Abstract
The head stabilization reflex (HSR) is a brain stem reflex which appears in the neck muscles in response to sudden head position changes and brings the head to its previous position. The reflex mechanism has not been understood. The afferent fibers come from cervical muscle spindles, vestibular structures, and the accessory nerve, the efferents from the accessory nerve. In this study, we aim to investigate the roles of supraspinal neural structures and the vestibular system on the HSR. The patient group consisted of 86 patients (33 cerebral cortical lesion, 14 cerebellar syndrome and 39 vestibular inexcitability or hypoexcitability); the control group was composed of 32 healthy volunteers. Concentric needle electrodes were inserted into the sternocleidomastoid muscle (SCM) and the accessory nerves were stimulated with the electrical stimulator. A reflex response of about 45–55 ms was obtained from the contralateral SCM muscle. 50 % of cases had bilateral loss whereas 37 % of cases with unilateral cerebellar lesions had an ipsilateral reflex loss. Bilateral HSR loss was detected in 84 % of cases with bilateral cerebellar lesions. Bilateral reflex loss was observed in 70 % of patients with unilateral cortical lesions and 94 % of those with bilateral vestibular dysfunction. Ipsilateral HSR loss was observed in 55 % of cases with unilateral vestibular dysfunction. It was discovered that supraspinal structures and the vestibular system may have an excitatory effect on HSR. This effect may be lost in supra-segmental and vestibular dysfunctions. The localization value of HSR was found to be rather poor in our study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The head stabilization reflex (HSR) is a reflex which appears in the neck muscles in response to a variety of sudden head position changes and brings the head back to its previous position. It is obtained by stimulating the accessory nerve and then recording the responses electromyographically from the contralateral sternocleidomastoid muscle (SCM) [1]. The information about the pathways of this reflex and suprasegmental effects on the reflex is insufficient.
The afferent fibers of HSR come from cervical muscle spindles, vestibular structures, and the accessory nerve and the efferent fibers from the accessory nerve. There is strong evidence of HSR not being a pure spinal segmental reflex and of the role of supraspinal structures on this segmental arch [1, 2]. The accessory nerve is not a spinal nerve and all reflex circuits undergo suprasegmental modulation proportionally to the number of synapses in the circuit, more or less. Structures which affect HSR appear mainly to have functions of equilibrium and delivering spatial position to the cortex.
The vestibular system includes the afferent receptors that transmit movements of the head and its spatial position to relevant cerebral structures. They relay information about position differences of the head and body in relation to each other to the central nervous system. In fact, this is a system in which mainly proprioceptive sensory inputs play an active role.
The primary route of reflexes associated with balance begins with vestibular nerves and then passes to both vestibular nuclei and the cerebellum. Thereafter, the signals are sent both to the brainstem reticular nuclei and spinal cord via vestibulospinal and reticulospinal tracts. Signals coming to the spinal cord ensure the control of balance automatically by regulating the interaction of facilitation and inhibition in the antigravity muscles [3].
Objective
The aim of the present study was to assess the roles of supraspinal neural structures and the vestibular system, which have possible impacts on the head stabilization reflex. The plan was to reveal possible supraspinal structures which have an effect on this reflex by studying HSR in patients with cerebral hemispheric and/or cerebellar lesions and in patients with vestibular dysfunction findings. If the neural structures which have excitatory and inhibitory effects on the head stabilization reflex and their connections can be shown, it may be possible both to understand the physiology of this reflex and clinically test it.
Materials and methods
Materials
Thirty-two normal, healthy individuals who gave written consent and 86 cases with central nervous system lesions or vestibular system dysfunction were enrolled in the study. The normal control group consisted of 18 females (mean age 44 ± 9 SD) and 14 males (mean age 48 ± 9 SD). Cases with previous vertigo attacks, any complaints that gave positive symptoms, chronic neck and back pain and those being treated with drugs were excluded from the study. Cases with central nervous system and vestibular system abnormalities (F/M = 1.4, mean age 53.7 ± 14.6 SD) were included in the patient group.
