Abstract
Inhibitory control is a term used to envelop a collection of processes that allow an organism to refrain from engaging in an inappropriate prepotent or responsive behavior. Studies have examined the propensity of inhibitory control by nonhuman animals, from the cognitively complex processes involved in self-control to potentially less cognitively taxing processes such as motoric self-regulation. Focusing on canines, research has suggested that the domestication process as well as experiences during ontogeny contribute to inhibitory control. Diet may also play an important role in an individual’s ability to self-regulate. This study examined this possibility by investigating motoric self-regulation in sled dogs, using three well-established tasks (i.e., A-not-B Bucket, Cylinder, and A-not-B Barrier tasks), performed after consumption of one of three dietary treatments with different glycemic index values. We also compared the performance of sled dogs during these tasks with results previously obtained from pet dogs. Overall, the results show many similarities in the performance of sled dogs and pet dogs on the motoric self-regulation tasks, with the notable exception that sled dogs may have a stronger spatial perseveration during the A-not-B Bucket task. Previous research findings reporting a lack of correlation among these tasks are also supported. Finally, during the early postprandial phase (period after consumption), dietary treatments with different glycemic index values did not influence self-regulatory performance for sled dogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhibitory control is composed of a collection of processes or behaviors that allow an individual to refrain from engaging in an impulsive and inappropriate response, but instead emit an alternative and more advantageous behavior or action (Beran 2015; Diamond 1990). As inhibitory control is thought to be an important foundational aspect of cognition, and particularly executive functioning, many researchers have examined inhibitory control abilities across nonhuman animals to better understand the evolution of this construct (e.g., Amici et al. 2008; MacLean et al. 2014; Kabadayi et al. 2016). However, inhibitory control is multi-faceted and includes a range of abilities from the cognitively complex, such as self-control, to the more “basic”, such as motoric self-regulation (Beran 2015). Self-control is thought to be more cognitively complex as it allows an individual to consider, or weigh, options when a decision is necessary, for instance, waiting for a more desirable food item that is to come in the future, if one is able to withhold consuming a less desirable but immediately available food item. At the other end of the spectrum, motoric self-regulation is thought to be less cognitively demanding, as the individual is only required to restrain a prepotent response. Although researchers have attempted to examine self-control by nonhuman animals (with many studies of nonhuman primates), the majority of studies investigating inhibitory control have examined motoric self-regulation (e.g., mammals and birds: MacLean et al. 2014; birds: Kabadayi et al. 2016; Vernouillet et al. 2016; fish: Lucon-Xiccato et al. 2017; primates: Amici et al. 2008; Glady et al. 2012; dogs: Bray et al. 2014; Brucks et al. 2017; Vernouillet et al. 2018).
Multiple tasks have been developed to examine the motoric self-regulation abilities of nonhuman animals, and among these A-not-B tasks, Cylinder tasks, reversal learning tasks, and several detour tasks have become most commonly used and standardized. The relative simplicity of these tasks, and the ease in which they may be administered, has allowed for their use across a range of species. Results from such studies have suggested quite interesting inter- and intra-species differences in motoric self-regulation abilities. For the purpose of the current study, studies of motoric self-regulation in canids have reported differences between wolves and pet dogs (Marshall-Pescini et al. 2015) as well as diverse groups of dogs, such as working/highly trained dogs and pet dogs (Barrera et al. 2018; Bray et al. 2015) or shelter dogs and pet dogs (Fagnani et al. 2016). Generally, these studies have shown that dogs afforded less opportunity for socialization perform poorer on tasks of inhibitory control, whereas those experienced in complex training and socialization procedures have better (or improved) inhibitory control abilities. Inhibitory control may be important to another group of dogs that have received considerably less research attention—sled dogs. Sled dogs typically receive experiences with handling and socialization early in life, task-specific training procedures which typically include a type of “leave it” command and team running exercises. Thus, sled dogs provide an intermediary group, in that owners of sled dog teams interact with their dogs on a regular basis, and more frequently during the racing season, but presumably individual dogs receive less one-on-one interaction with humans compared to many pet dogs (particularly those participating in voluntary research programs). Additionally, sled dogs are housed in outdoor kennels, whereas pet dogs are most often housed free range indoors. Therefore, we predicted that sled dogs may show higher inhibitory control compared to shelter dogs, but less inhibitory control compared to pet dogs.
Complicating the understanding of inhibitory control in canids, research findings show a lack of correlation, as well as intra-individual variability, across the tasks used to study motoric self-regulation (Vernouillet et al. 2018). Thus, a within-subjects design using a multi-task approach is necessary to not only evaluate an individual dog’s ability to self-regulate, but to draw comparative conclusions across dog breeds or groups. Such an approach may also allow for the accumulation of results to permit large-scale analyses to understand better the nature of the tasks themselves. Thus, in the current investigation of motoric self-regulation by sled dogs, the experimental methodology and behavioral tasks were replicated from a previous study of pet dogs completed by the same researchers (Vernouillet et al. 2018), allowing for correlation of the current results with those previously published. Adopting the same methodology and tasks as in the previous research also allowed for the evaluation of intra-individual variation in sled dogs and the comparison of these results with those reported for pet dogs.
Cognitive performance may also be influenced by the type and amount of carbohydrates in one’s diet (Philippou and Constantinou 2014). The glycemic index (GI) was developed to help people with diabetes manage their blood sugar levels (Jenkins et al. 1981). The GI ranks carbohydrate foods based on how much they raise blood glucose levels after consumption, as compared to a control (glucose solution or white bread) (Wolever et al. 1991). A value of 55 or less is considered a low GI carbohydrate source, a medium GI food has a value of 56–69, and a value of 70 or more is a high GI food, with glucose used as the control food (Atkinson et al. 2008). Low GI foods are more slowly digested, absorbed and metabolised, and have been shown in humans to offer health benefits, such as prevention and management of diabetes, cardiovascular disease and weight management (Barclay et al. 2008; Thomas et al. 2007). Conversely, there is significant evidence from the human literature showing that hyperglycemia is detrimental to health. High blood glucose levels are associated with obesity and may be an independent risk factor for cardiovascular disease (Kawano et al. 1999; Node and Inoue 2009; Wascher et al. 2005).
Relatively less is understood as to how glucose may influence cognitive function. Glucose is the main energy source for the brain and, for humans, complex cognitive tasks results in a measurable decline in peripheral blood glucose (Philippou and Constantinou 2014, also see McNay et al. 2000 for a study of rats). Indeed, poor blood glucose regulation is a risk factor for impaired cognitive functioning (Philippou and Constantinou 2014). However, performance during some cognitive tasks (such as working memory and attention) may be impacted more than others (Lamport et al. 2009). Glucose has also been shown to have memory enhancing effects that are dose dependent, with small and large (e.g., 10 and 500 mg/kg, respectively) doses showing little effect but moderate (e.g., 100 mg/kg) doses providing memory enhancement (Flint and Turek 2003; Smith et al. 2011). Whether glucose levels influence cognitive function (such as executive function and behavioral flexibility—as measured through a complement of behavioral responses, for instance extinction, inhibition, and reversal learning) is also not well understood (Riby et al. 2017).
