al. 1996).
Patients with Parkinson’s disease also exhibit abnormal muscle activation patterns in response to postural responses (Horak et al. 1992). For example, when using a feet-in-place ankle strategy, control subjects and subjects with Parkinson’s disease activate a distal to proximal pattern of muscle activation. Contrary to control subjects, individuals with PD frequently add short bursts of muscular activation in muscles that are antagonistic to one another, causing coactivation that would stiffen joints. However, antagonist muscle activation stops when people with Parkinson’s disease take levodopa medication. As a result, postural response muscle activation temporal patterns resemble normal, and joints are less rigid, even though levodopa does not increase the magnitude of postural responses to normal levels.
When responding to large forward perturbations, the body uses its hip, trunk, and knee joints and its ankle joint motion to restore equilibrium quickly. Individuals with PD keep their joints rigid, whereas control patients flex the hips/trunk in response to sideways perturbations and the knees in reaction to backward perturbations (Horak et al. 2005). Due to this rigidity, the same disturbances result in higher body displacements.
Lack of protective arm reaching reflexes to postural perturbations may also be caused by rigidity of joint mobility and decreased kinesthesia. When their postural stability is disturbed, healthy participants respond quickly and automatically, flexing or extending and abducting their shoulders. Hence, their arms lead their trunks and are likely to fall to the ground if their postural adjustments are ineffective. However, despite the PD’s much earlier beginning of deltoid muscle responses, this did not provide any practical protection since the PD’s arm motions were irregularly oriented, slower moving, and had smaller peak displacements (Carpenter et al. 2004).
5 Quiet Standing
For balance, the postural control system needs information about the relative positions of body segments and the magnitude of forces acting on the body. Simply put, the task of the postural control system is to maintain the horizontal projection of the individual’s CoM within the base of support defined by the area of the base of the feet during static upright posture. Stability is achieved by generating moments of force on the joints of the body to counteract the effect of gravity or any other disturbance in a continuous and dynamic process during the permanence of a certain posture. For balance regulation, the system needs information about the relative positions of body segments and the magnitude of forces acting on the body. For this, the body can use three sensors: somatosensory, visual, and vestibular. These sensors act in a complex, integrated, redundant, and differentiated way for each disturbance on the human body. The passive properties of the musculoskeletal system, especially the rigidity of biological structures, also play an important role in maintaining balance. The control of postural balance in a person is highly affected by the nature of the task, the environmental conditions, and the sensory information available. Body sway during upright posture is usually investigated using a force plate, a measuring instrument on which subjects remain standing during the experiments. The most common variable to analyze this oscillation is the position of the center of pressure (CoP), the point of application of the resultant forces acting on the support surface. The displacement of the CoP represents a sum of the actions of the postural control system and the force of gravity.
Sway is also increased in many neurological diseases impacting sensory and/or motor systems. Postural control during quiet standing is controversial in individuals with PD. While some studies show that postural sway can be abnormal before levodopa administration (Mancini et al. 2011) and even in the prodromal period (Maetzler and Hausdorff 2012), other studies show that it is similar to normal (Bronte-Stewart et al. 2002), at least at the earlier stages (Frenklach et al. 2009). The increased mediolateral sway in PD has been a more persistent result (Mitchell et al. 1995), associated with a history of falls (Visser et al. 2008). It is still unclear what the underlying mechanisms involved in freezing of gait (FoG) and postural control are. Quiet standing is largely similar between freezers and nonfreezeres, but the susceptibility for backward postural instability in people with FoG increases (for a review, see Bekkers et al. 2018a).
Individuals with severe PD frequently suffer from standing on unstable surfaces (Frenklach et al. 2009), which suggests reduced proprioception (Bronstein et al. 1990). In addition, there is growing evidence that the basal ganglia are related to reduced tactile discrimination (Tagliabue et al. 2009), suggesting that supraspinal circuits could be responsible for abnormal sensory integration for postural control in PD.
Patients with PD, whether in the ON or OFF state, exhibit an initially rigid strategy independent of the conditions of their surface support, changing gradually over time and with several repeats (Horak et al. 1992). This rigidity also explains why PD patients frequently experience less improvement than anticipated with manual assistance and find it challenging to benefit from canes and walkers without extensive training.
6 Prolonged Standing
Daily activities like waiting in line or talking to someone usually involve standing for a long time (more than a few minutes). In working conditions as well as in activities of daily living, some people remain standing for a long time, mostly confined to a small area. In the natural standing posture, people usually adopt asymmetrical postures and change their body position periodically while maintaining a relatively fixed body posture. The continuous, slow, low-amplitude oscillation is commonly interrupted by postural changes characterized by rapid and wide-ranging movements (Duarte et al. 2000). These postural changes are thought to be performed to alleviate the discomfort caused by psychological (tension, mental stress, and decreased motivation and concentration) and physiological factors (increase of venous pooling in the lower extremities, occlusion of blood flow, vertigo, muscular fatigue, and increased joint pressure) (Cavanagh et al. 1987; Edwards 1988; Kraemer et al. 2000). In this sense, during prolonged standing, postural shifts and an increase in body sway are considered effective responses of the postural control system to carry out the activity with the least amount of effort. Less postural changes and sway areas are conservative strategies (de Freitas et al. 2009). Aging reduces the adaptability of postural control, resulting in fewer postural shifts and, as a result, less body sway (Duarte and Sternad 2008). Even though standing still for a long period is a common task in people’s daily lives, it can present a high degree of difficulty for those with balance deficits, such as individuals with PD. Moretto et al. (2021) compared the postural control during the prolonged posture of individuals with PD to healthy individuals. Their results show that individuals with PD reduced body sway (smaller sway area), faster sway, and postural control complexity compared with the control group during prolonged standing. These results indicate impaired adaptation and a lack of postural control to produce a sufficient response in individuals with PD during prolonged standing. Faster sway during prolonged standing in Parkinson’s disease is an aging effect, implying a decline in neural processing and delayed muscle activation, which affects feedback and feedforward control and reduces sway.
7 Dynamic Balance During Walking
The gait pattern altered by PD is one of the motor signs that most affect the quality of life of individuals with PD, with more than 50% of falls in these individuals occurring during gait (Lord et al. 2017). The gait of individuals with PD tends to be slower, characterized by narrow and short steps, flexed trunk, little or no arm swing, and slow and spasmodic turning (Mancini et al. 2019). When analyzing the spatiotemporal parameters of gait in individuals with PD compared to healthy individuals (Fig. 3.4), studies have shown decreased speed (Cheng et al. 2014; Mondal et al. 2019; Morris et al. 1999), increased number of steps (Mondal et al. 2019), decrease in step length (Cheng et al. 2014; Mondal et al. 2019), and stride length (Mondal et al. 2019); shorter duration of the swing phase and unipedal stance phase (Mondal et al. 2019); and longer duration of the double support phase (Mondal et al. 2019; Sofuwa et al. 2005). Galna et al. (2015) evaluated 16 gait parameters of individuals with PD in the early stages and identified that 12 were different between older adults with PD and neurologically healthy older adults. These differences are in the measures of step speed, step length, variability of swing phase duration, stance phase duration, stride duration, stride length, and stride width, in stride duration time and stride phase duration, stance and asymmetry of step duration, and swing phase and stance phase.