Thirty-three patients with cerebral hemispheric lesions were enrolled in the study (2 with arteria cerebri anterior, 29 with arteria cerebri media and two with arteria cerebri posterior infarction). All of these patients were alert and awake and were able to sit in the chair. The presence of lesions in the central nervous system was shown by neurological examination and imaging (cranial MRI and/or CT). The time between the first symptom and enrollment in the study was 4–45 days (median 8 days). In accordance with the exclusion criteria used in the healthy control group, patients receiving vestibulosuppressant drugs, antidepressant drugs, excitatory or inhibitory drugs of the central nervous system and cases in which imaging and neurological symptoms were not matched were excluded from the study for the possible presence of different lesions.
Fourteen patients, in whom cerebellar infarction and hematoma were detected and cerebellar symptoms (dysmetria, dysdiadochokinesia, ataxia, dysarthria) were evident in the neurological examination, who could adapt to the method, were enrolled in the study [four patients with a chronic cerebellar syndrome (late-onset cerebellar ataxia, idiopathic and vitamin B12 deficiency), one with cerebellar hematoma and nine with cerebellar infarction in the acute or subacute phase].
A total of 39 patients who had presented to our department with the complaint of vertigo had vestibular dysfunction in the neurotologic examination and caloric testing with unilateral or bilateral, inexcitability or hypoexcitability (unilateral vestibular inexcitability/hypoexcitability in 22 and bilateral inexcitability/hypoexcitability in 17) and in whom central vertigo was excluded by cranial MRI were included in the study.
Methods
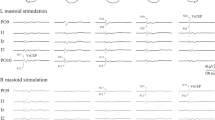
The same standard experimental protocol was applied to all patients. During the experiment, the subject was seated in the chair in an upright position and the head was adjusted to a neutral position, ensuring comfort (the head was erect and in the midline, eyes focused on the vanishing point). Then, concentric needle electrodes (Dantec, 37 mm) were inserted at the one/third subsegment of both SCM muscles, so that they would be away from the motor endplate. The accessory nerve was stimulated supramaximally (0.2 ms duration, square-wave shock, 300–400 volt intensity) in the posterior triangle with the superficial bipolar electrical stimulator on the registration system adjusted to two channels (Fig. 1). Four records were obtained separately from both muscles by triggering SCM muscle electromyographic recordings with this stimulator (Fig. 2). Keeping the sternocleidomastoid muscle at rest before stimulating the accessory nerve (AN) (absence of muscle activity while the needle was in the muscle, absence of electromyographic activity in the speakers of the EMG device) was ensured in order to avoid situations like quiet periods, which may produce artifacts in this study.
The accessory nerve is stimulated on the postauricular region (S1) with superficial electrodes in the case sitting in the chair. The M-response is recorded with a concentric needle from ipsilateral sternocleidomastoideus muscle (R1). Head stabilization reflex (HSR) response is recorded from contralateral sternocleidomastoideus muscle with concentric needle electrode (R2). G Ground
The stimulation of left accessory nerve and M-response recorded from ipsilateral sternocleidomastoideus muscle (Upper trace) and head stabilization reflex (HSR) with a latency of approximately 55 ms recorded from contralateral sternocleidomastoideus muscle (Lower trace). The superimposed form of 4 traces recorded with the stimulation of left accessory nerve 4 times in a normal individual is seen
All recordings were obtained with dual-channel simultaneous registrations using the “Nicolet Viking IVD” 4-channel EMG device. When AN was stimulated in the posterior triangle of the neck on one side, a reflex response was recorded from the contralateral SCM muscle electromyographically while an M-response was obtained from the ipsilateral SCM muscle. The same procedure was repeated on the other side.
Analysis of the data obtained
The amplitudes, shortest and longest latencies, short-longest latency difference (dispersion) and reflex times of the obtained reflex responses (four consecutive registrations) were recorded separately for left and right SCM muscles using manual measurement. In the experimental protocol consisted of eight stimulations; if reflex responses could not be obtained, the absence of reflex was confirmed by repeating the experiment protocol three times, in order to ensure the absence of the response. The data obtained were analyzed using the SPSS 22 statistical software package. The independent T test was used in the comparison of two independent groups for the variables in which normality was determined. The Mann–Whitney U test, one of the nonparametric tests, was used in binary comparisons of values for which normality was not determined.