In spite of the significant evidence for the role of GI in human nutrition, the effects of a high GI diet on dog health as well as cognition are mostly unknown. Studies of dogs with insulin-dependent diabetes have shown that the carbohydrate, fat and protein composition of the diet influence glucose metabolism (Graham et al. 1994). Furthermore, a study that examined the effects of different carbohydrate sources on glycemic and insulinemic responses in dogs found that diets containing sorghum, lentils and peas delayed and lengthened the glycemic and insulinemic responses (Carciofi et al. 2008). Another study that compared the glycemic responses of uncooked corn, wheat, barley, rice and sorghum found that rice produced the highest postprandial glycemic and insulinemic responses in dogs (Sunvold and Bouchard 1998). For dogs that have evolved to consume diets that are more moderate in protein and higher in carbohydrates than their ancestors (Axelsson et al. 2013), understanding whether diets differing in GI influences fundamental cognitive processes is important. Yet, few studies have examined whether glucose affects cognitive performance and, in particular, inhibitory control by nonhuman animals (Parrish et al. 2016; Miller et al. 2015). However, Miller and colleagues showed that glucose was able to replenish prior exertion as well as persistence during a self-control task requiring a dog to sit-and-stay (Miller et al. 2010, 2015, respectively; see also Beurms and Miller 2016). To our knowledge, no study has yet to use a motoric self-regulation task to examine the influence of glucose on inhibitory control. Determining whether carbohydrate levels in dogs’ diets and the resulting glycemic response affect the ability to engage in inhibitory control would provide beneficial information for working dog populations, where it is important to optimize cognitive processes and trainability, as well as pet dogs, since difficulties to inhibit often result in behavioral problems (Piotti et al. 2018). These are major reasons for surrender to humane shelters (Salman et al. 2000).
Commercial pet foods typically contain a mixture of carbohydrate sources, which can be categorized into three broad classes: traditional grains (e.g., wheat, corn, rice); novel whole grains (e.g., barley, oatmeal, rye), and non-grain carbohydrates (e.g., peas, lentils, chickpeas, beans, tapioca, potato, sweet potato). Pet food companies often market their products based on these categories of carbohydrate sources, with the grain-free category showing significant growth in recent years. Carbohydrate sources that are known to be low GI in humans, such as peas and lentils, are touted as being beneficial for dogs, though limited scientific data are available to back up these claims. One study found that a diet with peas as its primary carbohydrate source reduced postprandial insulin response after a glucose challenge in obese beagles when compared to a rice-based diet (Adolphe et al. 2014). Due to the convenience, safety and relatively low cost of kibble, the majority of dogs are fed dry extruded pet foods, which require a certain amount of carbohydrate to maintain the kibble structure. Yet, whether these diets differing in carbohydrate sources impact dogs’ cognitive processing is unknown. Thus, the current study examined whether diets differing in GI would influence sled dogs’ ability to show inhibitory control during tasks of motoric self-regulation. Overall, it was hypothesized that the low GI diet would enhance motoric self-regulation by providing a more constant postprandial blood glucose concentration and glucose supply for the brain.
The purpose of this study was threefold: (1) to evaluate performance of sled dogs during three well-established tasks of motoric self-regulations and determine whether performance measures during these tasks correlate, (2) to compare the performance of sled dogs during these tasks of motoric self-regulation with previously published performance of pet dogs, and (3) to evaluate whether dietary carbohydrate type/GI has an effect on performance during these motoric self-regulation tasks for sled dogs.
Methods
Subjects
During this study, 15 sled dogs [8 males (7 neutered and 1 intact) and 7 females (4 spayed and 3 intact); M ±SEM: 5.0 ± 0.7 years old] from the same kennel located in southeastern Ontario, Canada were used. The racing season concluded March 2017, and all dogs, with the exception of one, were involved in training or racing during the season. The dogs were from Siberian Husky racing lineages (2 males, 3 females), Seppala Siberian Husky lineages (2 females) or a combination of both (5 males, 3 females), as reported by the kennel owner (Table 1). Dogs had been with the owner of the kennel since they were less than 1 year old (except for one female who joined the kennel when she was 3 years old). The dogs were kept outdoors in their home enclosures for the duration of the study, in either individual pens (one male and one female) or in two group pens that contained both males and females (11 and 5 dogs in each enclosure). All dogs were deemed healthy before entry into the study based on a physical exam and routine blood work. Dogs had an ideal body condition score of 4–5 on a 9-point scale (Laflamme 1997). The kennel owner completed a brief behavior, temperament and medical questionnaire prior to the start of the experiment (modified from the Duke Canine Cognition Center questionnaire, see Supplementary Materials). Dogs were assigned to three dietary treatment groups (see below; Table 1), with at least two males and two females per group. During the study, dogs participated in a series of three tasks designed to measure motoric self-regulation. The study was completed during the month of June 2017. The study was approved by the Animal Care Committee at the University of Guelph (protocol #AUP3650).
Experimental conditions
Dietary conditions
Prior to the start of the study, each dog was assigned to one of the three dietary treatment groups (i.e., between measures factor) which it received each morning for the duration of the study (3 days). One group (n = 5, 2 females and 3 males) was fed 62 grams of Purina Dog Chow (see Table 2; subsequently referred to as “Traditional Grain Diet”, a high GI diet); a second group (n = 5, 3 females and 2 males) was fed 65 grams of Petcurean GO! SENSITIVITY + SHINE™ Limited Ingredient Duck recipe for dogs (see Table 2; subsequently referred to as “Grain-Free Diet”; a low GI diet); and a third group (n = 5, 2 females and 3 males) was given 50 g of a 50% (wt/vol) liquid glucose solution as control (subsequently referred to as “Glucose”). As reported by Rankovic (2018), the GI of the Traditional Grain Diet was 83 ± 17 (M ±SEM) and 41 ± 6 for the Grain-Free Diet [t(10) = 2.88 p = 0.016]. By definition, the glucose control had a GI of 100. The dietary treatments, including the glucose solution, were fed in amounts that provided 25 g of available carbohydrate, as determined through total starch and free sugar content of the foods (McCance and Lawrence 1929). The blood glucose levels of the sled dogs were not determined at the start of, or during the behavioral testing, so as to prevent a stress response that may negatively affect behavioral performance. Since GI is a property of the food, the high and low GI foods would be expected to elicit similar responses during the motoric self-regulation testing as were seen during the testing performed to determine the GI values of the test foods.
Prior to the study, the dogs were maintained on a standard background diet (GO! FIT + FREE™ Adult Dog Food, Petcurean Pet Nutrition, Chilliwack, BC, Canada) which was fed as one meal every evening. After an overnight fast, the dogs were fed the assigned treatment diet 30 min prior to each daily testing session. As these diets were selected to evaluate the potential effects of blood glucose concentrations on motoric self-regulation, the timing of the meal prior to the start of the task was critical to have consistent postprandial blood glucose levels, as in dogs, this has been shown to peak 30–60 min after ingestion of purified glucose (Adolphe et al. 2012). A small piece of boiled chicken (approximately 2 cm) was available during each experimental trial to provide positive reinforcement (subsequently referred to as “reward”), unless otherwise stipulated (see specific task procedures below). This reward was chosen due to its limited carbohydrate content. Water was available ad-libitum.
Motoric self-regulation tasks
Over the course of the study, each dog completed three well-studied motoric self-regulation tasks: A-not-B Bucket, Cylinder, and A-not-B Barrier. Dogs received only one task per day, for three consecutive days. The order of the tasks was pseudo-counterbalanced across dogs, and the order in which the dogs participated in each daily session was pseudo-counterbalanced (with a dog from each dietary condition participating as the first or last dog per daily session; see Table 1) to control for motivation across dietary conditions.
General procedures
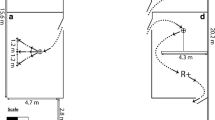
Dogs were tested individually, receiving one session per day within an enclosed room located on the main floor of the owners’ residence (4.5 m width × 7.46 m length × 2.3 m height; see Fig. 1). Although the dogs were housed outdoors, the study was conducted indoors to control for temperature and to provide a quiet, relatively distraction-free setting. Prior to the start of a testing session, the dog was led into the experimental area through a corridor (4.5 m length × 1.84 m width), which was separated from the experimental area by an opaque half-wall (1.61 m length × 1.26 m height). The dog was permitted 10 min to explore the entire experimental room and generally familiarize itself with the experimenter, the recorder, and the handler. During this familiarization phase, only the recording cameras were in place. Once this phase was completed, the dog was led back to the corridor, remaining behind the half-wall while the experimenter and the recorder set up the equipment for the motoric self-regulation task. A maximum of 30 min was allotted for the dog to complete all trials for each daily task. Once the task was completed, or 30 min elapsed, the dog was taken back to its outdoor holding area.
