Abstract
Patients with Alzheimer’s disease (AD) develop olfactory and gustatory disorders. However, the order of failure and relevance of the pathophysiology are unclear. We compared olfactory identification and whole mouth gustation in patients with AD to those with mild cognitive impairment (MCI) and to healthy controls (HC) and assessed correlations with pathophysiology. Patients with AD (n = 40), MCI (n = 34), and HC (n = 40) were recruited. We performed the Odor Stick Identification Test for Japanese (OSIT-J), gustatory test by the intraoral dropping method using taste solutions, Mini-Mental State Examination (MMSE), Alzheimer’s Disease Assessment Scale-cognitive subscale Japanese version (ADAS-J cog), Touch Panel-type Dementia Assessment Scale (TDAS), and measurement of amyloid β (Aβ) 42 and phosphorylated tau (p-tau) 181 levels in cerebrospinal fluid (CSF). Patients with AD and MCI had lower OSIT-J scores than did the HC. The OSIT-J score was correlated with the MMSE, ADAS-J cog, TDAS, and Aβ42 results. There were no significant differences in the gustatory test scores among the three groups. The gustatory test score was only correlated with the MMSE, ADAS-J cog, and TDAS results. Olfactory function decreased in AD and MCI patients and was associated with CSF biomarker levels and cognitive disorders. The results suggest that olfactory function is impaired in early stage of AD. Gustatory function was not correlated with CSF biomarkers, which suggests that it may not be impaired in early stage of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Alzheimer’s disease (AD), the two main pathologies are senile plaques, which are formed by extracellular accumulation of amyloid β (Aβ) protein, and neurofibrillary tangles, which are formed intracellularly by deposition of phosphorylated tau (p-tau) [1]. Although the main clinical symptom in AD is memory disturbance, Aβ and p-tau have been found to accumulate in olfactory-related areas before they accumulate in the hippocampus, which is involved in memory. Thal et al. [2] reported that Aβ accumulates in the entorhinal cortex at the early stage. Further, Braak et al. [3, 4] reported on staging of neurofibrillary tangles and found that p-tau occurs in the transentorhinal cortex at the earliest stage and spreads to the entorhinal cortex and hippocampus CA1 regions. It has also been confirmed that AD and mild cognitive impairment (MCI) in a pre-dementia state decrease the capability for identification of smells [5,6,7]. Furthermore, a previous study showed that olfactory impairment predicts incident amnestic MCI and progression from amnestic MCI to AD [8].
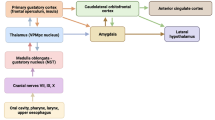
Gustatory disorder has been shown to occur in AD [9, 10]. Taste information in the human travels from cranial nerves VII, IX, and X in the periphery to the nucleus of the solitary tract, then to the ventral posterior medial nucleus of the thalamus, and then to primary gustatory cortex, the insula/frontal operculum [11]. AD is characterized progressive global and regional volume reductions in gray matter and white matte [12]. It was reported that decreased taste function in AD patients implies a defect that is more central than peripheral taste system [10]. However, there are only a few reports regarding the gustatory function of patients with AD, and the results of these reports are inconsistent.
To date, there have been several reports on olfaction and gustation in AD [5,6,7, 9, 10, 13]. However, few studies have examined both functions concurrently, and no study has investigated associations with the pathological changes of AD, such as in Aβ and p-tau in cerebrospinal fluid (CSF). Therefore, the relationship between pathological changes and the order of development of olfactory and gustatory disorders in AD are unclear. The aim of this study was to compare patients with olfactory dysfunction function and gustatory disorders in AD with MCI and healthy controls (HC), and to determine correlations with pathophysiology.
Methods
Patients
This study included patients with AD (n = 40), MCI (n = 34), and HC (n = 40). AD diagnosis was performed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [14], the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer’s Disease and Related Disorders Association [15]. MCI diagnosis was performed according to Petersen’s criteria [16, 17]. All patients with MCI in this study had amnestic MCI. HC have no evidence of cognitive impairment or psychiatric disorder at the time of clinical and neuropsychological assessment. Patients who had previously been diagnosed as having smell- and taste-related disease and/or who had taken anti-dementia drugs for AD and MCI and/or who cannot be performed olfactory and gustatory tests due to severe cognitive dysfunction were excluded.