Subdivision of the gait cycle illustrating the differences in the spatiotemporal parameters of the gait of an individual with Parkinson’s disease to neurologically healthy individuals. The red color indicates worse indices for PD. The up arrow indicates an increase, while the down arrow indicates a decrease
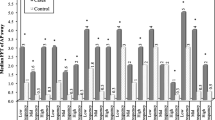
Regarding kinematic parameters of gait in PD, studies indicate a lower range of motion of the hips in the coronal plane and of the pelvic obliquity, lower range of flexion-extension of the knees with a high degree of flexion in the initial contact, and the stance phase; and greater ankle dorsiflexion during the stance phase, compared to healthy individuals (Pistacchi et al. 2017; Sale et al. 2013; Zanardi et al. 2021). Our group analyzed the joint angles of the lower limb of individuals with PD compared to healthy individuals (Fig. 3.5). Our results show greater differences in the distal joints to the proximal ones. Albani et al. (2014) found that individuals in the early stage of PD have lower ankle power during terminal stance compared to healthy individuals and a lower maximum dorsiflexion moment during the stance phase. Morris et al. (2005) found a significant reduction in range of motion in the sagittal plane of the hip, knee, and ankle joints, in addition to lower pelvic obliquity and rotation, and reduced hip abduction in individuals with PD compared to healthy older adults.
Angular kinematics during the gait of the Parkinson’s disease group in the ON medication (grey) and the age-matched healthy elderly control group (red). Mean and standard deviation of the angular displacement of the pelvis, hip, knee, and foot joints in the three joint axes of the most affected limb. The hatched areas indicate the difference between the groups
Individuals with PD have deficits in gait at a constant speed (Hausdorff 2009), and in gait planning and modulation in challenging tasks (Rochester et al. 2014; Vitorio et al. 2010, 2013, 2014). This indicates that PD affects gait automation, defined as the ability of the nervous system to stably control gait with minimal use of attentional resources (Clark et al. 2014; Schneider and Shiffrin 1977). Furthermore, it is suggested that this deficiency in gait automation is responsible for increased variability in spatiotemporal gait parameters and greater activation of prefrontal cortical regions during locomotion of individuals with PD compared to their neurologically healthy peers (Hausdorff 2009; Maidan et al. 2016; Peterson and Horak 2016).
Regarding the effect of the medication on the gait of individuals with PD, in general, during the ON state of the medication, the self-selected gait speed increases (Curtze et al. 2015; Mondal et al. 2019). However, during gait in the OFF state, individuals with PD show greater cortical activation when compared to healthy individuals (Stuart et al. 2019), which could be a possible explanation for the change in gait speed as an effect of medication. In a recent study, Orcioli-Silva et al. (2021) identified lower activation of the prefrontal cortex in subjects with PD in the OFF state during obstacle clearance compared to activation during unobstructed gait, an effect that was reversed after drug ingestion. On the other hand, the authors identified that individuals with PD in the ON state showed an increase in prefrontal cortex activation during gait with overcoming obstacles when compared with activation during unobstructed gait. Furthermore, it was identified that medication-related changes (i.e., ON–OFF) in posterior parietal cortex activation were associated with medication-related changes in step length for both walking conditions. These results suggest that dopaminergic medication may facilitate the recruitment of attentional-executive resources from the prefrontal cortex in challenging situations (overcoming obstacles), in addition to increasing sensorimotor integration (increased activation of the posterior parietal cortex) during gait. Furthermore, Gilat et al. (2017) showed that individuals in the ON state of the medication show improvement in gait automaticity, with greater cerebellar activation at times of greater gait variability. Furthermore, their results showed a high correlation between attentional cortical participation and step time variability in the OFF state, without the same correlation occurring in the ON state.
On the other hand, some authors argue that the increase in gait speed in the ON state may result not only from improved automatism but also increased task engagement from dopaminergic signaling in the perception of effort (Albin and Leventhal 2017). For example, Mazzoni et al. (2007) showed that individuals with PD, compared to healthy individuals, are more likely to move more slowly (lower motor vigor) when the energy demands of movement increase. That is, they present a possible alteration in the mechanism of perception of effort and reward to healthy individuals. On the other hand, Chong et al. (2015) showed that individuals with PD in the ON state were willing to invest more effort in a given task than individuals in the OFF state. The authors then concluded that medication motivated the behavior of individuals who chose to exert greater effort while performing the task. Thus, regardless of which dopaminergic signaling pathway acts (motor or motivational), there is a consensus in the literature that drug treatment can increase gait speed in individuals with PD.
Despite the consensus in the literature about the improvement in gait speed in people with PD treated with levodopa, when specifically analyzing spatiotemporal parameters such as stride length, cadence, step initiation, step time, gait phases, among others, the results are not always consistent. For example, Curtze et al. (2015) showed that individuals increased stride speed and length in the ON state without influencing cadence, step initiation, double support time, and swing time. Mondal et al. (2019) showed that in the ON state, there was a decrease in the number of steps, an increase in step length, an increase in stride length, and a decrease in double support time. In the same study, the medication did not affect cadence, single-leg stance time, step time, cycle time, swing time, and base of support width. This variety of results makes it difficult to conclude anything about the effect of dopaminergic medication on spatiotemporal gait parameters. While Curtze et al. (2015) argued that levodopa improves gait without changing parameters related to its dynamic stability, Mondal et al. (2019) discussed that parameters related to gait rhythm are resistant to levodopa and parameters that require caloric expenditure (i.e., stride length) are sensitive to medication.
The pathological substrates of gait impairments in PD are still not fully understood. However, it is suggested that the degeneration of dopaminergic neurons in the substantia nigra with projection to the basal nuclei affects gait, as these brain structures are responsible, among other functions, for context-specific adaptation, motor coordination, body posture, and gait automation (Mancini et al. 2019). Furthermore, alterations in the cerebral cortex in the generation and propagation of neural rhythms have also been identified as involved in the locomotor deficits of individuals with PD (Arbuthnott and Garcia-Munoz 2009; Galna et al. 2015; la Fougere et al. 2010).