Schematic of the experimental room where the three motoric self-regulation tasks were conducted. Dark gray shading indicates zones inaccessible to the dogs. Light gray shading indicates the experimental arena. Each dog was brought from outside to the experimental arena through the corridor, where it waited with the handler to begin each session
The experimenter, the recorder and the dog handler (individuals retained the same role throughout the study) were present in the experimental room throughout the entirety of the study. The experimenter was responsible for baiting the apparatuses during each trial and interacting with the dog as required by the task procedures. The recorder was responsible for manually documenting the outcome of each trial. The handler was responsible for holding the dog in a stay at the starting position, releasing the dog on direction from the experimenter, and retrieving the dog on direction from the recorder.
Each motoric self-regulation task started with the dog and the handler waiting behind the half-wall. Once the experimenter and the recorder completed setting up the apparatus, the digital recording devices were started and the handler was directed to lead the dog to the starting position. Once the experimenter completed the necessary procedures for the specific task (see specific task procedures below), she directed the handler to release the dog by dropping the leash. Once the dog was released, the experimenter, the recorder, and the handler averted their eyes from the dog and the apparatus, remaining motionless to avoid any unintentional cueing. Once the dog completed the criteria for the task, the handler secured the dog, and both returned to the starting position until the next trial was initiated.
Experimental sessions were digitally recorded using three recording devices to ensure complete coverage of the experimental apparatus and the dog’s responses. A FujiFilm Finepix XP60 digital camera focused on the apparatus, a Hero 6 GoPro video camera recorded the experimental arena, and a Cannon EOS Rebel Ti5 focused on the dog at the starting position. The outcome of each trial was documented in real time by the recorder (see below). Additionally, all trials were re-analyzed offline by an individual naïve to the experimental conditions to provide inter-rater reliability.
Motoric self-regulation task procedures
A-not-B Bucket task
Materials
Three opaque plastic buckets measuring 35.6 cm in height × 17.8 cm in diameter were used for the A-not-B Bucket task. The three buckets were placed along a hypothetical line running perpendicular to a predetermined starting position. The middle bucket on the line was placed 200 cm from the starting position and the other two buckets were placed 120 cm on either side of the middle bucket (see Fig. 2). To prevent potential movement of the buckets, a layer of rocks was placed in the base of each bucket and covered with a plastic plate. A perforated small plastic sphere (9.5 cm in diameter), containing one inaccessible piece of reward, was attached to the plastic plate in each bucket. These spheres were present to ensure odor cues could not be used to differentiate the rewarded bucket and were not visible to the dog from the starting position.
Procedures
Training phase
During the training phase, a dog was led to the starting position by the handler. The experimenter stood behind the middle bucket, called the dog’s name and showed it the food reward. She then moved behind one of the three buckets placing the reward in it, while continuing to call the dog’s name. Once the food was in the bucket, the experimenter positioned herself 100 cm behind the middle bucket, signaled to the handler to release the dog, and proceeded to remain stationary. The dog was given an opportunity to explore each bucket as necessary to locate the food reward during each trial. If the dog retrieved the reward from the baited bucket with its first choice (herein referred to as a “successful choice”), a different bucket was baited during the subsequent trial. If the dog did not retrieve the reward with its first choice, the same bucket was baited during the following trial. A choice was defined as when the dog’s snout passed over the rim of a bucket. If the dog failed to interact with the apparatus within a minute, the trial was aborted, and the next trial began. If the dog did not interact with the apparatus within 10 min or if the dog appeared unmotivated (e.g., did not search for the reward or look at the experimenter when called), it was removed from the task (and data not used). Once a dog successfully retrieved a reward from each of the three buckets, it progressed to the Testing phase.
Testing phase
The initial trials of the testing phase, referred to as “A-trials”, were procedurally similar to the training phase, with the exception that only one predetermined bucket was consistently rewarded per dog (e.g., the left or right bucket). A-trials continued until the dog made a successful choice during three trials, not necessarily consecutive. Once the criterion was met, “A-not-B” testing trials began. Each A-not-B testing trial began with the experimenter standing behind the middle bucket. She held a reward, and while showing it to the dog and calling its name, she stepped behind the same side bucket that was baited during the previous A-trial (herein referred to as “Bucket A”) and placed the reward in the bucket. After one second, the experimenter reached back into the bucket, removed the reward and stepped laterally to the other side bucket (herein referred to as “Bucket B”), while maintaining visual contact with the dog. The experimenter then baited Bucket B with the reward while continuing to call the dog’s name. The experimenter completed the procedure by stepping to a position 1 m behind the middle bucket and signaled to the handler to release the dog. In this fashion, A-not-B testing trials continued until the dog chose Bucket B as its first choice during a single trial. The assignment of Buckets A and B was counterbalanced across dogs.
Behavioral measures
For this task, we collected four dependent measures for each dog: (1) Training Performance—the total number of training trials required for the dog to retrieve a reward out of each baited bucket, (2) A-Trial Performance—the number of A-trials required for a dog to successfully retrieve the reward from Bucket A for three trials, (3) First Testing Trial Performance—the success (or failure) during the first A-not-B testing trial, and (4) A-not-B Bucket Task Score—the number of A-not-B testing trials needed until the dog successfully chose Bucket B as its first choice.
Cylinder task
Materials
Two cylindrical tubes (20 cm diameter × 22 cm length) were each secured to a wooden platform (61 cm length × 31 cm width × 3 cm height; see Fig. 2). A pillowcase filled with sand was placed under the wooden platform to prevent the cylinder from moving. One cylinder (herein referred to as the “Opaque Cylinder”) was constructed from cardboard, whereas the other cylinder (herein referred to as the “Transparent Cylinder”) was constructed from clear acrylic. Depending on the experimental phase, either the Transparent or Opaque cylinder was positioned with the solid sides perpendicular to the starting position. Hence, the reward in the Opaque Cylinder was never visible from the starting position, whereas the reward in the Transparent Cylinder was always visible from the starting position.
Procedure
Training phase
To begin a trial, the experimenter stood 100 cm behind the cylinder with the reward hidden from the dog’s view. The handler brought the dog to the starting position, and once in position the experimenter moved in front of the cylinder, called the dog’s name and showed the reward. When the dog looked toward the experimenter, she stepped behind the cylinder and placed the reward in the center of the cylinder through one of the side openings, while continuing to call the dog’s name. The side through which the experimenter placed the reward was consistent across trials for each individual dog, but counterbalanced across dogs. Once the reward was placed, the experimenter took one step backward, looked toward the ceiling and gave the release cue to the handler.
Each dog was first given training trials with the Opaque Cylinder prior to testing with the Transparent Cylinder, as results from previous studies have shown that individuals first trained using an Opaque Cylinder are more likely to be successful detouring with a Transparent Cylinder (e.g., Santos et al. 1999; Vernouillet et al. 2018). Additionally, training trials with an Opaque Cylinder ensure the dogs are able to solve the task without a needing to inhibit their proponent response to a visual food reward. During training, a trial was scored as successful if the dog’s snout entered directly into either side of the cylinder without touching the outer surface of the cylinder. A trial was scored as unsuccessful if the dog used its paw or any other body part to manipulate the cylinder prior to its snout entering the cylinder. A trial was ended when the dog retrieved the reward, at which point the handler brought the dog back to the starting position. A trial was aborted if the dog failed to interact with the apparatus within a minute, at which point the next trial was started. The task was aborted if the dog did not interact with the apparatus within 10 min or if the dog appeared unmotivated to continue (e.g., did not look at the reward nor responded when called). A dog was required to successfully retrieve the reward from the Opaque Cylinder during four of five consecutive training trials to achieve the training criteria.