The following clinical and demographic characteristics were assessed: age, sex, smoking, drinking habits, medications, and the presence of hypertension, hyperlipidemia, and diabetes mellitus.
The study design was approved by the ethics committee of Tottori University. The research protocol was explained to the patients and/or relatives, and informed consent for their participation was obtained.
Olfactory test
We performed the Odor Stick Identification Test for Japanese (OSIT-J) as an olfactory test [18]. The experimenter applied an odorous semisolid cream from an odor stick to a 2-cm circle on a thin paraffin paper, folded the paper in half, rubbed it to grind the microcapsules, and passed it to the patient. The patient then opened and sniffed the paper and chose one of six possible answers: four items plus “detectable but not recognized” and “no smell detected.” There were 12 kinds of smells familiar to Japanese (India ink, wood, perfume, menthol, Japanese orange, curry, cooking gas, rose, Japanese cypress, fermented beans/sweaty socks, condensed milk, and roasted garlic). A score of 12 points indicated that the patient answered all questions correctly and a score of 0 point indicated that the patient’s answers were all incorrect. Finally, the subjects were asked if odorless sticks had a smell. If the subject answered that they detected a smell, the subject was excluded.
Gustatory test
We performed a gustatory test by the intraoral dropping method using taste solutions. There were four kinds of taste solutions: sweet (0.003, 0.025, 0.1, 0.2, and 0.8 g/ml sucrose), salty (0.003, 0.0125, 0.05, 0.1, and 0.2 g/ml sodium chloride), sour (0.0002, 0.002, 0.02, 0.04, and 0.08 g/ml tartaric acid), and bitter (0.00001, 0.0002, 0.001, 0.005, and 0.04 g/ml quinine hydrochloride). The experimenter placed a drop of solution into the oral cavity at the lowest concentration. The patient chose one of six possible answers: sweet, salty, sour, bitter, unidentifiable taste, and no taste. If the patient answered correctly, the concentration was taken as the recognition threshold. If the choice was incorrect, the concentration was increased at the next trial. If the patient recognized the lowest concentration, they received 1 point. If the patient recognized the highest concentration, they received 5 points. If the patient did not recognize the highest concentration, they received 6 points.
Neuropsychological tests
To assess global cognitive function, we used the Mini-Mental State Examination (MMSE) [19], Alzheimer’s Disease Assessment Scale-cognitive subscale Japanese version (ADAS-J cog) [20], and Touch Panel-type Dementia Assessment Scale (TDAS) [21]. The MMSE and ADAS-J cog are face-to-face neuropsychological tests. The TDAS is a modified part of the ADAS and answers are entered into a touch panel-type computer. In the MMSE, which is a screening test, a maximum score of 30 points is possible if the subject answers all questions correctly. In the ADAS-J cog and TDAS, which are used to evaluate the degree of AD progression, a score of 0 point indicates that all answers were correct.
CSF tests
CSF samples were collected by lumbar puncture and placed into a polypropylene container. Ethylenediaminetetraacetic acid was added to CSF samples for p-tau181 to prevent dephosphorylation. The collected CSF samples were stored immediately at − 80 °C until use. Collection of CSF samples could not be performed for difficult cases of lumbar puncture and patients who refused the examination. The Aβ42 and p-tau181 levels in CSF were measured by performing a sandwich enzyme-linked immunosorbent assay (Human Amyloid β 1-42 Assay kit; Immuno-Biological, Gunma, Japan, and INNOTEST PHOSPHO-TAU (181p); Innogenetics, Ghent, Belgium, respectively).
Questionnaire
We administered a questionnaire about the subjective olfactory function or subjective gustatory function. These functions were self-rated on a scale of 1 (worst = subjective symptoms were present) to 5 points (best = no subjective symptoms).
