Abstract
The aim of this study was to investigate the effects of the somatosensory system on the vestibular system and the interconnected ways they work together to maintain balance. The study was conducted on 54 individuals (27 females and 27 males), aged between 18–25 years. vHIT as well as cVEMP tests were used to evaluate the participants. Tests were carried out while sitting, standing on firm surface and standing on foam respectively. According to the posterior vHIT results, there was a significant difference between VOR gains obtained while sitting and standing on firm surface in right side as well as on the left side (p < 0,01). Moreover, when VOR gains in standing on firm and standing on foam results were compared to each other, statistical significance was found right and left posterior canals (p < 0,05). Concerning the results obtained from VEMP, a statistically significant difference was seen in the comparison of P1-N1 amplitudes of the right side on firm surface and standing on foam (p < 0,01). When the inputs from somatosensorial system are disturbed, the parts of the vestibular system that are primarily affected are the posterior SSC, saccule and inferior vestibular nerve. This can be interpreted as the inferior vestibular nerve being more affected than the superior vestibular nerve when posture is disturbed due to somatosensory cues being unavailable or unstable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posture is a complex mechanism that is maintained by the integration of signals from visual, somatosensorial and vestibular systems [1]. The vestibular nucleus checks and integrates these inputs for balance and coordination with the help of other areas in the brain. If there is a problem at any stage of the process, it may present itself as complaints of dizziness or imbalance [2]. This can be the result of abnormalities in many different places: vestibular system, vestibulo-spinal reflex (VSR), vestibulo-ocular reflex (VOR) and vestibulo-collic reflex (VCR) pathways. The primary function of the vestibular-spinal reflex is to maintain posture while the vestibulo-ocular reflex (VOR) functions to maintain visual stability during head movements [3].
To assess the function of the sensory organs in the vestibular system, such as the otolith organs and the semicircular canals (SSCs), we can use the cervical vestibular-evoked myogenic potential (cVEMP) and the video head impulse test (vHIT) [4]. vHIT enables the objective evaluation of VOR gains of each SSC individually [5, 6]. cVEMP test is the recording of short latency muscle reflex responses of the sternocleidomastoid muscle triggered by sound stimulation of the vestibular otolith organs (esp. saccule) [7, 8]. During standing, both the SSCs and otolith organs sense head motion [9].
Studies in the literature have shown that caloric or galvanic stimulation to the vestibular system affects the somatosensory system [10,11,12]. According to the study by Horak et al., although vestibular inputs have little effect when somatosensory information is the major source of information, vestibular signals greatly affect lower extremity motor outputs when somatosensory information is absent or unstable. (76). However, when somatosensory cues are absent or unstable, the specifically affected areas in the vestibular system are not well established.
In this study we utilized a foam surface to disrupt the somatosensory inputs and evaluated the vestibular system using vHIT and cVEMP. We evaluated VOR gains of each SSC bilaterally with vHIT. With this method, the effect of insufficient somatosensory cues on the angular VOR gains were investigated. Similarly, the effect of the impaired somatosensory inputs on saccule was investigated using the cVEMP test. Thus, we examined the vestibular system in more detail when the posture is disturbed due to unstable somatosensory cues.
Material Method
Ethical committee approval was obtained from the Clinical Research Ethics Committee of Istanbul Medipol University. The study was conducted at the Audiology Laboratory of Istanbul Medipol University between October and July 2018. Fifty-four individuals (27 males and 27 females) aged between 18–25 years (mean: 21.07 ± 1.35) participated in this study in the study. Individuals who did not suffer from dizziness or imbalance in the last six months were included in the study. Participants who had vision and neck problems, physical disability that which may prevent from standing on a foam surface, a history of psychological and neurological disorders, and regular use of alcohol and drug were excluded from the study. Before the experiment, the study was explained to each participant and a written informed consent was obtained.