Testing phase
The procedure and criteria for testing were similar to training, but the Transparent Cylinder replaced the Opaque Cylinder and only five testing trials were administered.
Behavioral measures
Four dependent measures were collected for each dog: (1) Training Performance—the total number of training trials with the Opaque Cylinder required for a dog to successfully retrieve the reward out of the cylinder for four trials, (2) First Testing Trial Performance—the success (or failure) during the first testing trial, (3) Cylinder Task Score—the number of testing trials completed prior to making the first successful choice, and (4) Overall Test Performance—the number of successful trials out of the five testing trials with the Transparent Cylinder.
A-not-B Barrier task
Materials
A barrier, constructed from a collapsed metal exercise pen (90 cm height × 220 cm length), was extended in a straight line from one wall of the testing arena to 70 cm from the other side of the arena (see Fig. 2). The barrier was movable such that the gap between itself and nearest wall could be positioned to the left or right side of the arena. The barrier was positioned perpendicular and 200 cm away from the dog’s starting position.
Procedure
Training phase
The experimenter was positioned 100 cm behind the barrier, centered with the starting position, while the handler stood with the dog at the starting position. The experimenter called the dog’s name, showing it a reward which she placed on the floor directly in front of her. She then took one step backwards and instructed the handler to release the dog. The dog was permitted 1 min to retrieve the reward by passing through the open gap in the barrier (herein referred to as “Side A”; the location of Side A was counterbalanced across dogs). Once the dog had retrieved the reward, the experimenter secured the dog, transferring the leash to the handler who remained on the opposite side of the barrier. This procedure was followed to ensure the dog did not experience either the handler or the experimenter moving through the gap in the barrier (i.e., detouring around the barrier). A choice was defined as when the dog’s entire body passed over a hypothetical line connecting the starting position to the barrier. A training trial was scored as successful if the dog’s first choice was made to Side A. Three successful trials, not necessarily consecutive, were required to meet training criteria and procedure to the testing phase.
Testing phase
The A-not-B testing trials were identical to training trials, with the exception that the barrier was shifted such that the gap was located at the opposite wall (herein referred to as “Side B”). To ensure that the dog was unable to see the barrier being shifted, prior to the start of the testing phase the handler and the dog waited in the corridor behind the half-wall while the barrier was moved. Dogs continued to experience A-not-B testing trials until Side B was selected as the first choice.
Behavioral measures
Three dependent measures were collected: (1) Training Performance—the total number of training trials required for a dog to successfully choose Side A as its first choice for three trials (not necessarily consecutive), (2) First Testing Trial Performance—the success (or failure) during the first A-not-B testing trial, and (3) A-not-B Barrier Task Score—the number of A-not-B testing trials that a dog required before successfully choosing Side B as its first choice.
Statistical analyses
All behavioral analyses were completed using the R program (version 3.3.2, R Core Team) with the lme4 package (Bates et al. 2015). Significant alpha value was considered ≤ 0.05.
Within-task analyses
We examined the influence of sex, diet, and task order on motoric self-regulation. First, we conducted generalized linear mixed models (GLMs) on the Training Performance for each task. Second, the First Testing Trial Performance for each task was examined using logistic regressions. Third, GLMs were conducted using the Task Score for each specific task (i.e., A-not-B Bucket Task Score, Cylinder Task Score, and A-not-B Barrier Task Score). Finally, we examined A-Trial Performance for the A-not-B Bucket task and Overall Test Performance for the Cylinder task using GLMs. GLMs were fit with a Poisson distribution. Parameter estimations for the GLMs were achieved using a Chi square test comparing models with the factor of interest to a null model. Parameter estimations in the logistic regressions were achieved using a residual maximum likelihood test that compared models with the factor of interest to a null model. Degrees of freedom were estimated using a Satterthwaite approximation. Tukey HSD post hoc analyses were conducted when a factor was found significant. The analyses procedures used here were adopted to allow for comparison to our previous research examining inhibition by pet dogs (Vernouillet et al. 2018).
Between-task analyses
We examined whether the Task Score for each task correlated by performing Spearman’s rank-order correlations.
Comparisons with pet dogs
We compared the Task Scores of sled dogs during this current study to the same dependent measures from pet dogs that completed the same Motoric Self-regulation Tasks, using identical performance criterion, from a previous study (Vernouillet et al. 2018) by performing Mann-Whitney U tests. Unlike sled dogs, pet dogs were not given specific diets and they performed all the tasks within a single 1-h session.
Results
For the motoric self-regulation tasks, all experimental trials were re-analyzed offline by an independent reviewer who was blind to the treatment groups, with high inter-rater reliability (r = 0.95). The number of dogs who completed all trials varied depending on the task (A-not-B Bucket: n = 14, Cylinder: n = 13, and A-not-B Barrier: n = 13). For within-task analyses, only data from dogs that completed all training and testing trials within the given task were used (see Table 1). For between-task analyses, only data were used from the dogs that completed enough trials until they performed a successful testing trial during all three tasks.
Within-task analyses
A-not-B Bucket task
Training phase
Examining Training Performance, the dogs required 4.2 ± 0.4 (M ±SEM) trials to meet criterion. Neither sex (GLM: \(\chi_{(1)}^{2}\) = 0.02, p = 0.896), diet (GLM: \(\chi_{(2)}^{2}\) = 1.80, p = 0.407) nor task order (GLM: \(\chi_{(2)}^{2}\) = 0.04, p = 0.843) influenced Training Performance.
Testing phase
-
a)
A-Trial Performance. Examining A-Trial Performance, the dogs required 4.1 ± 0.5 (M ±SEM) trials to meet criterion to advance to testing. Neither sex (GLM: \(\chi_{(1)}^{2}\) = 1.43, p = 0.232), diet (GLM: \(\chi_{(2)}^{2}\) = 1.79, p = 0.408) nor task order (GLM: \(\chi_{(2)}^{2}\) = 0.02, p = 0.878) influenced A-Trial Performance.
-
b)
First Testing Trial Performance. Six dogs (43%) successfully chose Bucket B during the first A-not-B testing trial, which was not significantly different from chance (chance = 4.6, n = 6, 2, and 6 for Bucket A, middle bucket, and Bucket B, respectively; Goodness of fit: \(\chi_{(2)}^{2}\) = 2.31, p = 0.315).
Neither diet (logistic regression: \(\chi_{(2)}^{2}\) = 0.12, p = 0.944) nor task order (logistic regression: \(\chi_{(2)}^{2}\) = 3.26, p = 0.071) influenced First Testing Trial Performance. However, males were more successful than females, as five out of seven males, but only one out of seven females chose Bucket B as their first choice (Logistic regression: \(\chi_{(1)}^{2}\) = 5.00, p = 0.025).
Out of the eight dogs that were unsuccessful during the first testing trial, six consistently chose Bucket A, and the other two split their choices between Bucket A and the middle bucket (n = 6 and 2 for Bucket A and middle bucket, respectively; Binomial: p = 0.109).
Overall, more dogs made successful choices during the first A-trial compared to the first A-not-B testing trial, but this did not reach significance (79% and 43% for A-trial and A-not-B trial, respectively; z = 1.93, p = 0.054).
-
c)
A-not-B Bucket Task Score. Examining A-not-B Bucket Task Score, the dogs required 2.2 ± 0.4 (M ±SEM) trials before successfully retrieving the reward from Bucket B as their first choice (see Fig. 3 for individual dog performance). Sex (GLM: \(\chi_{(1)}^{2}\) = 0.03, p = 0.858), diet (GLM: \(\chi_{(2)}^{2}\) = 0.51, p = 0.775) and task order (GLM: \(\chi_{(2)}^{2}\) = 3.89, p = 0.143) did not influence the A-not-B Bucket Task Score.