8 Conclusion
Poor balance control and postural instability are among the most incapacitating aspects of PD. Balance issues in PD go beyond simply being a symptom of motor failure. Balance issues in PD result from dysfunctions in the cognitive, emotional, sensory, and autonomic systems. The regulation of balance involves a wide variety of brain networks. The basal ganglia play four key functions: posture-movement coupling, energization/scaling (vigor), automatization, and context-dependent adaptability. Multiple components of balance regulation, such as standing balance, reactive postural responses, anticipatory postural changes before gait initiation, and dynamic balance during walking and turning, are all affected by PD:
-
(a)
FoG and falls go together frequently. More balance impairments are present in freezers than in nonfreezers, especially during dynamic balance during walking and anticipatory postural adjustments.
-
(b)
PD have delayed, hypometric, and rigid anticipatory postural adjustments before step initiations.
-
(c)
Although PD has normal onset latency, postural responses to external perturbations are weak and slow.
-
(d)
Limits of stability are lowered, particularly in the forward motion.
-
(e)
The axial tone is higher than usual, and increased neck tone is associated with decreased functional movement.
-
(f)
Proprioception may be difficult for PD to integrate for standing balance swiftly.
-
(g)
Parkinsonian gait shows slow, shuffling, and variable steps.
References
Albani, G., Cimolin, V., Fasano, A., Trotti, C., Galli, M., & Mauro, A. (2014, Apr–Jun). “Masters and servants” in parkinsonian gait: a three-dimensional analysis of biomechanical changes sensitive to disease progression. Functional Neurology, 29(2), 99–105. https://www.ncbi.nlm.nih.gov/pubmed/25306119
Albin, R. L., & Leventhal, D. K. (2017, Jul). The missing, the short, and the long: Levodopa responses and dopamine actions. Annals of neurology, 82(1), 4–19. doi: https://doi.org/10.1002/ana.24961
Arbuthnott, G., & Garcia-Munoz, M. (2009, Dec). Dealing with the devil in the detail – some thoughts about the next model of the basal ganglia. Parkinsonism & related disorders, 15 Suppl 3, S139–142. doi: https://doi.org/10.1016/S1353-8020(09)70801-6
Bastide, M. F., Meissner, W. G., Picconi, B., Fasano, S., Fernagut, P. O., Feyder, M., Francardo, V., Alcacer, C., Ding, Y., Brambilla, R., Fisone, G., Jon Stoessl, A., Bourdenx, M., Engeln, M., Navailles, S., De Deurwaerdere, P., Ko, W. K., Simola, N., Morelli, M., Groc, L., Rodriguez, M. C., Gurevich, E. V., Quik, M., Morari, M., Mellone, M., Gardoni, F., Tronci, E., Guehl, D., Tison, F., Crossman, A. R., Kang, U. J., Steece-Collier, K., Fox, S., Carta, M., Angela Cenci, M., & Bezard, E. (2015, Sep). Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Progress in neurobiology, 132, 96–168. doi: https://doi.org/10.1016/j.pneurobio.2015.07.002
Bekkers, E. M. J., Dijkstra, B. W., Dockx, K., Heremans, E., Verschueren, S. M. P., & Nieuwboer, A. (2017, Jul). Clinical balance scales indicate worse postural control in people with Parkinson’s disease who exhibit freezing of gait compared to those who do not: A meta-analysis. Gait & Posture, 56, 134–140. doi: https://doi.org/10.1016/j.gaitpost.2017.05.009
Bekkers, E. M. J., Dijkstra, B. W., Heremans, E., Verschueren, S. M. P., Bloem, B. R., & Nieuwboer, A. (2018a, Nov). Balancing between the two: Are freezing of gait and postural instability in Parkinson’s disease connected? Neuroscience and biobehavioral reviews, 94, 113–125. doi: https://doi.org/10.1016/j.neubiorev.2018.08.008
Bekkers, E. M. J., Van Rossom, S., Heremans, E., Dockx, K., Devan, S., Verschueren, S. M. P., & Nieuwboer, A. (2018b). Adaptations to Postural Perturbations in Patients With Freezing of Gait. Frontiers in Neurology, 9, 540. doi: https://doi.org/10.3389/fneur.2018.00540
Bloem, B. R., Hausdorff, J. M., Visser, J. E., & Giladi, N. (2004, Aug). Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Movement Disorders, 19(8), 871–884. doi: https://doi.org/10.1002/mds.20115
Breniere, Y., Cuong Do, M., & Bouisset, S. (1987, Mar). Are dynamic phenomena prior to stepping essential to walking? Journal of motor behavior, 19(1), 62–76. doi: https://doi.org/10.1080/00222895.1987.10735400
Breniere, Y., & Do, M. C. (1991, Dec). Control of gait initiation. Journal of motor behavior, 23(4), 235–240. doi: https://doi.org/10.1080/00222895.1991.9942034
Bronstein, A. M., Hood, J. D., Gresty, M. A., & Panagi, C. (1990, Jun). Visual control of balance in cerebellar and parkinsonian syndromes. 113 (Pt 3), 767–779. doi: https://doi.org/10.1093/brain/113.3.767
Bronte-Stewart, H. M., Minn, A. Y., Rodrigues, K., Buckley, E. L., & Nashner, L. M. (2002, Sep). Postural instability in idiopathic Parkinson’s disease: the role of medication and unilateral pallidotomy. 125(Pt 9), 2100–2114. http://www.ncbi.nlm.nih.gov/pubmed/12183355
Butler, J. S., Fearon, C., Killane, I., Waechter, S. M., Reilly, R. B., & Lynch, T. (2017, Mar). Motor preparation rather than decision-making differentiates Parkinson’s disease patients with and without freezing of gait. 128(3), 463–471. doi: https://doi.org/10.1016/j.clinph.2016.12.019
Carpenter, M. G., Allum, J. H., Honegger, F., Adkin, A. L., & Bloem, B. R. (2004, Sep). Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. 75(9), 1245–1254. doi: https://doi.org/10.1136/jnnp.2003.021147 75/9/1245 [pii]