Results
Normal group
Reflex responses with a mean latency of 50 ms (42–58 ms) were obtained in all the normal controls. No significant difference was observed between the minimum and maximum latency differences obtained from the right and left SCM muscles (p = 0.58). The HSR amplitude of the left was 80 V higher on average than that of the right in this group (Table 1). However, since evaluation of amplitude with the needle electrode is not an accepted practice in electrophysiological methodology, the amplitude difference between right and left was ignored.
Group with hemispheric lesions
Bilateral head stabilization reflex was found to be lost in 70 % of a total of 33 patients (20 with left hemispheric lesions and 13 with right); there was a statistically significant difference from the healthy group (p = 0.001) (Table 2).
When the effects of right and left hemispheric lesions on the reflex were compared, there was no significant statistical difference between the two sides in terms of loss of HSR (p = 0.73).
Cerebellar syndrome
Bilateral loss of reflexes was observed in approximately 50 % of the patients with unilateral cerebellar lesion. Loss of reflex in the ipsilateral side of the lesion was found to be 37.5 %. Bilateral loss of reflexes was observed in 83.4 % of bilateral cerebellar lesions (p = 0.0007). Loss of the reflex was bilateral in 50 % of the patients with unilateral lesions and unilateral in 37 % (Table 3).
Group with vestibular dysfunction
Table 4 shows HSR properties according to the type of vestibular dysfunction. Ipsilateral HSR loss was detected in 55 % in unilateral lesions and bilateral HSR loss in 94 % of cases with bilateral excitability. These data were highly statistically significant (p = 0.0006).
Discussion
When the head stabilization reflex was first described by Uludag, the authors suggested that the supraspinal structures had effects on this reflex [1]. The remarkable finding in patients with unilateral cerebellar dysfunction was the bilateral inhibition of the reflex in about half of the cases, even though the lesions or clinical findings were unilateral. This may suggest that the effect shows an ipsilateral excitatory behavior on the reflex or cerebellar excitatory impact occurs on this reflex without being crossed. Another speculation at this point may be that there is excitation of the common generator by both hemispheres of the cerebellum and the blockage of the reflex even with the disappearance of one of the excitations. It is unlikely that cases with unilateral cerebellar lesions will allow us to reveal fully the physiological contribution of the cerebellum on this reflex. This is because, considering the anatomical structure and functional units of the cerebellum, it is impossible to tell the isolated effect of these sections on the reflex. Demonstrating the full contribution of the cerebellum to the reflex physiologically seems to require experimental animal models.
In the study of Uludag et al., reflex responses with normal latency and amplitude were obtained in patients with pure sensory neuropathy and areflexia [1]. The authors explained this by disuse of the normal stretch reflex pathways of the reflex. Although the present study does not include these kinds of cases, this information is significant for the physiology of the reflex.
A reflex arc consists of afferent and efferent systems. In a case where the probable afferent system is fully blocked, achievement of the reflex can be explained by the use of another afferent route by this reflex simultaneously or individually. It is likely that this afferent pathway is the vestibular structures [1].
Unilateral or bilateral vestibulocochlear dysfunction often occurs in Meniere’s syndrome. It is known that lateralization of the head to the side of vestibulocochlear dysfunction can be seen in the examination of patients with Meniere’s syndrome. These data strongly suggest vestibular activity in HSR. Furthermore, they suggest that the impact on the reflex may be ipsilateral and inhibitory or contralateral and excitatory.