When an unsuccessful choice was made during testing, dogs preferentially chose the previously baited Bucket A as their first choice (M: 82% and 18%, for Bucket A and the middle bucket, respectively; Binomial: p = 0.005).
Cylinder task
Training phase
Examining Training Performance, dogs successfully retrieved the reward from the Opaque Cylinder during 4.6 ± 0.1 (M ±SEM) out of the first five training trials. Thus, all dogs met the criterion to proceed to the testing phase within the minimum number of training trials required, so no further analysis was conducted.
Testing phase
-
a)
First Testing Trial Performance. Seven dogs (54%) successfully detoured around the Transparent Cylinder during the first testing trial, but this result was not significantly different from chance (Binomial: p = 0.209).
Sex (logistic regression: \(\chi_{(1)}^{2}\) = 0.58, p = 0.446), diet (logistic regression: \(\chi_{(2)}^{2}\) = 2.263, p = 0.323) and task order (logistic regression: \(\chi_{(2)}^{2}\) = 0.41, p = 0.525) did not influence the First Testing Trial Performance.
First Testing Trial Performance with the Transparent Cylinder was not significantly different from first trial performance during training with the Opaque Cylinder (first trial performance: 54% and 77% with the Transparent and Opaque Cylinders, respectively; z = 1.24, p = 0.215). However, when we compared performance during the last training trial with the Opaque Cylinder and First Testing Trial Performance, dogs showed a significant decline in performance (performance during last training trial of training: 100%, performance during first testing trial: 50%; z = 2.228, p = 0.026).
-
b)
Cylinder Task Score. Examining Cylinder Task Score, dogs required 1.8 ± 0.3 (M ±SEM) testing trials before successfully detouring through one of the side openings during testing with the transparent cylinder (see Fig. 3 for individual dog performance).
Sex (GLM: \(\chi_{(1)}^{2}\) = 0.618, p = 0.432), diet (GLM: \(\chi_{(2)}^{2}\) = 0.390, p = 0.823) and task order (GLM: \(\chi_{(2)}^{2}\) = 1.708, p = 0.426) did not influence the Cylinder Task Score.
-
c)
Overall Test Performance. Examining the Overall Test Performance, the dogs made successful choices during 3.5 ± 0.34 trials (M ±SEM) out of the five testing trials. Sex (GLM: \(\chi_{(1)}^{2}\) = 0.69, p = 0.406), diet (GLM: \(\chi_{(2)}^{2}\) = 0.01, p = 0.996) and task order (GLM: \(\chi_{(2)}^{2}\) = 0.15, p = 0.927) did not influence the Overall Test Performance.
A-not-B barrier task
Training phase
Examining Training Performance, the dogs required 3.7 ± 0.19 (M ±SEM) trials to meet criteria. Sex (GLM: \(\chi_{(1)}^{2}\) = 0.13, p = 0.719), diet (GLM: \(\chi_{(2)}^{2}\) = 0.15, p = 0.929) and task order (GLM: \(\chi_{(2)}^{2}\) = 0.25, p = 0.620) did not influence Training Performance.
Testing phase
-
a)
First Testing Trial Performance. Eight dogs (53%) successfully detoured around the barrier during the first A-not-B testing trial, which was not significantly different from chance (chance = 7.5, n = 7 and 8 for Side A and B, respectively; Binomial: p = 0.796).
Sex (logistic regression: \(\chi_{(1)}^{2}\) = 0.58, p = 0.446), diet (logistic regression: \(\chi_{(2)}^{2}\) = 0.54, p = 0.764) and task order (logistic regression: \(\chi_{(2)}^{2}\) = 1.65, p = 0.199) did not influence the First Testing Trial Performance.
Overall, fewer dogs made successful choices during the first A-not-B testing trial compared to the first training trial (53% and 87% for first A-not-B testing trial and first training trial, respectively; z = 1.99, p = 0.023).
-
b)
A-not-B Barrier Task Score. Examining A-not-B Barrier Task Score, dogs required 1.6 ± 0.24 (M ±SEM) trials to successfully choose Side B as their first choice (see Fig. 3 for individual dog performance). Sex (GLM: \(\chi_{(1)}^{2}\) = 0.00, p = 0.973), diet (GLM: \(\chi_{(2)}^{2}\) = 0.52, p = 0.769), and task order (GLM: \(\chi_{(2)}^{2}\) = 0.24, p = 0.888) did not influence A-not-B Barrier Task Score.
Correlations between tasks
Task Scores were correlated between the A-not-B Bucket and the A-not-B Barrier tasks (Spearman’s correlation: rho = 0.60, p = 0.040), but not between the A-not-B Bucket and the Cylinder tasks (Spearman’s correlation: rho = − 0.45, p = 0.122) nor between the A-not-B Barrier and the Cylinder tasks (Spearman’s correlation: rho = − 0.10, p = 0.763).
Comparisons with pet dogs
A-not-B Bucket task
During the A-not-B Bucket task, there was no difference in the number of training trials, or the number of A-trials needed for the pet dogs and sled dogs to meet criteria (U = 166.5, p = 0.976, and U = 157, p = 0.912, respectively; see Table 3). First Testing Trial Performance as well as A-not-B Bucket Task Score also did not differ between the two groups of dogs (z = − 0.29, p = 0.772 and U = 155.5, p = 0.872, respectively). However, the response distribution of unsuccessful choices differed between pet dogs and sled dogs. Sled dogs chose the previously baited Bucket A more often than did pet dogs (choice toward Bucket A: 82% and 52% for sled dogs and pet dogs, respectively; z = − 2.128, p = 0.033).
Cylinder task
During the Cylinder task, there was no difference in the number of trials needed to meet training criteria for pet dogs (which received training with the Opaque Cylinder) and sled dogs (U = 86.5, p = 0.631; see Table 3). Furthermore, there were no differences in performance measures during testing between sled dogs and pet dogs that received training with the Opaque Cylinder (First Testing Trial performance: z = − 0.81, p = 0.418, Cylinder Task Score: U = 69.5, p = 0.459, and Overall Test Performance: U = 53, p = 0.112).
A-not-B barrier task
During the A-not-B Barrier task, pet dogs required significantly fewer training trials compared to sled dogs to meet criteria (U = 130, p = 0.032; see Table 3). However, sled dogs tended to have better First Trial Performance compared to pet dogs, but this failed to reach significance (z = 1.78, p = 0.075). A-not-B Barrier Task Score did not significantly differ (U = 149.5, p = 0.368).
Discussion
This study was the first to examine motoric self-regulation in sled dogs, using three well-established tasks, and to evaluate potential dietary effects on performance during these tasks. Additionally, potential correlations in performance of the sled dogs during the motoric self-regulation tasks with those collected from pet dogs (published previously from our laboratory, Vernouillet et al. 2018) were investigated. Overall, and similar to several previous studies, little correlational support from the sled dogs was found which suggests that these well-established tasks are measuring different constructs of motoric self-regulation. The results showed few differences between performance measures of sled dogs and pet dogs. However, one robust difference was that sled dogs showed a strong A-not-B error during the A-not-B Bucket task, which was not noted with pet dogs. Also, others have reported that pet dogs do not show an A-not-B error (Bray et al. 2014). Finally, the results did not support that the dietary manipulations influenced motoric self-regulation performance for the sled dogs.