The subjects were randomly divided into two groups with similar numbers of males and females. For the first group cVEMP was performed and a week later vHIT was applied and vice versa. This method was followed to make sure that the participants` neck muscles do not get tired and contracted and affect the results of the second test.
vHIT
Participants wore the Interacoustics VisualEyes Videonystagmography (Micromedical Technologies, Illinois, USA) lightweight goggle frame with a built-in accelerometer to record head movements with a camera to record eye movements. Participants sat approximately 1 m from a wall with a visual fixation target. The visual fixation target the participants looked at during testing was adjusted according to their height. Clinician tilted the participant’s head 30° below the horizontal plane to bring the horizontal SSC parallel to the ground and then head stimulatation were performed (10 to 15 head impulses with small amplitudes (15 to 20°) to the right and left, with peak velocity changing from 150 to 250° per second). Horizontal vestibulo-ocular reflex (VOR) gain was calculated as the ratio of the eye velocity to the head velocity 60 ms after the given head impulse [13] All six SSCs were tested using the appropriate modules (Lateral vHIT, LARP vHIT and RALP vHIT). All modules tested in an individual while "Sitting—Standing on Firm Surface (SFS)—Standing on Foam (SF)" positions (Fig. 1). During SFS and SF positions participants feet were adjusted according to their shoulder width.
cVEMP
Interacoustics Eclipse (Interacoustics A/S, Middelfart, Denmark) device was used while performing the cVEMP test. Electrodes was placed at the midpoint of the sternocleidomastoid muscle (SCM), the sternoclavicular junction, and at the manubrium sternum. During sitting cVEMP participants were instructed to hold their heads upright and sideways to provide tonic SCM activity during stimulation. The sound stimuli consisted of 500 Hz, 100 dB SPL tone burst with a linear envelope (1 ms rise/fall time, 2 ms plateau), at a repetition rate of 5 Hz. Acoustic stimuli were delivered monaurally through insert headphones. Same process was repeated for the other side as well. Two recordings were taken on both side and their average were used for the results. The same procedure was performed in the SFS and SF positions for as well.
Statistical Analysis
The data analysis of our study was performed using the Statistical Package for Social Sciences (SPSS) Version 22.0 (SPSS inc., Chicago, IL, USA). Mean and standard deviation (SD) are given in descriptive statistical information. “Kolmogorov – Smirnov Test” was used to find out whether the independent parameters were normally distributed. The triple comparison between groups was measured using the "Friedman Test". The differences between sitting—SFS, sitting-SF and SFS—SF in vHIT and cVEMP tests were compared using the "Wilcoxon Sign Rank Test". The statistical significance level was accepted as 0.05 in the analysis results of the Wilcoxon Sign Rank Test. In the Friedman Test, the statistical significance level was accepted as 0.017.
Results
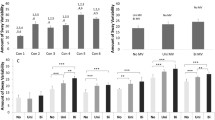
There was no significant difference between the groups in all lateral and anterior SSCs VOR gain results. In the triple comparison, a statistically significant difference was observed only for right posterior SSC results (p = 0,002), while the difference on the left side did not show a statistically significant difference (p = 0,057) (Table 1). In addition, differences were observed between the right and left sides in both sitting-SFS comparison and SFS-SF for the posterior SSC VOR gains (p < 0.05) (Fig. 2).
According to the results of the Friedman test performed for the triple comparison of the groups, no statistically significant difference was observed between the cVEMP parameters (Table 2). No significant difference was found on the paired comparison of left and right sides in sitting-SFS and sitting-SF comparisons in cVEMP parameters (Table 2). Likewise, there was no significant difference on the left side in the comparison of SFS and SF. A statistically significant difference was observed in the right side when comparing SFS and SF (p = 0.009) (Fig. 3). Apart from this, no significant differences were observed between latency values and asymmetry values among cVEMP parameters.