Carpinella, I., Crenna, P., Calabrese, E., Rabuffetti, M., Mazzoleni, P., Nemni, R., & Ferrarin, M. (2007, Dec). Locomotor function in the early stage of Parkinson’s disease. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society, 15(4), 543–551. doi: https://doi.org/10.1109/TNSRE.2007.908933
Cavanagh, P. R., Rodgers, M. M., & Iiboshi, A. (1987, Apr). Pressure distribution under symptom-free feet during barefoot standing. 7(5), 262–276. doi: https://doi.org/10.1177/107110078700700502
Cheng, K. Y., Lin, W. C., Chang, W. N., Lin, T. K., Tsai, N. W., Huang, C. C., Wang, H. C., Huang, Y. C., Chang, H. W., Lin, Y. J., Lee, L. H., Cheng, B. C., Kung, C. T., Chang, Y. T., Su, C. M., Chiang, Y. F., Su, Y. J., & Lu, C. H. (2014, Jan). Factors associated with fall-related fractures in Parkinson’s disease. Parkinsonism & related disorders, 20(1), 88–92. doi: https://doi.org/10.1016/j.parkreldis.2013.09.024
Chong, T. T., Bonnelle, V., Manohar, S., Veromann, K. R., Muhammed, K., Tofaris, G. K., Hu, M., & Husain, M. (2015, Aug). Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex; a journal devoted to the study of the nervous system and behavior, 69, 40–46. doi: https://doi.org/10.1016/j.cortex.2015.04.003
Clark, D. J., Christou, E. A., Ring, S. A., Williamson, J. B., & Doty, L. (2014, Nov). Enhanced somatosensory feedback reduces prefrontal cortical activity during walking in older adults. The journals of gerontology. Series A, Biological sciences and medical sciences, 69(11), 1422–1428. doi: https://doi.org/10.1093/gerona/glu125
Coelho, D. B., Ribeiro de Souza, C., de Lima-Pardini, A. C., Treza, R. C., Shida, T. K. F., Silva-Batista, C., & Teixeira, L. A. (2021, Feb). Is freezing of gait correlated with postural control in patients with moderate-to-severe Parkinson’s disease? European Journal of Neuroscience, 53(4), 1189–1196. doi: https://doi.org/10.1111/ejn.15010
Cohen, R. G., Klein, K. A., Nomura, M., Fleming, M., Mancini, M., Giladi, N., Nutt, J. G., & Horak, F. B. (2014). Inhibition, executive function, and freezing of gait. Journal of Parkinson’s Disease, 4(1), 111–122. doi: https://doi.org/10.3233/JPD-130221
Cohen, R. G., Nutt, J. G., & Horak, F. B. (2011, Jun). Errors in postural preparation lead to increased choice reaction times for step initiation in older adults. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 66(6), 705–713. doi: https://doi.org/10.1093/gerona/glr054
Cohen, R. G., Nutt, J. G., & Horak, F. B. (2017). Recovery from Multiple APAs Delays Gait Initiation in Parkinson’s Disease. Frontiers in Human Neuroscience, 11, 60. doi: https://doi.org/10.3389/fnhum.2017.00060
Curtze, C., Nutt, J. G., Carlson-Kuhta, P., Mancini, M., & Horak, F. B. (2015, Sep). Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Movement Disorders, 30(10), 1361–1370. doi: https://doi.org/10.1002/mds.26269
de Freitas, P. B., Freitas, S. M., Duarte, M., Latash, M. L., & Zatsiorsky, V. M. (2009, Aug). Effects of joint immobilization on standing balance. 28(4), 515–528. doi: https://doi.org/10.1016/j.humov.2009.02.001
de Lima-Pardini, A. C., Coelho, D. B., Nucci, M. P., Boffino, C. C., Batista, A. X., de Azevedo Neto, R. M., Silva-Batista, C., Barbosa, E. R., Cohen, R. G., Horak, F. B., Teixeira, L. A., & Amaro, E., Jr. (2020). Brain networks associated with anticipatory postural adjustments in Parkinson’s disease patients with freezing of gait. NeuroImage. Clinical, 28, 102461. doi: https://doi.org/10.1016/j.nicl.2020.102461
de Souza Fortaleza, A. C., Mancini, M., Carlson-Kuhta, P., King, L. A., Nutt, J. G., Chagas, E. F., Freitas, I. F. J., & Horak, F. B. (2017, Jul). Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait & Posture, 56, 76–81. doi: https://doi.org/10.1016/j.gaitpost.2017.05.006
Del Tredici, K., & Braak, H. (2016, Feb). Review: Sporadic Parkinson’s disease: development and distribution of alpha-synuclein pathology. Neuropathology and applied neurobiology, 42(1), 33–50. doi: https://doi.org/10.1111/nan.12298
Delval, A., Krystkowiak, P., Blatt, J. L., Labyt, E., Destee, A., Derambure, P., & Defebvre, L. (2005, Nov-Dec). [Differences in anticipatory postural adjustments between self-generated and triggered gait initiation in 20 healthy subjects]. Neurophysiologie clinique = Clinical neurophysiology, 35(5–6), 180–190. doi: https://doi.org/10.1016/j.neucli.2006.01.002 (Caracterisation des ajustements posturaux lors d’une initiation de la marche declenchee par un stimulus sonore et autocommandee chez 20 sujets sains.)
Donker, S. F., Roerdink, M., Greven, A. J., & Beek, P. J. (2007, Jul). Regularity of center-of-pressure trajectories depends on the amount of attention invested in postural control. Experimental Brain Research, 181(1), 1–11. doi: https://doi.org/10.1007/s00221-007-0905-4
Duarte, M., Harvey, W., & ZatsiorskyVm. (2000, Nov). Stabilographic analysis of unconstrained standing. 43(11), 1824–1839. doi: https://doi.org/10.1080/00140130050174491
Duarte, M., & Sternad, D. (2008, Nov). Complexity of human postural control in young and older adults during prolonged standing. 191(3), 265–276. doi: https://doi.org/10.1007/s00221-008-1521-7
Edwards, R. H. (1988). Hypotheses of peripheral and central mechanisms underlying occupational muscle pain and injury. 57(3), 275–281. doi: https://doi.org/10.1007/BF00635985