Task-specific results
A-not-B Bucket task
The A-not-B task has been developed to examine whether animals attend to the spatial displacement of a rewarded item. Typically, the subject learns that a desirable item is placed at one location (Bucket A). Once this initial response is learned, the individual is shown on a subsequent trial that the item is first placed in the initial location, but subsequently moved to another (Bucket B). To successfully retrieve the item, the subject must inhibit searching in Bucket A and instead directly search in Bucket B. The sled dogs from the present study quickly learned the task requirements, but when presented with the first displacement testing trial, their first choice was not significantly better than chance. Furthermore, although the sled dogs needed few testing trials to learn to search first in Bucket B, when an error was committed, it was more often the A-not-B error.
During this study, testing procedures were replicated from a previous study with pet dogs completed by the same researchers. Specifically, dogs were required to complete multiple test trials until a correct choice was made to Bucket B. This differs from previous studies (Amici et al. 2008; MacLean et al. 2014; Bray et al 2014) which only presented the subjects with a single test trial. Administering multiple test trials allowed for the examination of not only the dogs’ initial response to the displacement procedure, but also allowed for assessment of choice perseverance with additional testing experience. Interesting group differences were discovered by examining which bucket the sled dogs and pet dogs were more likely to choose during an unsuccessful testing trial. When making an incorrect choice, the sled dogs showed A-not-B errors—they initially chose Bucket A. This contrasts with the pet dogs in our previous study, as well as the study by Bray et al. (2014), which reported that overall pet dogs tend not to commit the A-not-B error. Thus, pet dogs must notice the displacement procedure [as they are very accurate with the displacement (Bray et al. 2014), or they search both Bucket A and the middle bucket after a displacement (Vernouillet et al. 2018)], whereas the sled dogs either did not notice the displacement, or they were unable to self-regulate their proponent response (they showed preservative behavior). The standard procedures of the A-not-B Bucket task, as well as the modified version, do not allow for differentiation between these two interpretations. However, both potential differences are worthy of future research to differentiate whether sled dogs are less attentive to displacements or are less able to show motoric self-regulation during this task.
Few additional differences were observed between sled dogs and pet dogs. Male sled dogs were more successful than females during the first testing trial. This difference is compelling and worthy of future study, but should be interpreted with caution given the relatively small sample size contributing to these particular effects, which typically require much larger sample sizes and there was no prior reason to expect males to perform better than females. [Note: Five out of seven males and one out of seven females chose Bucket B as their first choice.]
Cylinder task
The Cylinder task evaluates whether subjects are able to inhibit reaching directly for a reward situated within a cylindrical tube, and encountering a barrier, by detouring to one of two side openings. The Cylinder task has been conducted either by immediately providing subjects with a Transparent Cylinder, or providing initial training experience with an Opaque Cylinder. The present study used this latter procedure.
The sled dogs in this study quickly learned to detour to one of the side openings when learning with an Opaque Cylinder, and this was similar to the previously reported results from pet dogs (group Opaque-Transparent, Vernouillet et al. 2018). Overall, and similar to the pet dogs, the sled dogs appeared to show detour behavior. However, when examining the sled dogs’ detour performance at a finer scale, it was noted that approximately half of the sled dogs failed to make the correct detour response during their first trial with the Transparent Cylinder—and this performance was significantly lower than the previous trial with the Opaque Cylinder (i.e., the last training trial). These results are similar to those reported with pet dogs. Together these results show that when trained with an Opaque Cylinder, only examining overall performance may suggest quick learning and successful transfer of detouring behavior when subsequently experiencing a Transparent Cylinder. However, this conclusion would not accurately represent the learning process dogs seem to show with this task. Rather it seems that when learning with an Opaque Cylinder, sled dogs and pet dogs alike may learn the process in two steps. First, they learn the required task response—the detour response. Subsequently, they learn the self-regulatory behavior—to inhibit directly reaching for the visible reward. The learning process is likely different than when trained initially with a Transparent Cylinder, in which case dogs need to learn both components simultaneously and may require more trials to learn the task (Vernouillet et al. 2018). Overall, sled dogs and pet dogs showed no differences in their detour behavior during the Cylinder task, when receiving initial experience with an Opaque Cylinder.
A-not-B Barrier task
During the A-not-B Barrier task, a subject learns to take a consistent path around a barrier to locate a hidden reward. Once accurate path learning has been established, the original path is blocked, requiring the subject to inhibit taking the learned route, and instead detour using a novel path.
The sled dogs quickly learned the initial training requirements for the task, and although they learned to successfully detour when the original path was blocked, they did not show immediate detour behavior. Although, the sled dogs required more training trials to learn the task compared to the pet dogs, during testing the performance of the two groups was quite similar. Together these results support that generally, dogs may not be able to immediately (at first experience) inhibit their initial response, but a small amount of experience allows dogs to overcome this spatial perseveration. It is interesting that no differences were detected between sled dogs and pet dogs during the testing phase of this task, whereas the sled dogs showed stronger perseveration compared to pet dogs during the A-not-B Bucket task. Additional research with sled dogs will be necessary to understand the performance differences between these two tasks. However, one likely influence is the saliency differences between the two tasks; the large continuous surface blocking a once accessible path during the A-not-B Barrier task may have caused the sled dogs to notice the displacement or provided a robust visual stimulus that allowed the sled dogs to break from perseveration.
Comparison between tasks
Previous studies have reported a lack of correlation among tasks purporting to examine motoric self-regulation by dogs. Although the current study found a significant correlation between task score performance during the A-not-B Bucket task and the A-not-B Barrier task, no other significant correlations were found. The accumulation of studies showing a lack of correlation across tasks of motoric self-regulation is concerning, and an area in need of future investigation. In doing so, several task-related features need to be considered to determine the source(s) of these performance differences, including task demands (Bray et al. 2014), experience with transparency (Fagnani et al. 2016; Stow et al. 2018), type of cueing (Sümegi et al. 2014), ceiling effects due to high performance (Vernouillet et al. 2018), or perceptual differences (Brucks et al. 2017; Kabadayi et al. 2018).
Carbohydrate sources and motoric self-regulation
The type and/or quality of dietary carbohydrates is known to affect cognition in humans (e.g., healthy adults: Philippou and Constantinou 2014, children and adults with Type 1 diabetes: Ryan et al. 2016). However, less is understood about how carbohydrates (and hyperglycemia) influence canine health and cognition. Thus, the aim of this study was to determine if motoric self-regulation would be acutely affected by feeding a low or high GI diet. The present study used two commercial diets that had high and low GI values to examine the effect of self-regulation in dogs: a Traditional Grain Diet that included corn and wheat as the main carbohydrate sources (high GI diet; 83 ± 17) and a Grain-Free Diet that included pulses (peas, lentils, chickpeas; low GI diet; 41 ± 6) as the primary carbohydrate sources. Moreover, a high glycemic control (purified glucose solution) was included. Still, an effect of dietary GI levels on performance during the three tasks of motoric self-regulation was not detected.
All dogs were housed in kennels at the same facility, received the same background diet and were deemed healthy at the start of the study. Still, although all test diets were fed in quantities that provide equal amounts of available carbohydrates, previous research that measured the GI of the test diets observed an apparent variability in blood glucose levels among dogs in response to a meal (Rankovic 2018), which might be a reason for the absence of a dietary effect on cognitive task performance. Also, this study focused on the effect of dietary GI levels during the early postprandial phase. As the brain, and hence its influence on cognitive performance, is sensitive to glucose, the focus was on potential early effects of dietary manipulations. Research in humans has shown that changes in cognitive function may also be seen during the late postprandial phase (Benton et al. 2003; Lamport et al 2011). Therefore, future studies are needed to determine if diets with different GI values have an effect on learning and/or performance during motoric self-regulation tasks by dogs at a later time point than examined here, or if these diets are fed for a longer-term. Furthermore, how high the blood glucose levels rise after a meal containing carbohydrates and how long it stays high depend not only on the quality of the carbohydrates (GI), which was investigated in the present study, but also on the quantity. Therefore, the effect of glycemic load, the product of GI and the total amount of carbohydrate in a food on cognitive function also require future investigation. Few research studies have addressed the potential influence of diet on cognitive functioning in dogs. Yet, such knowledge may have substantial impact, such as enhancing the human/dog relationship with pets (perhaps reducing the number of dogs surrendered due to trainability issues), canine performance for competitive sports, and trainability and sustainability of focus for working dogs, as just a few examples.