Discussion
In electrophysiological studies, neuron responses to vestibular stimuli have been recorded in areas such as the parieto-insular vestibular cortex (PIVC), somatosensory cortex, and intraparietal region [14,15,16]. These studies confirm the multidimensional structure of the vestibular cortical network by identifying neurons that respond to combinations of tactile, visual, and vestibular inputs. In our study, in vHIT, VOR gains decrease when people shift from sitting to standing position. The reason for this may be that nervous system requires the somatosensory system to be more active when standing up thus resulting in a more subdued vestibular system. When the participants moved from the standing position to the foam surface, the gain still increases. This may be the result of the failing trust in the somatosensory system because of the disruption of the ground surface. To compensate for the postural disturbance on the foam surface the vestibular system becomes more active. The fact that the vestibular system becomes more active may have been the reason for the increased the VOR gain. Supporting our research, Naranjo et al. [17] found, vestibular reflexes have been shown to increase under postural challenge.
Moran and Cochrane [18] said better results were obtained when the eyes were open whilst head shaking and looking at a fixed point, that is when the VOR worked, compared to balancing on the foam with the eyes closed. However, no difference was found between these two conditions on a firm surface. This suggested that VOR contributes to posture when somatosensory cues are reduced. Similarly, in our study, there was an increase in VOR gain in all canals (only statistically significant one being the posterior canal) in the SF condition compared to the SFS condition. This result objectively demonstrates that the increase in VOR gain is compatible with the decrease in somatosensory input.
Bottini et al. found that in a patient with decreased tactile perception, left hemianesthesia temporarily appeared after the administration of cold-water caloric stimulation. They also observed activation in the right hemisphere (insula, right putamen, inferior frontal gyrus in the premotor cortex) for said patient [19]. These data were interpreted as a modulation of somatosensory perception induced by vestibular stimulation and mediated by a right hemispheric neural network involved in awareness with somatosensory processing [19, 20]. Ferre et al. found that the modulation of tactile processing was more dominant in the right hemisphere in their study with galvanic stimulation on normal individuals [21]. In addition, similar results were obtained in studies using galvanic stimulation and/or caloric stimulation and using fMRI and/or PET [22,23,24]. A significant difference was found only on the right side in the comparison of SFS and SF during the cVEMP test phase in our study. This result is also compatible with the right hemisphere where vestibular-somatosensory senses are dominant.
In our study, there were significant differences in the gain of posterior canal in vHIT and saccule responses in cVEMP, when we disrupted the surface and reduced the somatosensorial cues. The fact that these two anatomical structures relay information via the inferior vestibular nerve to the vestibular nucleus and concerning is statistically significant difference in obtained both tests suggests that the inferior vestibular nerve is related to the efferent vestibular system. Chagnaud et al. [25] showed that efferent vestibular nerve fibers are active during swimming in larval Xenopus frogs. In addition, the fact that the efferent system did not work well in these creatures whose spinal cords have been removed has shown that there is a connection between the efferent vestibular system and the spinal cord [25]. This suggested that there may be a relationship between the efferent system and VSR. Studies have also shown that the efferent vestibular system influences the VOR [26]. Another study in frogs showed that the efferent vestibular system plays an active role in the stimulation of posterior canal afferent fibers. When we evaluate the studies in the literature and our results together, it is thought that the efferent vestibular system influences VOR and VSR when people spend more energy to maintain their balance.
Öztürk et al. [27], found statistically significant differences in the superior and horizontal responses that project to the superior vestibular nerve when a visual illusion was given to disrupt the visual system [27]. In our study, when we influenced the somatosensory system the posterior canal which is connected to the inferior vestibular nerve had shown statistically significant difference. This suggests that problems that may occur in the posterior canal or inferior vestibular nerve may affect the somatosensory system more, while problems that may occur in other canals and/or superior vestibular nerve may have a more dire effect on the visual system.