Ehgoetz Martens, K. A., Hall, J. M., Georgiades, M. J., Gilat, M., Walton, C. C., Matar, E., Lewis, S. J. G., & Shine, J. M. (2018, Apr 1). The functional network signature of heterogeneity in freezing of gait. Brain, 141(4), 1145–1160. doi: https://doi.org/10.1093/brain/awy019
Fling, B. W., Cohen, R. G., Mancini, M., Carpenter, S. D., Fair, D. A., Nutt, J. G., & Horak, F. B. (2014). Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. 9(6), e100291. doi: https://doi.org/10.1371/journal.pone.0100291
Fling, B. W., Cohen, R. G., Mancini, M., Nutt, J. G., Fair, D. A., & Horak, F. B. (2013, Aug). Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain: a journal of neurology, 136(Pt 8), 2405–2418. doi: https://doi.org/10.1093/brain/awt172
Fonoff, E. T., de Lima-Pardini, A. C., Coelho, D. B., Monaco, B. A., Machado, B., Pinto de Souza, C., Dos Santos Ghilardi, M. G., & Hamani, C. (2019). Spinal Cord Stimulation for Freezing of Gait: From Bench to Bedside. Frontiers in Neurology, 10, 905. doi: https://doi.org/10.3389/fneur.2019.00905
Frenklach, A., Louie, S., Koop, M. M., & Bronte-Stewart, H. (2009, Feb 15). Excessive postural sway and the risk of falls at different stages of Parkinson’s disease. 24(3), 377–385. doi: 10.1002/mds.22358
Gallardo, M. J., Cabello, J. P., Corrales, M. J., Torres-Donaire, J., Bravo, J. J., Talavera, M. P., Leon, A., & Vaamonde-Gamo, J. (2018, Oct). Freezing of gait in Parkinson’s disease: functional neuroimaging studies of the frontal lobe. Neurological Research, 40(10), 900–905. doi: https://doi.org/10.1080/01616412.2018.1484985
Galna, B., Lord, S., Burn, D. J., & Rochester, L. (2015, Mar). Progression of gait dysfunction in incident Parkinson’s disease: impact of medication and phenotype. Movement disorders: official journal of the Movement Disorder Society, 30(3), 359–367. doi: https://doi.org/10.1002/mds.26110
Gao, C., Liu, J., Tan, Y., & Chen, S. (2020). Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Translational neurodegeneration, 9, 12. doi: https://doi.org/10.1186/s40035-020-00191-5
Gazibara, T., Kisic Tepavcevic, D., Svetel, M., Tomic, A., Stankovic, I., Kostic, V. S., & Pekmezovic, T. (2017, Oct). Near-falls in people with Parkinson’s disease: Circumstances, contributing factors and association with falling. Clinical Neurology and Neurosurgery, 161, 51–55. doi: https://doi.org/10.1016/j.clineuro.2017.08.008
Gilat, M., Bell, P. T., Ehgoetz Martens, K. A., Georgiades, M. J., Hall, J. M., Walton, C. C., Lewis, S. J. G., & Shine, J. M. (2017, May 15). Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. NeuroImage, 152, 207–220. doi: https://doi.org/10.1016/j.neuroimage.2017.02.073
Gilat, M., Ehgoetz Martens, K. A., Miranda-Dominguez, O., Arpan, I., Shine, J. M., Mancini, M., Fair, D. A., Lewis, S. J. G., & Horak, F. B. (2018, Mar 15). Dysfunctional Limbic Circuitry Underlying Freezing of Gait in Parkinson’s Disease. Neuroscience, 374, 119–132. doi: https://doi.org/10.1016/j.neuroscience.2018.01.044
Gilat, M., Shine, J. M., Walton, C. C., O’Callaghan, C., Hall, J. M., & Lewis, S. J. G. (2015). Brain activation underlying turning in Parkinson’s disease patients with and without freezing of gait: a virtual reality fMRI study. NPJ Parkinsons Disease, 1, 15020. doi: https://doi.org/10.1038/npjparkd.2015.20
Hallett, M. (2008). The intrinsic and extrinsic aspects of freezing of gait. Movement Disorders, 23 Suppl 2, S439–443. doi: https://doi.org/10.1002/mds.21836
Hass, C. J., Waddell, D. E., Fleming, R. P., Juncos, J. L., & Gregor, R. J. (2005, Nov). Gait initiation and dynamic balance control in Parkinson’s disease. Archives of physical medicine and rehabilitation, 86(11), 2172–2176. doi: https://doi.org/10.1016/j.apmr.2005.05.013
Hausdorff, J. M. (2009, Jun). Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos, 19(2), 026113. doi: https://doi.org/10.1063/1.3147408
Hawkes, C. H., Del Tredici, K., & Braak, H. (2010, Feb). A timeline for Parkinson’s disease. Parkinsonism & related disorders, 16(2), 79–84. doi: https://doi.org/10.1016/j.parkreldis.2009.08.007
He, S. Q., Dum, R. P., & Strick, P. L. (1995, May). Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. The Journal of Neuroscience, 15(5 Pt 1), 3284–3306. https://www.ncbi.nlm.nih.gov/pubmed/7538558
Hoehn, M. M., & Yahr, M. D. (1967, May). Parkinsonism: onset, progression and mortality. Neurology, 17(5), 427–442. doi: https://doi.org/10.1212/wnl.17.5.427
Honeine, J. L., Schieppati, M., Crisafulli, O., & Do, M. C. (2016). The Neuro-Mechanical Processes That Underlie Goal-Directed Medio-Lateral APA during Gait Initiation. Frontiers in human neuroscience, 10, 445. doi: https://doi.org/10.3389/fnhum.2016.00445
Horak, F. B., Dimitrova, D., & Nutt, J. G. (2005). Direction-specific postural instability in subjects with Parkinson’s disease. Experimental Neurology, 193(2), 504–521. doi: https://doi.org/10.1016/j.expneurol.2004.12.008
Horak, F. B., Frank, J., & Nutt, J. (1996, Jun). Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. Journal of Neurophysiology, 75(6), 2380–2396. http://www.ncbi.nlm.nih.gov/pubmed/8793751 (J. Neurophysiol.)
Horak, F. B., Nutt, J. G., & Nashner, L. M. (1992, Aug). Postural inflexibility in parkinsonian subjects. Journal of the Neurological Sciences, 111(1), 46–58. doi: https://doi.org/10.1016/0022-510X(92)90111-W (J. Neurol. Sci.)
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of Neural Transmission, 114(10), 1339–1348. doi: https://doi.org/10.1007/s00702-007-0657-0 (J. Neural Transm.)