In the present study, bilateral reflex responses were not obtained in approximately 70 % of the cases with hemispheric lesions. The fact that there was no difference between right and left hemispheric lesions in causing HSR loss indicates that both hemispheres have the same effect on this reflex. Bilateral loss of the reflex in unilateral cerebral hemispheric lesions suggests the bilateral inhibition of HSR. Nevertheless, there are no enough data to explain the bilateral loss of HSR and its connections in cases of unilateral cerebral lesions. Furthermore, a specific region which leads to complete loss of HSR in cerebral lesions has not been demonstrated. The reflex does not disappear in all isolated cortical, subcortical, anterior and posterior cerebral lesions. HSR dysfunction in such broad and various lesions indicates somehow the existence of connections with relevant supraspinal and vestibular structures, but does not help with lesion localization.
Cervical proprioceptive sensory inputs not only regulate body posture, but also help to protect the position of the head relative to the body via cervicocollic reflexes [5–7]. Although there have been attempts to show reflex pathways like the ventral trigeminocervical reflex in some animal experimental models, the availability of the same pathways in humans is controversial [8, 9]. Sensory signals both stimulate motor neurons that innervate flexor muscles of the stimulated limb and inhibit motor neurons that innervate the extensor muscles of the same limb. These reflexes are modulated according to peripheral requirements with reciprocal innervation. Ia intermediate neurons play a role in the formation of these reflexes; flexor muscle contraction is precluded while the extensor muscle contracts. Cervical reflexes constitute one part of the multisensory intersegmental postural control mechanisms in healthy humans. Tonic neck–eye reflex in humans can only be seen in newborns or rarely in patients with extensive and advanced lesions of the central nervous system. Local anesthesia of deep posterolateral neck tissue causes ipsilateral increased extensor muscle tone and contralateral decreased extensor muscle tone temporarily and so it leads to falls and gait deviations. Unilateral irritation or deficit of the cervical afferents cause cervical tone imbalance due to the occurrence of an asymmetry in somatosensory inputs as a result of anesthesia or incisions of cervical roots or cervical muscles and consequently the integration of the inputs from the vestibular and neck deteriorates [4, 7, 10–13]. In the animal studies of Masaki et al., vestibular connections of the cervical reflex and the role of the AS and SCM muscles in these connections have been shown [14]. Uludag et al. [1] have shown that the AS carries efferent fibers in HSR and this reflex has been recorded in the SCM muscle. This study supports Uludag and Ertekin’s study.
When HSR is evaluated in the light of the above research, this reflex is thought to be produced as a result of the mechanisms for balance and posture protection. Although peripheral inputs are important for HSR, central effects (which we think are more excitatory) seem to play an essential role [1]. In cerebellar lesions, not regaining HSR in the acute and chronic period suggests the absence of an adaptation mechanism. Although some regained cerebellar functions can be explained by improved plasticity, we observed that HSR did not return (was not improved). Unimproved HSR is suggestive of a significant block or inhibition on these reflex pathways [15, 16].
Both the present study and Uludag’s study suggest that HSR is a polysynaptic reflex [1]. In these studies, having a long latency of 40–60 ms and showing variability of latency gradually with consecutive stimuli support our thesis. It seems impossible to explain the intermediate neurons involved in the polysynaptic reflex arc and their supraspinal connections with our current knowledge. Here, we think that inhibitory intermediate neurons have a special role in reflex coordination. The convergence of inputs within intermediate neurons enhances the flexibility of reflex responses. There is a convergence of information from various sources on the intermediate neurons in the spinal cord and brain stem. Ib inhibitory intermediate neurons are the most studied of the neurons which receive widespread convergence. They receive their basic inputs from the Golgi tendon organs, which are sensitive to muscle tension. Reflex activities of Ib afferent fibers from Golgi tendon organs are modulated by a variety of inputs to Ib inhibitory intermediate neurons [13, 17, 18].
The feature which distinguishes HSR from other brain stem reflexes (such as the blink reflex, corneal reflex, jaw reflex, masseter inhibitory reflex and corneomandibular reflex) is the rapid disappearance of HSR in cortical, subcortical, cerebellar, brainstem and limited vestibular lesions. On the other hand, the other above-mentioned brainstem reflexes are mostly lost in severe lesions of the brain stem and associated cranial nerves [2, 19].