The testing frequency ranges of the otoliths and SSCs are different; therefore, it is believed that they contribute to the different aspects of postural control [28]. The majority of standing sway power is in the lower frequency range, while the video head impulse VOR gain measurements of SSC function is in the high frequency range [28]. Current results suggest that VOR gain serves as an indirect measure for the angular vestibular contributions to balancing one’s self while standing [9]. This may be the reason why there were clearer significant differences between the VEMP results and the vHIT results in our study.
Conclusion
In our study, the SFS versus SF in cVEMP, the decrease in the amplitude results on the foam surface indicate that the body's activity decreases in difficult situations. Especially forcing the VSR on the foam surface may have been effective in decreasing the amplitudes. vHIT results show that the gains were lower during standing in the comparison of Sitting-SFS result in all SSCs, while the gain was higher in the SFS-SF comparison. While it is thought that the reason for low gain while standing is related to more active functioning of the somatosensory system and the decrease in the activity of the vestibular system, it is thought that the reason for the increase in gain on the foam is the active role of the vestibular system instead of the struggling somatosensory system.
Limitations of the Study
Since we do not have an equipment that measures foot pressures, the pressure rates of the people could not be measured. For this reason, the oscillations made by people whose balance is disturbed, especially in the positions they stand on the sponge, could not be evaluated through an objective evaluation.
References
Hain TC, Helminski JO (2007) Anatomy and physiology of the normal vestibular system. In: Herdman SJ (ed) Vestibular rehabilitation, 3rd edn. F. A Davis Company, USA, pp 2–18
Plishka CM (2015) A clinician’s guide to balance and dizziness: evaluation and treatment. SLACK Incorporated, USA
Mucha A, Collins MW, Elbin RJ et al (2014) A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. https://doi.org/10.1177/0363546514543775
Apeksha K, Singh S, Rathnamala M et al (2020) Balance assessment of children with sensorineural hearing loss. Indian J Otolaryngol Head Neck Surg 73:12–17. https://doi.org/10.1007/s12070-020-01797-x
Halmagyi GM, Curthoys IS (1988) A clinical sign of canal paresis. Arch Neurol 45:737–739. https://doi.org/10.1001/archneur.1988.00520310043015
Aalling M, Skals RK, Abrahamsen ER, Hougaard DD (2020) Comparison of test results from two separate video head impulse test systems in a cohort of patients diagnosed with a unilateral vestibular schwannoma. Eur Arch Oto-Rhino-Laryngology 277:3185–3193. https://doi.org/10.1007/s00405-020-06116-2
Quaranta N, Longo G, Dadduzio S et al (2020) Ocular and cervical vestibular-evoked myogenic potentials in idiopathic sudden sensorineural hearing loss (ISSHL) without vertigo: VEMPs in ISSHL. Eur Arch Oto-Rhino-Laryngology 277:409–414. https://doi.org/10.1007/s00405-019-05724-x
Dorbeau C, Bourget K, Renard L et al (2021) Vestibular evoked myogenic potentials. Eur Ann Otorhinolaryngol Head Neck Dis. https://doi.org/10.1016/j.anorl.2021.01.001
Anson E, Bigelow RT, Studenski S et al (2019) Failure on the foam eyes closed test of standing balance associated with reduced semicircular canal function in healthy older adults. Ear Hear 40:340–344. https://doi.org/10.1097/AUD.0000000000000619
Ramachandran VS, McGeoch PD, Williams L, Arcilla G (2007) Rapid relief of thalamic pain syndrome induced by vestibular caloric stimulation. Neurocase. https://doi.org/10.1080/13554790701450446
McGeoch PD, Ramachandran VS (2008) Vestibular stimulation can relieve central pain of spinal origin. Spinal Cord. https://doi.org/10.1038/sc.2008.47