Jacobs, J. V., Lou, J. S., Kraakevik, J. A., & Horak, F. B. (2009a, Dec 1). The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience, 164(2), 877–885. doi: https://doi.org/10.1016/j.neuroscience.2009.08.002 (Neuroscience)
Jacobs, J. V., Nutt, J. G., Carlson-Kuhta, P., Stephens, M., & Horak, F. B. (2009b, Feb). Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Experimental Neurology, 215(2), 334–341. doi: https://doi.org/10.1016/j.expneurol.2008.10.019
Jankovic, J. (2008, Apr). Parkinson’s disease: clinical features and diagnosis. Journal of neurology, neurosurgery, and psychiatry, 79(4), 368–376. doi: https://doi.org/10.1136/jnnp.2007.131045
Jellinger, K. A. (2003, Sep). Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution – a pilot study. Acta neuropathologica, 106(3), 191–201. doi: https://doi.org/10.1007/s00401-003-0725-y
Kalia, L. V., & Lang, A. E. (2015). Parkinson’s disease. 386(9996), 896–912. doi: https://doi.org/10.1016/s0140-6736(14)61393-3
Kehagia, A. A., Barker, R. A., & Robbins, T. W. (2013). Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neuro-degenerative diseases, 11(2), 79–92. doi: https://doi.org/10.1159/000341998
Keizer, K., & Kuypers, H. G. (1989). Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Experimental Brain Research, 74(2), 311–318. doi: https://doi.org/10.1007/BF00248864
Kraemer, W. J., Volek, J. S., Bush, J. A., Gotshalk, L. A., Wagner, P. R., Gomez, A. L., Zatsiorsky, V. M., Duarte, M., Ratamess, N. A., Mazzetti, S. A., & Selle, B. J. (2000, Nov). Influence of compression hosiery on physiological responses to standing fatigue in women. 32(11), 1849–1858. doi: https://doi.org/10.1097/00005768-200011000-00006
la Fougere, C., Zwergal, A., Rominger, A., Forster, S., Fesl, G., Dieterich, M., Brandt, T., Strupp, M., Bartenstein, P., & Jahn, K. (2010, May 1). Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. 50(4), 1589–1598. doi: https://doi.org/10.1016/j.neuroimage.2009.12.060 S1053-8119(09)01349-4 [pii]
Lagravinese, G., Pelosin, E., Bonassi, G., Carbone, F., Abbruzzese, G., & Avanzino, L. (2018, Apr). Gait initiation is influenced by emotion processing in Parkinson’s disease patients with freezing. Movement disorders: official journal of the Movement Disorder Society, 33(4), 609–617. doi: https://doi.org/10.1002/mds.27312
Lewis, S. J., & Barker, R. A. (2009, Jun). A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism & related disorders, 15(5), 333–338. doi: https://doi.org/10.1016/j.parkreldis.2008.08.006
Lira, J. L. O., Ugrinowitsch, C., Coelho, D. B., Teixeira, L. A., de Lima-Pardini, A. C., Magalhaes, F. H., Barbosa, E. R., Horak, F. B., & Silva-Batista, C. (2020, Apr). Loss of presynaptic inhibition for step initiation in parkinsonian individuals with freezing of gait. The Journal of Physiology, 598(8), 1611–1624. doi: https://doi.org/10.1113/JP279068
Lord, S., Galna, B., Yarnall, A. J., Morris, R., Coleman, S., Burn, D., & Rochester, L. (2017, Nov). Natural history of falls in an incident cohort of Parkinson’s disease: early evolution, risk and protective features. Journal of neurology, 264(11), 2268–2276. doi: https://doi.org/10.1007/s00415-017-8620-y
Lord, S. R., Bindels, H., Ketheeswaran, M., Brodie, M. A., Lawrence, A. D., Close, J. C. T., Whone, A. L., Ben-Shlomo, Y., & Henderson, E. J. (2020). Freezing of Gait in People with Parkinson’s Disease: Nature, Occurrence, and Risk Factors. Journal of Parkinson’s Disease, 10(2), 631–640. doi: https://doi.org/10.3233/JPD-191813
Maetzler, W., & Hausdorff, J. M. (2012, Apr 15). Motor signs in the prodromal phase of Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society, 27(5), 627–633. doi: https://doi.org/10.1002/mds.24973
Maidan, I., Nieuwhof, F., Bernad-Elazari, H., Reelick, M. F., Bloem, B. R., Giladi, N., Deutsch, J. E., Hausdorff, J. M., Claassen, J. A., & Mirelman, A. (2016, Nov). The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: an fNIRS study. Neurorehabilitation and Neural Repair, 30(10), 963–971. doi: https://doi.org/10.1177/1545968316650426 (Neurorehabil. Neural Repair.)
Mancini, M., Horak, F. B., Zampieri, C., Carlson-Kuhta, P., Nutt, J. G., & Chiari, L. (2011, Aug). Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism & Related Disorders, 17(7), 557–562. doi: https://doi.org/10.1016/j.parkreldis.2011.05.010
Mancini, M., Nutt, J. G., & Horak, F. B. (2019). Balance dysfunction in Parkinson’s disease: basic mechanisms to clinical management. Cambridge, MA, USA. https://doi.org/10.1016/C2017-0-00054-X
Mancini, M., Zampieri, C., Carlson-Kuhta, P., Chiari, L., & Horak, F. B. (2009, Sep). Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson’s disease: an accelerometer-based approach. 16(9), 1028–1034. doi: https://doi.org/10.1111/j.1468-1331.2009.02641.x
Massion, J. (1992). Movement, posture and equilibrium: interaction and coordination. Progress in Neurobiology, 38(1), 35–56. doi: 0301-0082(92)90034-C [pii]
Matsui, H., Udaka, F., Miyoshi, T., Hara, N., Tamaura, A., Oda, M., Kubori, T., Nishinaka, K., & Kameyama, M. (2005, Oct). Three-dimensional stereotactic surface projection study of freezing of gait and brain perfusion image in Parkinson’s disease. Movement Disorders, 20(10), 1272–1277. doi: https://doi.org/10.1002/mds.20520
Mazzoni, P., Hristova, A., & Krakauer, J. W. (2007, Jul 4). Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. The Journal of neuroscience: the official journal of the Society for Neuroscience, 27(27), 7105–7116. doi: https://doi.org/10.1523/JNEUROSCI.0264-07.2007
Michalowska, M., Fiszer, U., Krygowska-Wajs, A., & Owczarek, K. (2005, Oct-Dec). Falls in Parkinson’s disease. Causes and impact on patients’ quality of life. Functional neurology, 20(4), 163–168. http://www.ncbi.nlm.nih.gov/pubmed/16483454
Mitchell, S. L., Collins, J. J., De Luca, C. J., Burrows, A., & Lipsitz, L. A. (1995, Sep 8). Open-loop and closed-loop postural control mechanisms in Parkinson’s disease: increased mediolateral activity during quiet standing. 197(2), 133–136. doi: 030439409511924L [pii]
Mondal, B., Choudhury, S., Banerjee, R., Chatterjee, K., Ghosal, S., Anand, S. S., & Kumar, H. (2019). Analysis of gait in Parkinson’s disease reflecting the effect of l-DOPA. Annals of Movement Disorders, 2(1), 21–27. doi: https://doi.org/10.4103/AOMD.AOMD_19_18