Statistical analysis
Statistical analyses were performed by using IBM SPSS Statistics 23 (IBM Japan, Tokyo, Japan) and GraphPad Prism7 (MDF Inc., Tokyo, Japan). To compare the age, medications, subjective symptoms, MMSE, ADAS-J cog, TDAS, Aβ42, p-tau181, and p-tau181/Aβ42 ratio results between the AD and MCI patients and the HC, one-way analysis of variance was used, and the Tukey method was used for multiple comparisons. The chi-square test was used to compare differences in the distribution of demographics. Olfactory test and gustatory test scores were compared among three groups by analysis of covariance, with sex and age as covariates. A receiver operating characteristic (ROC) analysis was performed to estimate the capability of the olfactory and gustatory tests. The area under the curve (AUC), sensitivity, and specificity were calculated to assess the discriminative capability of those tests. To evaluate correlations between the olfactory and gustatory tests and other continuous tests, Pearson’s correlation coefficient was used.
Results
Characteristics of subjects
The subject characteristics are summarized in Table 1. There were no significant differences in the distribution of age, sex, smoking, drinking habits, medications, and the presence of hypertension, hyperlipidemia, and diabetes mellitus between AD, MCI, and HC subjects. Neuropsychological test results (MMSE, ADAS-J cog, and TDAS) were significantly worse with progression to MCI and AD. Of the CSF biomarkers, p-tau181 levels were increased in AD patients relative to the levels in HC, and Aβ42 levels were decreased in AD and MCI patients relative to the levels in the HC, and p-tau181/Aβ42 levels were increased in AD patients relative to the levels in MCI and HC. There were no significant differences in the subjective olfactory function and subjective gustatory function between AD, MCI, and HC subjects.
Olfactory test
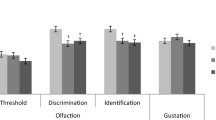
The olfactory test results are shown Fig. 1a and Table 2. The total OSIT-J scores were significantly lower in AD and MCI patients than in the HC (p < 0.0001, p = 0.026, respectively). Although there was no significant difference in the total OSIT-J score between AD and MCI patients, a downward trend was observed in those suffering from AD (p = 0.060). The various OSIT-J smell categories [18], i.e., good odors (perfume, Japanese orange, rose, and condensed milk), plant odors (India ink, wood, menthol, and Japanese cypress), spice odors (curry and roasted garlic), and offensive odors (cooking gas and fermented beans/sweaty socks), were compared. The scores for good odors, plant odors, spice odors, and offensive odors were significantly lower in AD patients than in HC (p = 0.003, p < 0.0001, p < 0.0001, p = 0.002, respectively). Furthermore, the scores of plant odors were significantly lower in AD than in MCI patients (p = 0.044).
Results of olfactory test (a) and gustatory test (b) in patients with Alzheimer’s disease (AD), mild cognitive impairment (MCI), and healthy controls (HC). Olfactory test total score of AD and MCI exhibited a significant decrease compared with HC (p < 0.0001, p = 0.026, respectively). Gustatory test total score was not significant among three groups. Error bars represent standard error of the mean (SEM)
In the ROC analysis of the total OSIT-J scores (Fig. 2a), the sensitivity and specificity in distinguishing AD subjects from HC with a cutoff point of 6.5 were 82.5 and 67.5% (AUC = 0.809), respectively. Similarly, in the comparison between MCI and HC subjects, the sensitivity and specificity with a cutoff point of 7.5 were 76.5 and 62.5% (AUC = 0.711), respectively, and, in the comparison between AD and MCI subjects, the sensitivity and specificity with a cutoff point of 5.5 were 75.0 and 50.0% (AUC = 0.645), respectively.
A receiver operating characteristic (ROC) analysis for olfactory test (a) and gustatory test (b) in patients with Alzheimer’s disease (AD), mild cognitive impairment (MCI), and healthy controls (HC). The area under the ROC curve of the olfactory test was 0.809 for AD versus HC group, 0.711 for MCI versus HC group, and 0.645 for AD versus MCI group, whereas that for the gustatory test was 0.656, 0.534, and 0.633, respectively
Gustatory test
The results of the gustatory test are shown Fig. 1b and Table 2. There were no significant differences in the total gustatory test scores between AD, MCI, and HC subjects. Similarly, the gustatory test sub-items, which are four basic tastes of sweet, salty, sour, and bitter, did not change among the three groups. However, the mean gustatory test score in AD was the worst.