In conclusion, hemispheric lesions, cerebellar and vestibular structures were found to have an effect on HSR in this study. This effect is thought to be an excitatory effect but it is believed that excitation disappears with the occurrence of lesions and HSR is inhibited. The interest lies in the complexity of the systems that regulate the head body balance.
References
Uludağ B, Ertekin C (1999) The head stabilization reflex by stimulation of accessory nerve. J Neurol Sci 167:68–72
Kizilay F, Gungor H, Ozkaynak S (2009) Head stabilization reflex in patients with brain stem vascular lesions. J Turk Neurol 15:19–23
Guyton MD, Arthur C (2000) Textbook of medical physiology, 10th edn. Saunders Elsevier, Philadelphia, pp 640–646
Petersen Jens A, Straumann Dominik, Webe Konrad P (2013) Clinical diagnosis of bilateral vestibular loss: three simple bedside tests. Ther Adv Neurol Disord 6:41–45
Peterson BW, Goldberg J, Bilotto G, Fuller JH (1985) Cervicocollic reflex: its dynamic properties and interaction with vestibular reflexes. J Neurophysiol 54:90–109
Kanaya T, Gresy MA, Bronstein AM, Buckwell D, Day B (1995) Control of the head in response to tilt of the body in normal and labyrinthine—defective human subjects. J Physiol 489:895–910
Bikeles F, Ruttin E (1915) Uber die reflektorischen kompensatorischen Augenbewegungen bei beiderseitiger Ausschaltung des N vestibularis. Neurol Zbl 34:807–810
Kato S, Papuashvili N, Okada YC (2003) Identification and functional characterization of the trigemi ventral cervical reflex pathway in the swine. Clin Neurophysiol 114(2):263–271
Katoh A, Shin SL, Kimpo RR, Rinaldi JM, Raymond JL (2015) Purkinje cell responses during visually and vestibularly driven smooth eye movements in mice. Brain Behav 5(3):e00310
Barany R (1906) Augenbewegungen, durch Thoraxbewegungen ausgelost. Zbl Physiol 20:298–302
Bronstein AM, Hood JD (1985) Cervical nystagmus due to loss of cerebellar inhibition on the cervico-ocular reflex: a case report. J Neurol Neurosurg Psychiatry 48:128–131
Biguer B, Donaldson IML, Hein A, Jeannerod M (1989) Neck muscle vibration modifies the representation of visuel motion and direction in man. Brain 111:1405–1424
Pearson K, Gordon J (2000) Chapter 36. In: Kandel RE, Schwartz JH, Jessell TM (eds) Principles of neural science. McGraw-Hill Companies, New York, pp 713–736
Masaki Y, Ogasawara K, Yoshikawa H, Watanabe M, Furukawa T, Ando I, Ichikawa G (2002) Cervical reflex induced by click stimuli in cats. Acta Otolaryngol 122(6):607–612
Hirata Y, Highstein SM (2002) Plasticity of the vertical vestibulo-ocular reflex: a system identification appro localizing the adaptive sites. Ann NY Acad Sci 978:480–495
Baek SH, Choi JY, Jung JM, Kwon DY, Park MH, Choi J, Kim JS (2015) Abnormal head impulse test in a unilateral cerebellar lesion. J Clin Neurol 11:279 (PMID: 25749819)
Starose’tseva NG, İvanichev GA (2002) Spinal-bulbo-spinal polysynaptic reflex in clinical neurology. Zh Nevrol Psikhiatr İm S S Korsakova 102(9):26–31
Mendell LM, Henneman E (1971) Terminals of single Ia fibers; location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol 34:171–187
Ding BC, Wang P (1998) Role of ventrolateral medullary areas in depressor reflex. Sheng Li Ke Xue Jin Zhan 29(3):271–274
Conflict of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bademkiran, F., Uludag, B., Guler, A. et al. The effects of the cerebral, cerebellar and vestibular systems on the head stabilization reflex. Neurol Sci 37, 737–742 (2016). https://doi.org/10.1007/s10072-015-2459-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2459-8