Moretto, G. F., Santinelli, F. B., Penedo, T., Mochizuki, L., Rinaldi, N. M., & Barbieri, F. A. (2021, Jan). Prolonged Standing Task Affects Adaptability of Postural Control in People With Parkinson’s Disease. 35(1), 58–67. doi: https://doi.org/10.1177/1545968320971739
Morris, M., Iansek, R., McGinley, J., Matyas, T., & Huxham, F. (2005, Jan). Three-dimensional gait biomechanics in Parkinson’s disease: evidence for a centrally mediated amplitude regulation disorder. Movement Disorders, 20(1), 40–50. doi: https://doi.org/10.1002/mds.20278
Morris, M. E., McGinley, J., Huxham, F., Collier, J., & Iansek, R. (1999). Constraints on the kinetic, kinematic and spatiotemporal parameters of gait in Parkinson’s disease. Human Movement Science, 18(2–3), 461–483. doi: https://doi.org/10.1016/s0167-9457(99)00020-2
Nakano, K., Kayahara, T., Tsutsumi, T., & Ushiro, H. (2000, Sep). Neural circuits and functional organization of the striatum. Journal of neurology, 247 Suppl 5, V1-15. doi: https://doi.org/10.1007/pl00007778
Naugle, K. M., Hass, C. J., Bowers, D., & Janelle, C. M. (2012, Mar). Emotional state affects gait initiation in individuals with Parkinson’s disease. Cognitive, affective & behavioral neuroscience, 12(1), 207–219. doi: https://doi.org/10.3758/s13415-011-0071-9
Nieuwboer, A., & Giladi, N. (2013, Sep 15). Characterizing freezing of gait in Parkinson’s disease: models of an episodic phenomenon. Movement disorders: official journal of the Movement Disorder Society, 28(11), 1509–1519. doi: https://doi.org/10.1002/mds.25683
Nutt, J. G., Bloem, B. R., Giladi, N., Hallett, M., Horak, F. B., & Nieuwboer, A. (2011, Aug). Freezing of gait: moving forward on a mysterious clinical phenomenon. The Lancet Neurology, 10(8), 734–744. doi: https://doi.org/10.1016/S1474-4422(11)70143-0
Nutt, J. G., Carter, J. H., Lea, E. S., & Sexton, G. J. (2002, Jun). Evolution of the response to levodopa during the first 4 years of therapy. Annals of neurology, 51(6), 686–693. doi: https://doi.org/10.1002/ana.10189
Nutt, J. G., Carter, J. H., Van Houten, L., & Woodward, W. R. (1997, Sep). Short- and long-duration responses to levodopa during the first year of levodopa therapy. Annals of neurology, 42(3), 349–355. doi: https://doi.org/10.1002/ana.410420311
Orcioli-Silva, D., Vitorio, R., Nobrega-Sousa, P., Beretta, V. S., Conceicao, N. R. D., Oliveira, A. S., Pereira, M. P., & Gobbi, L. T. B. (2021, May). Cortical Activity Underlying Gait Improvements Achieved With Dopaminergic Medication During Usual Walking and Obstacle Avoidance in Parkinson Disease. Neurorehabilitation and neural repair, 35(5), 406–418. doi: https://doi.org/10.1177/15459683211000736
Pahapill, P. A., & Lozano, A. M. (2000, Sep). The pedunculopontine nucleus and Parkinson’s disease. Brain: a journal of neurology, 123 (Pt 9), 1767–1783. doi: https://doi.org/10.1093/brain/123.9.1767
Pelykh, O., Klein, A. M., Botzel, K., Kosutzka, Z., & Ilmberger, J. (2015, Sep). Dynamics of postural control in Parkinson patients with and without symptoms of freezing of gait. Gait & Posture, 42(3), 246–250. doi: https://doi.org/10.1016/j.gaitpost.2014.09.021
Peterson, D. S., & Horak, F. B. (2016, Mar). Neural Control of Walking in People with Parkinsonism. Physiology, 31(2), 95–107. doi: https://doi.org/10.1152/physiol.00034.2015
Peterson, D. S., Pickett, K. A., Duncan, R., Perlmutter, J., & Earhart, G. M. (2014). Gait-related brain activity in people with Parkinson disease with freezing of gait. 9(3), e90634. doi: https://doi.org/10.1371/journal.pone.0090634
Peterson, D. S., Van Liew, C., Stuart, S., Carlson-Kuhta, P., Horak, F. B., & Mancini, M. (2020). Relating Parkinson freezing and balance domains: A structural equation modeling approach. Parkinsonism & Related Disorders. doi: https://doi.org/10.1016/j.parkreldis.2020.08.027
Pistacchi, M., Gioulis, M., Sanson, F., De Giovannini, E., Filippi, G., Rossetto, F., & Zambito Marsala, S. (2017, Jan/Mar). Gait analysis and clinical correlations in early Parkinson’s disease. Functional Neurology, 32(1), 28–34. doi: https://doi.org/10.11138/fneur/2017.32.1.028
Plotnik, M., Giladi, N., & Hausdorff, J. M. (2012). Is freezing of gait in Parkinson’s disease a result of multiple gait impairments? Implications for treatment. Parkinson’s disease, 2012, 459321. doi: https://doi.org/10.1155/2012/459321
Plotnik, M., & Hausdorff, J. M. (2008). The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Movement Disorders, 23 Suppl 2, S444–450. doi: https://doi.org/10.1002/mds.21984
Rochester, L., Galna, B., Lord, S., & Burn, D. (2014, Apr 18). The nature of dual-task interference during gait in incident Parkinson’s disease. Neuroscience, 265, 83–94. doi: https://doi.org/10.1016/j.neuroscience.2014.01.041
Sale, P., De Pandis, M. F., Vimercati, S. L., Sova, I., Foti, C., Tenore, N., Fini, M., Stocchi, F., Albertini, G., Franceschini, M., & Galli, M. (2013, Apr). The relation between Parkinson’s disease and ageing. Comparison of the gait patterns of young Parkinson’s disease subjects with healthy elderly subjects. European Journal of Physical and Rehabilitation Medicine, 49(2), 161–167. https://www.ncbi.nlm.nih.gov/pubmed/22569487
Schaafsma, J. D., Balash, Y., Gurevich, T., Bartels, A. L., Hausdorff, J. M., & Giladi, N. (2003, Jul). Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. European Journal of Neurology, 10(4), 391–398. doi: https://doi.org/10.1046/j.1468-1331.2003.00611.x
Schlenstedt, C., Mancini, M., Horak, F., & Peterson, D. (2017, Jul). Anticipatory Postural Adjustment During Self-Initiated, Cued, and Compensatory Stepping in Healthy Older Adults and Patients With Parkinson Disease. Archives of physical medicine and rehabilitation, 98(7), 1316–1324 e1311. doi: https://doi.org/10.1016/j.apmr.2017.01.023
Schlenstedt, C., Mancini, M., Nutt, J., Hiller, A. P., Maetzler, W., Deuschl, G., & Horak, F. (2018). Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson’s Disease? Frontiers in Aging Neuroscience, 10, 36. doi: https://doi.org/10.3389/fnagi.2018.00036
Schneider, W., & Shiffrin, R. M. (1977). Controlled and automatic human information processing: I. Detection, search, and attention. 84(1), 1–66. doi: https://doi.org/10.1037/0033-295x.84.1.1