In the ROC analysis of the total gustatory test score (Fig. 2b), the sensitivity and specificity for distinguishing AD subjects from HC with a cutoff point of 9.5 were 60.0 and 70.0% (AUC = 0.656), respectively. Similarly, in the comparison between MCI patients and HC, the sensitivity and specificity with a cutoff point of 7.5 were 73.5 and 27.5% (AUC = 0.534), respectively, and in the comparison between the AD and MCI subjects, the sensitivity and specificity with a cutoff point of 8.5 were 75.0 and 35.3% (AUC = 0.633), respectively.
Comparison of test results
Comparisons of results for the olfactory test, gustatory test, CSF biomarkers, and neuropsychological test are shown in Table 3. There was a significant correlation between the OSIT-J total score and the MMSE, ADAS-J cog, TDAS, Aβ42, and p-tau181/Aβ42 results. In the comparison of the smell categories, the MMSE, ADAS-J cog, and TDAS results correlated with all odor category scores, and Aβ42 and p-tau181/Aβ42 levels correlated with plant odor and spice odor scores. There was a significant correlation between the total gustatory test score and the MMSE, ADAS-J cog, and TDAS results. In the comparisons with the four gustatory test sub-items, the MMSE, ADAS-J cog, TDAS, p-tau181, Aβ42, and p-tau181/Aβ42 results correlated with the scores for salty taste, and TDAS correlated with the score for sour and bitter taste. In addition, there was a significant correlation between the total OSIT-J score and the gustatory test results (r = − 0.290, p = 0.002).
Discussion
In this study, we found that olfactory function was related to CSF biomarkers and cognitive disorders. The results suggested that olfactory function is likely to be impaired in the early stage of AD. It is known that AD and MCI develop from a preclinical AD state in which there are no subjective symptoms but Aβ and p-tau accumulate in the brain [22, 23]. The Dominantly Inherited Alzheimer Network (DIAN) study showed that Aβ42 in the CSF appeared to decrease approximately 15 to 20 years before expected symptoms onset [24]. Therefore, it has been suggested that evaluation of olfactory function may be useful for detection of preclinical AD and MCI [25]. In addition, the total OSIT-J score of patients with AD and MCI significantly decreased relative to that of the HC. However, there was no significant difference in the total OSIT-J score between AD and MCI patients. Therefore, olfactory function may decline from the early stage of AD and continue to decline slowly until the late stage of AD. In a previous study, it was reported that patients with AD showed decreased recognition of India ink, wood, rose, Japanese cypress, and roasted garlic [5]. Our study showed similar decreases in recognition of plant odors in patients with AD than in MCI and HC. Therefore, the ability to identify plant odors was thought to be relatively closely in AD pathology. In addition, the ability to identify good or offensive odors was not correlated with CSF biomarkers, which suggests that it may not be affected by AD pathophysiology. However, because the stimulus of an offensive odor may be too strong, it may stimulate the trigeminal nerve rather than the olfactory nerve [26]. It may be difficult to accurately evaluate olfactory function.
In a previous study, it was reported that olfactory function was significantly lower in AD than in MCI individuals [27], but there was no significant difference in the total OSIT-J score between AD and MCI patients in this study. That study used Sniffin’ sticks, which can evaluate identification and discrimination abilities, as well as smell threshold levels [28]. We used the OSIT-J because it was easy to use in this study, so the discrepancies in the results were thought to occur because of differences in the olfactometric ability. We did not use the Sniffin’ sticks because we wanted to use smells that were familiar in Japanese environment and culture. However, we found that the results of the OSIT-J, which is a simple olfactometric test, correlated with CSF biomarker and neuropsychological test results. Therefore, if a new high-performance olfactory test that includes smells familiar to Japanese is developed, we may be able to distinguish among AD, MCI, and HC more accurately.