Ferrè ER, Bottini G, Haggard P (2011) Vestibular modulation of somatosensory perception. Eur J Neurosci 34:1337–1344. https://doi.org/10.1111/j.1460-9568.2011.07859.x
Agrawal Y, Schubert MC, Migliaccio AA et al (2014) Evaluation of quantitative head impulse testing using search coils versus video-oculography in older individuals. Otol Neurotol 35:283–288. https://doi.org/10.1097/MAO.0b013e3182995227
Grüsser OJ, Pause M, Schreiter U (1990) Localization and responses of neurones in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis). J Physiol 430:537–557. https://doi.org/10.1113/jphysiol.1990.sp018306
Schwarz DW, Fredrickson JM (1971) Rhesus monkey vestibular cortex: a bimodal primary projection field. Science 172:280–281. https://doi.org/10.1126/science.172.3980.280
Bremmer F, Klam F, Duhamel J-R et al (2002) Visual±vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur J Neurosci 16:1569–1586. https://doi.org/10.1046/j.1460-9568.2002.02206.x
Naranjo EN, Allum JHJ, Inglis JT, Carpenter MG (2015) Increased gain of vestibulospinal potentials evoked in neck and leg muscles when standing under height-induced postural threat. Neuroscience 293:45–54. https://doi.org/10.1016/j.neuroscience.2015.02.026
Moran RN, Cochrane G (2020) Preliminary study on an added VOR visual conflict task for postural control. J Clin Transl Res 5:155–160 https://doi.org/10.18053/jctres.05.2020s4.001
Bottini G, Paulesu E, Sterzi R et al (1995) Modulation of conscious experience by peripheral sensory stimuli. Nature 376:778–781. https://doi.org/10.1038/376778a0
Bottini G, Paulesu E, Gandola M et al (2005) Left caloric vestibular stimulation ameliorates right hemianesthesia. Neurology 65:1278–1283. https://doi.org/10.1212/01.wnl.0000182398.14088.e8
Ferrè ER, Day BL, Bottini G, Haggard P (2013) How the vestibular system interacts with somatosensory perception: a sham-controlled study with galvanic vestibular stimulation. Neurosci Lett 550:35–40. https://doi.org/10.1016/j.neulet.2013.06.046
Eickhoff SB, Weiss PH, Amunts K et al (2006) Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp 27:611–621. https://doi.org/10.1002/hbm.20205
Eulenburg P, Caspers S, Roski C, Eickhoff SB (2012) Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage 60:162–169. https://doi.org/10.1016/J.NEUROIMAGE.2011.12.032
Lopez C, Blanke O, Mast FW (2012) The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 212:159–179. https://doi.org/10.1016/j.neuroscience.2012.03.028
Chagnaud BP, Banchi R, Simmers J, Straka H (2015) Spinal corollary discharge modulates motion sensing during vertebrate locomotion. Nat Commun. https://doi.org/10.1038/ncomms8982
Rossi ML, Martini M (1991) Efferent control of posterior canal afferent receptor discharge in the frog labyrinth. Brain Res 555:123–134. https://doi.org/10.1016/0006-8993(91)90868-V
Öztürk ŞT, Şerbetçioğlu MB, Ersin K, Yılmaz O (2021) The impact of optical illusions on the vestibular system. J Audiol Otol. https://doi.org/10.7874/jao.2021.00080
Carriot J, Jamali M, Brooks JX, Cullen KE (2015) Integration of canal and otolith inputs by central vestibular neurons is subadditive for both active and passive self-motion: implication for perception. J Neurosci 35:3555–3565. https://doi.org/10.1523/JNEUROSCI.3540-14.2015
Funding
This clinical report received no specific grant from any funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study contains only human participants.
Consent for Publication
Informed Patient Consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ersin, K., Şerbetçioğlu, M.B., Öztürk, Ş.T. et al. The Effect of Somatosensorial System on Vestibular System. Indian J Otolaryngol Head Neck Surg 74 (Suppl 3), 4138–4143 (2022). https://doi.org/10.1007/s12070-021-02867-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-021-02867-4