As the current study is the first to investigate whether GI levels influence motoric self-regulation in dogs, a few limitations have to be acknowledged. The blood glucose levels of the sled dogs were not determined at the start of the behavioral testing, so as not to potentially interfere with the cognitive tasks (e.g., blood draw causing an increase in the dog’s stress-levels that may negatively affect behavioral performance). Although the glycemic index of the diets was determined (Rankovic 2018), due to the apparent variability of postprandial blood glucose in dogs, an association between blood glucose levels and performance on the cognitive tasks could not be determined. It is possible that an effect of diet was not observed because at the time of behavioral testing there may not have been significant differences in blood glucose levels, although the study focused on the early postprandial phase when differences in blood glucose levels are more likely. In addition, testing was performed on the sled dogs during the summer months when physical training was minimal. Although the sled dogs had ideal body condition scores (4–5 on a 9-point scale) and none were considered to be overweight, it is possible that an effect of diet may be observed if the dogs were in a more physically active state due to enhanced insulin sensitivity and blood glucose control (Schnurr et al. 2014; Davis et al. 2018). To gain an understanding of whether blood glucose levels affect cognitive function in dogs, future studies could use non-invasive, real-time interstitial glucose monitoring as a possible technique to determine actual physiological glucose levels when tasks are being performed (Wiedmeyer et al. 2003).
In conclusion, the present study provides the first insights into motoric self-regulation in sled dogs and evaluated potential dietary effects of performance during these tasks. When comparing the performance of sled dogs to data previously collected with pet dogs, strong similarities were observed between the two groups of dogs, with the noted interesting exception that sled dogs showed a robust spatial perseveration during the A-not-B Bucket test; a result that warrants further investigation. The present study showed that GI levels may not influence dogs’ motoric self-regulation in the early postprandial phase. Future research is needed to evaluate whether GI levels influence self-regulation during the late postprandial phase, or whether the quantity of carbohydrates (glycemic load) or long-term dietary levels are important. Additional research is needed to evaluate whether dietary GI levels impact other cognitive functions, and for other groups of dogs.
References
Adolphe JL, Drew MD, Huang Q, Silver TI, Weber LP (2012) Postprandial impairment of flow-mediated dilation and elevated methylglyoxal after simple but not complex carbohydrate consumption in dogs. Nutr Res 32(4):278–284. https://doi.org/10.1016/j.nutres.2012.03.002
Adolphe J, Silver T, Childs H, Drew M, Weber L (2014) Short-term obesity results in detrimental metabolic and cardiovascular changes that may not be reversed with weight loss in an obese dog model. Brit J Nutr 112(4):647–656. https://doi.org/10.1017/S0007114514001214
Amici F, Aureli F, Call J (2008) Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol 18(18):1415–1419. https://doi.org/10.1016/j.cub.2008.08.020
Atkinson FS, Foster-Powell K, Brand-Miller JC (2008) International tables of glycemic index and glycemic load values: 2008. Diabetes Care 31(12):2281–2283. https://doi.org/10.2337/dc08-1239
Axelsson E, Ratnakumar A, Arendt ML, Maqbool K, Webster MT, Perloski M et al (2013) The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495(7441):360–364. https://doi.org/10.1038/nature11837
Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC (2008) Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 87(3):627–637. https://doi.org/10.1093/ajcn/87.3.627
Barrera G, Alterisio A, Scandurra A, Bentosela M, D’Aniello B (2018) Training improves inhibitory control in water rescue dogs. Anim Cogn 22(1):127–131. https://doi.org/10.1007/s10071-018-1224-9
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Benton D, Ruffin M, Lassel T, Nabb S, Messaoudi M, Vinoy S et al (2003) The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharm 166(1):86–90. https://doi.org/10.1007/s00213-002-1334-5
Beran MJ (2015) The comparative science of “self-control”: what are we talking about? Front Psychol 6:51. https://doi.org/10.3389/fpsyg.2015.00051
Beurms S, Miller HC (2016) Sharing more than the sofa: what dogs can teach us about human self-control. Curr Dir Psychol Sci 25(5):351–356. https://doi.org/10.1177/0963721416664392
Bray E, MacLean E, Hare B (2014) Context specificity of inhibitory control in dogs. Anim Cogn 17(1):15–31. https://doi.org/10.1007/s10071-013-0633-z
Bray E, MacLean E, Hare B (2015) Increasing arousal enhances inhibitory control in calm but not excitable dogs. Anim Cogn 18(6):1317–1329. https://doi.org/10.1007/s10071-015-0901-1
Brucks D, Marshall-Pescini S, Wallis L, Huber L, Range F (2017) Measures of dogs’ inhibitory control abilities do not correlate across tasks. Front Psychol 8:849. https://doi.org/10.3389/fpsyg.2017.00849
Carciofi AC, Takakura FS, de Oliveira LD et al (2008) Effects of six carbohydrate sources on dog diet digestibility and post-prandial glucose and insulin response. J Anim Physiol Anim Nutr 92(3):326–336. https://doi.org/10.1111/j.1439-0396.2007.00794.x
Davis MS, Geor RJ, Williamson KK (2018) Effect of endurance conditioning on nsulin-mediated glucose clearance in dogs. Med Sci Sports Exerc 50:2494–2499
Diamond A (1990) Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann N Y Acad Sci 608:637–669
Fagnani J, Barrera G, Carballo F, Bentosela M (2016) Is previous experience important for inhibitory control? A comparison between shelter and pet dogs in A-not-B and cylinder tasks. Anim Cogn 19(6):1165–1172. https://doi.org/10.1007/s10071-016-1024-z
Flint R, Turek C (2003) Glucose effects on a continuous performance test of attention in adults. Behav Brain Res 142(1–2):217–228. https://doi.org/10.1016/S0166-4328(03)00002-0
Glady Y, Genty E, Roeder J (2012) Brown Lemus (Eulemus fulvus) can master the qualitative version of the reverse-reward contingency. PLoS One 7(10):1–7. https://doi.org/10.1371/jounal.pone.0048378
Graham PA, Maskell IE, Nash AS (1994) Canned high fiber diet and postprandial glycemia in dogs with naturally occurring diabetes mellitus. J Nutr 124(12 Suppl):2712S–2715S. https://doi.org/10.1093/jn/124.suppl_12.2712S
Hall JA, Melendez LD, Jewell DE, Kaltenboeck B (2013) Using gross energy improves metabolizable energy predictive equations for pet foods whereas undigested protein and fiber content predict stool quality. PLoS ONE 8(1):e54405
Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM et al (1981) Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 34(3):362–366. https://doi.org/10.1093/ajcn/34.3.362
Kabadayi C, Taylor LA, von Bayern AM, Osvath M (2016) Ravens, New Caledonian crows and jackdaws parallel great apes in motor self-regulation despite smaller brains. R Soc Open Sci 3(4):160104. https://doi.org/10.1098/rsos.160104
Kabadayi C, Bobrowicz K, Osvath M (2018) The detour paradigm in animal cognition. Anim Cogn 21(1):21–35. https://doi.org/10.1007/s10071-017-1152-0
Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T et al (1999) Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 34(1):146–154
Laflamme D (1997) Development and validation of a body score system for dogs. Canine Pract 22(4):10–15
Lamport DJ, Lawton CL, Mansfield MW, Dye L (2009) Impairments in glucose tolerance can have a negative impact on cognitive function: a systematic research review. Neurosci Biobehav Rev 33(3):394–413. https://doi.org/10.1016/j.neubiorev.2008.10.008
Lamport DJ, Hoyle E, Lawton CL, Mansfield MW, Dye L (2011) Evidence for a second meal cognitive effect: glycaemic responses to high and low glycaemic index evening meals are associated with cognition the following morning. Nutr Neurosci 14(2):66–71. https://doi.org/10.1179/1476830511Y.0000000002
Lucon-Xiccato T, Gatto E, Bisazza A (2017) Fish perform like mammals and birds in inhibitory motor control tasks. Sci Rep 7(1):13144. https://doi.org/10.1038/s41598-017-13447-4
MacLean EL, Hare B, Nunn CL, Addessi E, Amici F, Anderson RC et al (2014) The evolution of self-control. Proc Natl Acad Sci USA 111(20):E2140–E2148. https://doi.org/10.1073/pnas.1323533111
Marshall-Pescini S, Viranyi Z, Range F (2015) The effect of domestication on inhibitory control: wolves and dogs compared. PLoS One 10(2):e0118469. https://doi.org/10.1371/journal.pone.0118469
McCance RA, Lawrence RD (1929) The carbohydrate content of foods. Lancet 213(5520):1264–1265
McNay EC, Fries TM, Gold PE (2000) Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci USA 97(6):2881–2885. https://doi.org/10.1073/pnas.050583697
Miller HC, Pattison KF, DeWall CN, Rayburn-Reeves R, Zentall TR (2010) Self-control without a “self”? Common self-control process in humans and dogs. Psych Sci 21(4):534–538. https://doi.org/10.1177/0956797610364968
Miller HC, Pattison KF, Laude JR, Zentall TR (2015) Self-regulatory depletion in dogs: insulin release is not necessary for the replenishment of persistence. Behav Processes, 110:22–26. https://www.ncbi.nlm.nih.gov/pubmed/25264236. https://doi.org/10.1016/j.beproc.2014.09.030
Node K, Inoue T (2009) Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc Diabetol 8:23. https://doi.org/10.1186/1475-2840-8-23
Parrish AE, Emerson ID, Rossettie MS, Beran MJ (2016) Testing the glucose hypothesis among capuchin monkeys: Does glucose boost self-control? Behav Sci (Basel) 6(3):16. https://doi.org/10.3390/bs6030016
Philippou E, Constantinou M (2014) The influence of glycemic index on cognitive functioning: a systematic review of the evidence. Adv Nutr 5(2):119–130. https://doi.org/10.3945/an.113.004960
Piotti P, Satchell L, Lockhart T (2018) Impulsivity and behaviour problems in dogs: a reinforcement sensitivity theory perspective. Behav Processes 151:104–110. https://doi.org/10.1016/j.beproc.2018.03.012
Rankovic A (2018) The acute effects of starch sources on glycemic index, glycemic response, insulinemic response and satiety-related hormones in dogs. Master’s thesis dissertation, University of Guelph, Guelph, Canada
Riby LM, Lai Teik Ong D, Azmie NBM, Ooi EL, Regina C, Yeo EKW et al (2017) Impulsiveness, postprandial blood glucose, and glucoregulation affect measures of behavioral flexibility. Nutr Res 48:65–75. https://doi.org/10.1016/j.nutres.2017.10.011
Ryan CM, van Duinkerken E, Rosano C (2016) Neurocognitive consequences of diabetes. Am Psychol 71(7):563–576. https://doi.org/10.1037/a0040455
Salman MD, Hutchison J, Ruch-Gallie R, Kogan L, New JC, Kaas PH, Scarlett JM (2000) Behavioral reasons for relinquishment of dogs and cats to 12 shelters. J Appl Anim Welf Sci 3(2):93–106. https://doi.org/10.1207/S15327604JAWS0302_2
Santos L, Ericson B, Hauser M (1999) Constraints on problem solving and inhibition: object retrieval in cotton-top tamarins (Saguinus oedipus oedipus). J Comp Psychol 113(2):186–193. https://doi.org/10.1037/0735-7036.113.2.186
Schnurr TM, Reynolds AJ, Gustafson SJ, Duffy LK, Dunlap KL (2014) Conditioning causes an increase in glucose transporter-4 levels in mononuclear cells in sled dogs. Int J Biochem Cell Biol 55:227–231
Smith MA, Riby LM, Eekelen JA, Foster JK (2011) Glucose enhancement of human memory: a comprehensive research review of the glucose memory facilitation effect. Neurosci Biobehav Rev 35(3):770–783. https://doi.org/10.1016/j.neubiorev.2010.09.008
Stow MK, Vernouillet A, Kelly DM (2018) Neophobia does not account for motoric self-regulation performance as measured during the detour-reaching cylinder task. Anim Cogn 21(4):565–574. https://doi.org/10.1007/s10071-018-1189-8
Sümegi Z, Kis A, Miklosi A, Topal J (2014) Why do adult dogs (Canis familiaris) commit the A-not-B search error? J Comp Psychol 128(1):21–30. https://doi.org/10.1037/a0033084
Sunvold GD, Bouchard GB (1998) The glycemic response to dietary starch. In: Reinhart GA, Carey DP (eds) Recent advances in canine and feline nutrition: iams nutrition symposium proceedings, vol II. Orange Frazer Press, Wilmington
Thomas DE, Elliott EJ, Baur L (2007) Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd005105.pub2
Vernouillet A, Anderson J, Clary D, Kelly DM (2016) Inhibition in Clark’s nutcrackers (Nucifraga columbiana): results of a detour-reaching test. Anim Cogn 19(3):661–665. https://doi.org/10.1007/s10071-016-0952-y
Vernouillet A, Stiles LR, Andrew McCausland J, Kelly DM (2018) Individual performance across motoric self-regulation tasks are not correlated for pet dogs. Learn Behav 46(4):522–536. https://doi.org/10.3758/s13420-018-0354-x
Wascher TC, Schmoelzer I, Wiegratz A, Stuehlinger M, Mueller-Wieland D, Kotzka J, Enderle M (2005) Reduction of postchallenge hyperglycaemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance. Eur J Clin Invest 35(9):551–557. https://doi.org/10.1111/j.1365-2362.2005.01550.x
Wiedmeyer CE, Johnson PJ, Cohn LA, Meadows RL (2003) Evaluation of a continuous glucose monitoring system for use in dogs, cats, and horses. J Am Vet Med Assoc 223(7):987–992
Wolever TMS, Jenkins DJA, Jenkins AL, Josse RG (1991) The glycemic index: methodology and clinical implications. Am J Clin Nutr 54(5):846–854
Acknowledgments
We would very much like to thank the owner’s of the sled dogs for lending us the facilities and access to their sled dogs to perform our experiments. We would like to thank Laura Stiles for assistance with data scoring. AdV, DMK, JA designed the study; DMK, AlV and JAM conducted the behavioral experiments; AdV, JA, AR designed the nutritional components, AlV and DMK analyzed the data, and all authors contributed to writing and editing the manuscript.
Funding
This study was funded by a Natural Science & Engineering Research Council Collaborative Research Development grant (#CRDPJ488705–15) in partnership with Petcurean Pet Nutrition to AdV, DMK and JA. JA is an employee with Petcurean Pet Nutrition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors AdV, DMK and JA received funding by a Natural Science & Engineering Research Council Collaborative Research Development grant (#CRDPJ488705–15) in partnership with Petcurean Pet Nutrition to JA, who is an employee with Petcurean Pet Nutrition. Authors AdV, DMK and JA declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kelly, D.M., Adolphe, J.L., Vernouillet, A. et al. Motoric self-regulation by sled dogs and pet dogs and the acute effect of carbohydrate source in sled dogs. Anim Cogn 22, 931–946 (2019). https://doi.org/10.1007/s10071-019-01285-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-019-01285-y