The total gustatory test score was associated with cognitive function but did not correlate with CSF biomarker levels. Furthermore, there were no significant differences among AD and MCI patients and HC. In a previous study, it was reported that AD and MCI patients had taste disorders [9, 10]. For reasons that did not affect our study, there were differences in the density of the taste solutions and gustometric techniques. We evaluated taste function by using the intraoral falling drop method because the filter paper disk method, which involves placing a filter paper on the tongue and oral cavity, has a risk of accidental ingestion, whereas the intraoral falling drop method can be performed quickly. Therefore, because there is a larger amount of substances that can be tasted in the solution placed in the oral cavity than in the filter paper disk method, our method may not have been able to detect fine differences. Furthermore, the concentrations of taste solutions did not match the concentrations used in the other study, which may have also caused discrepancies in the results between the studies. However, because the taste is felt throughout the oral cavity, we thought that the intraoral falling drop method is suitable for evaluating the taste of daily life. As a limitation of the intraoral falling drop method, individual cranial nerves cannot be evaluated. However, since we found that gustatory function correlated with cognitive function, our results agreed with those of a previous study [9], which showed that gustatory function decreased with progression to clinical AD. We think that gustatory function did not change earlier because we found no association with CSF biomarker levels.
A previous study showed that older people had significantly lower recognition thresholds for salty, sour, and bitter tastes than did young adults [29]. The salt and sour receptors perceive these tastes through ion channels, whereas sweet and bitter taste compounds interact with G-protein coupled receptors [30]. However, whether this difference in transmission format is associated with a failure caused by disease is unknown. Our study showed a correlation between CSF biomarkers and salty taste, as well as between cognitive function and salty, sour, and bitter tastes. Specifically, because the ability to detect salty tastes decreases with progression of AD, this may affect how much seasoning is used in a person’s food and thus, their salt intake may increase. In a previous study, 81.4% of patients with AD showed some eating disturbance such as changes in appetite and eating habits [31], which may have been caused by taste disturbances. However, because aging is related to cognitive dysfunction [32], it may be that gustatory test score was similar to change due to age [29].
Our results suggest that olfactory and taste functions deteriorate with progression of AD pathology. Olfactory function was related to CSF biomarkers, but gustatory function was unrelated. Therefore, we thought that olfactory function likely to be impaired in the early stage of pathology, but gustatory function may not impaired in the early stage of disease. Especially for olfaction, stimulating the sense of smell can enhance generated granule cells in the olfactory bulb [33] and possibly lead to improvement in cognitive function [34]. Regarding gustation, it has been reported that the forebrain region including limbic system in rats responded to intra-gastric administration of glucose, L-glutamate, and NaCl [35] and supplementation with monosodium glutamate improved behaviors of hospitalized elderly [36]. Therefore, it is important to find the olfactory and gustatory decline in the early stage and to intervene. However, several limitations of this study should be considered. First, the olfactory and gustatory tests used were insufficient as diagnostic auxiliary tools because they are not specialized for dementia. Second, only a subset of subjects underwent lumbar puncture; therefore, correlation with pathology is partially undermined by the small sample size. Third, HC tended to be higher age and number of female than AD and MCI. It is known that olfactory and taste dysfunctions are both associated with gender and with normal aging [29, 37]. In the future, further development of sensitive olfactory and gustatory tests and large-scale study should contribute to early diagnosis and understanding of the conditions present in AD.