Shine, J. M., Matar, E., Ward, P. B., Bolitho, S. J., Gilat, M., Pearson, M., Naismith, S. L., & Lewis, S. J. (2013a, Apr). Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain, 136(Pt 4), 1204–1215. doi: https://doi.org/10.1093/brain/awt049
Shine, J. M., Matar, E., Ward, P. B., Bolitho, S. J., Pearson, M., Naismith, S. L., & Lewis, S. J. (2013b). Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load. Plos One, 8(1), e52602. doi: https://doi.org/10.1371/journal.pone.0052602
Shine, J. M., Matar, E., Ward, P. B., Frank, M. J., Moustafa, A. A., Pearson, M., Naismith, S. L., & Lewis, S. J. (2013c, Dec). Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain: a journal of neurology, 136(Pt 12), 3671–3681. doi: https://doi.org/10.1093/brain/awt272
Smulders, K., Esselink, R. A., De Swart, B. J., Geurts, A. C., Bloem, B. R., & Weerdesteyn, V. (2014, Feb). Postural inflexibility in PD: does it affect compensatory stepping? Gait & Posture, 39(2), 700–706. doi: https://doi.org/10.1016/j.gaitpost.2013.10.003
Sofuwa, O., Nieuwboer, A., Desloovere, K., Willems, A. M., Chavret, F., & Jonkers, I. (2005, May). Quantitative gait analysis in Parkinson’s disease: comparison with a healthy control group. Archives of physical medicine and rehabilitation, 86(5), 1007–1013. doi: https://doi.org/10.1016/j.apmr.2004.08.012
Stins, J. F., & Beek, P. J. (2011, Jun). Organization of voluntary stepping in response to emotion-inducing pictures. Gait & posture, 34(2), 164–168. doi: https://doi.org/10.1016/j.gaitpost.2011.04.002
Stuart, S., Belluscio, V., Quinn, J. F., & Mancini, M. (2019). Pre-frontal Cortical Activity During Walking and Turning Is Reliable and Differentiates Across Young, Older Adults and People With Parkinson’s Disease. Frontiers in neurology, 10, 536. doi: https://doi.org/10.3389/fneur.2019.00536
Tagliabue, M., Ferrigno, G., & Horak, F. (2009, Feb 18). Effects of Parkinson’s disease on proprioceptive control of posture and reaching while standing. 158(4), 1206–1214. doi: S0306-4522(08)01763-6 [pii] https://doi.org/10.1016/j.neuroscience.2008.12.007
Takakusaki, K. (2017, Jan). Functional Neuroanatomy for Posture and Gait Control. Journal of Movement Disorders, 10(1), 1−17. doi: https://doi.org/10.14802/jmd.16062
Tarazi, F. I., Sahli, Z. T., Wolny, M., & Mousa, S. A. (2014, Nov). Emerging therapies for Parkinson’s disease: from bench to bedside. 144(2), 123−133. doi: https://doi.org/10.1016/j.pharmthera.2014.05.010
Thompson, P. D., & Marsden, C. D. (1995). Walking disorders. In W. G. Bradley, R. B. Daroff, & G. M. M. Fenichel, C. D. (Eds.), Neurology in Clinical Practice – Principles of Diagnosis and Management (pp. 321−334). Butterworth-Heinemann.
Vandenbossche, J., Deroost, N., Soetens, E., Coomans, D., Spildooren, J., Vercruysse, S., Nieuwboer, A., & Kerckhofs, E. (2012). Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Frontiers in Human Neuroscience, 6, 356. doi: https://doi.org/10.3389/fnhum.2012.00356
Vercruysse, S., Spildooren, J., Heremans, E., Wenderoth, N., Swinnen, S. P., Vandenberghe, W., & Nieuwboer, A. (2014, Dec). The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait. Cerebral Cortex, 24(12), 3154−3166. doi: https://doi.org/10.1093/cercor/bht170
Visser, J. E., Carpenter, M. G., van der Kooij, H., & Bloem, B. R. (2008, Nov). The clinical utility of posturography. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 119(11), 2424−2436. doi: https://doi.org/10.1016/j.clinph.2008.07.220
Vitorio, R., Lirani-Silva, E., Barbieri, F. A., Raile, V., Stella, F., & Gobbi, L. T. (2013, Jun). Influence of visual feedback sampling on obstacle crossing behavior in people with Parkinson’s disease. Gait & Posture, 38(2), 330−334. doi: https://doi.org/10.1016/j.gaitpost.2012.12.019
Vitorio, R., Lirani-Silva, E., Pieruccini-Faria, F., Moraes, R., Gobbi, L. T., & Almeida, Q. J. (2014, Sep 26). Visual cues and gait improvement in Parkinson’s disease: which piece of information is really important? Neuroscience, 277, 273−280. doi: https://doi.org/10.1016/j.neuroscience.2014.07.024
Vitorio, R., Pieruccini-Faria, F., Stella, F., Gobbi, S., & Gobbi, L. T. (2010, Jan). Effects of obstacle height on obstacle crossing in mild Parkinson’s disease. Gait & posture, 31(1), 143−146. doi: https://doi.org/10.1016/j.gaitpost.2009.09.011
Wang, M., Jiang, S., Yuan, Y., Zhang, L., Ding, J., Wang, J., Zhang, J., Zhang, K., & Wang, J. (2016, Aug). Alterations of functional and structural connectivity of freezing of gait in Parkinson’s disease. 263(8), 1583−1592. doi: https://doi.org/10.1007/s00415-016-8174-4
Weiss, A., Herman, T., Giladi, N., & Hausdorff, J. M. (2015, Mar). New evidence for gait abnormalities among Parkinson’s disease patients who suffer from freezing of gait: insights using a body-fixed sensor worn for 3 days. Journal of Neural Transmission, 122(3), 403−410. doi: https://doi.org/10.1007/s00702-014-1279-y
Welter, M. L., Do, M. C., Chastan, N., Torny, F., Bloch, F., du Montcel, S. T., & Agid, Y. (2007, Sep). Control of vertical components of gait during initiation of walking in normal adults and patients with progressive supranuclear palsy. Gait & posture, 26(3), 393−399. doi: https://doi.org/10.1016/j.gaitpost.2006.10.005
Yiou, E., Caderby, T., Delafontaine, A., Fourcade, P., & Honeine, J. L. (2017, Nov 18). Balance control during gait initiation: State-of-the-art and research perspectives. World journal of orthopedics, 8(11), 815−828. doi: https://doi.org/10.5312/wjo.v8.i11.815
Zanardi, A. P. J., da Silva, E. S., Costa, R. R., Passos-Monteiro, E., Dos Santos, I. O., Kruel, L. F. M., & Peyre-Tartaruga, L. A. (2021, Jan 12). Gait parameters of Parkinson’s disease compared with healthy controls: a systematic review and meta-analysis. Scientific Reports, 11(1), 752. doi: https://doi.org/10.1038/s41598-020-80768-2
Conflict of Interest Statement
None.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Coelho, D.B. et al. (2023). Postural Control in Parkinson’s Disease. In: Lombello, C.B., da Ana, P.A. (eds) Current Trends in Biomedical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-38743-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-38743-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-38742-5
Online ISBN: 978-3-031-38743-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)