References
Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM (2013) Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers. Clin Chem 59:903–916
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Jimbo D, Inoue M, Taniguchi M, Urakami K (2011) Specific feature of olfactory dysfunction with Alzheimer’s disease inspected by the Odor Stick Identification Test. Psychogeriatrics 11:196–204
Peters JM, Hummel T, Kratzsch T, Lötsch J, Skarke C, Frölich L (2003) Olfactory function in mild cognitive impairment and Alzheimer’s disease: an investigation using psychophysical and electrophysiological techniques. Am J Psychiatry 160:1995–2002
Eibenstein A, Fioretti AB, Simaskou MN, Sucapane P, Mearelli S, Mina C et al (2005) Olfactory screening test in mild cognitive impairment. Neurol Sci 26:156–160
Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M et al (2016) Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 73:93–101
Steinbach S, Hundt W, Vaitl A, Heinrich P, Förster S, Bürger K et al (2010) Taste in mild cognitive impairment and Alzheimer’s disease. J Neurol 257:238–246
Ogawa T, Irikawa N, Yanagisawa D, Shiino A, Tooyama I, Shimizu T (2017) Taste detection and recognition thresholds in Japanese patients with Alzheimer-type dementia. Auris Nasus Larynx 44:168–173
Sewards TV (2004) Dual separate pathways for sensory and hedonic aspects of taste. Brain Res Bull 62:271–283
Kim S, Youn YC, Hsiung GY, Ha SY, Park KY, Shin HW et al (2011) Voxel-based morphometric study of brain volume changes in patients with Alzheimer’s disease assessed according to the Clinical Dementia Rating score. J Clin Neurosci 18:916–921
Lang CJ, Leuschner T, Ulrich K, Stössel C, Heckmann JG, Hummel T (2006) Taste in dementing diseases and parkinsonism. J Neurol Sci 248:177–184
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, D.C.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939–944
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194
Petersen RC (2011) Clinical practice. Mild cognitive impairment. N Engl J Med 364:2227–2234
Saito S, Ayabe-Kanamura S, Takashima Y, Gotow N, Naito N, Nozawa T et al (2006) Development of a smell identification test using a novel stick-type odor presentation kit. Chem Senses 31:379–391
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Homma A, Fukuzawa K, Tsukada Y, Ishii T, Hasegawa K (1992) Development of a Japanese version of Alzheimer’s disease assessment scale (ADAS). Jpn J Geriatr Psychiatry 3:647–655 (in Japanese)
Inoue M, Jimbo D, Taniguchi M, Urakami K (2011) Touch Panel-type Dementia Assessment Scale: a new computer-based rating scale for Alzheimer’s disease. Psychogeriatrics 11:28–33
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:280–292
Berti V, Polito C, Lombardi G, Ferrari C, Sorbi S, Pupi A (2016) Rethinking on the concept of biomarkers in preclinical Alzheimer’s disease. Neurol Sci 37:663–672
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC et al (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367:795–804
Lafaille-Magnan ME, Poirier J, Etienne P, Tremblay-Mercier J, Frenette J, Rosa-Neto P et al (2017) Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 89:327–335
Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD (1978) Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav 20:175–185
Williams SS, Williams J, Combrinck M, Christie S, Smith AD, McShane R (2009) Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J Neurol Neurosurg Psychiatry 80:667–670
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52
Yoshinaka M, Ikebe K, Uota M, Ogawa T, Okada T, Inomata C et al (2016) Age and sex differences in the taste sensitivity of young adult, young-old and old-old Japanese. Geriatr Gerontol Int 16:1281–1288
Frank ME, Hettinger TP, Mott AE (1992) The sense of taste: neurobiology, aging, and medication effects. Crit Rev Oral Biol Med 3:371–393
Kai K, Hashimoto M, Amano K et al (2015) Relationship between eating disturbance and dementia severity in patients with Alzheimer’s disease. PLoS One 10:e0133666. https://doi.org/10.1371/journal.pone.0133666
Crum RM, Anthony JC, Bassett SS, Folstein MF (1993) Population-based norms for the mini-mental state examination by age and educational level. JAMA 269:2386–2391
Yamaguchi M, Mori K (2005) Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci U S A 102:9697–9702
Jimbo D, Kimura Y, Taniguchi M, Inoue M, Urakami K (2009) Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics 9:173–179
Tsurugizawa T, Kondoh T, Torii K (2008) Forebrain activation induced by postoral nutritive substances in rats. Neuroreport 19:1111–1115
Tomoe M, Inoue Y, Sanbe A, Toyama K, Yamamoto S, Komatsu T (2009) Clinical trial of glutamate for the improvement of nutrition and health in the elderly. Ann N Y Acad Sci 1170:82–86
Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L (1984) Smell identification ability: changes with age. Science 226:1441–1443
Acknowledgments
We thank the patients who participated in our study and their families. We also thank the staff at Shinsei Hospital (Kurayoshi, Japan) who cooperated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study design was approved by the ethics committee of Tottori University. The research protocol was explained to the patients and/or relatives, and informed consent for their participation was obtained.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kouzuki, M., Suzuki, T., Nagano, M. et al. Comparison of olfactory and gustatory disorders in Alzheimer’s disease. Neurol Sci 39, 321–328 (2018). https://doi.org/10.1007/s10072-017-3187-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3187-z