Abstract
Place learning is thought to be an adaptive and flexible facet of navigation. Due to the flexibility of this learning, it is thought to be more complex than the simpler strategies such as learning a particular route or navigating through the use of cues. Place learning is crucial in a familiar environment as it allows an individual to successfully navigate to the same endpoint, regardless of where in the environment the journey begins. Much of the research to date focusing on different strategies employed for navigation has used human subjects or other mammals such as rodents. In this series of experiments, the spatial memory of four different species of fish (goldfish, killifish, zebrafish and Siamese fighting fish) was analysed using a plus maze set-up. Results suggest that three of the species showed a significant preference for the adoption of a place strategy during this task, whereas zebrafish showed no significant preference. Furthermore, zebrafish took significantly longer to learn the task than the other species. Finally, results suggest that zebrafish took the least amount of time (seconds) to complete trials both during training and probe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the animal kingdom, spatial memory is important for many reasons including avoiding predation, prey detection and finding a mate (Wolbers and Hegarty 2010; White and Brown 2014). There are two categories associated with spatial memory and navigation (van Gerven et al. 2012). The first are called “egocentric processes” and involve encoding features of the environment in relation to where the individual is currently located (Shelton and McNamara 2001). These processes involve learning to navigate to a location using landmarks in the environment as beacons or by learning a series of turning responses (O’Keefe and Nadel 1978; van Gerven et al. 2012). Egocentric navigation is relatively simple and therefore allows spatial memories to be formed easily (van Gerven et al. 2012). Such methods do, however, lack flexibility, particularly in response to changes within the environment, meaning the individual will be less likely to reach their desired location from a novel start point (Rodriguez et al. 1994).
O’Keefe and Nadel argued that animals are also able to navigate using another set of processes which, although more cognitively demanding, are able to adapt more readily to changes within the environment (O’Keefe and Nadel 1978; Rodriguez et al. 1994). These are called “allocentric processes”. Cognitive mapping, first proposed by Tolman, is the best known example of an allocentric process and is described as a complex mental representation of a familiar environment that can be used to influence spatial behaviour and decision-making (Tolman 1948; O’Keefe and Nadel 1978). One of the key features of such an allocentric process is that an individual is able to navigate based on the spatial relationships between multiple landmarks, sensory features and possible routes within a particular environment (Iaria et al. 2009). As the individual moves though the environment, its cognitive map is continuously updated, making for more robust spatial memory that is also more relevant and current (Cheeseman et al. 2014). This means that changes within the environment, such as removal of specific landmarks or attempting to reach the same goal location from a novel start point, will not significantly affect the individual’s spatial memory (Wolbers and Hegarty 2010).
Many experiments have been conducted using maze tests to analyse spatial memory in a variety of species, with mammals and birds being the most popular animals to study (Tolman et al. 1946; O’Keefe and Nadel 1978; Clayton and Krebs 1995; Shettleworth and Westwood 2002; Hamilton et al. 2009). Both taxa possess a hippocampal structure in the brain which is thought to be a key area associated with complex spatial memory abilities (see Pravosudov and Roth 2013). Some studies have also shown that individuals who navigate through complex environments on a regular basis, or perform complex spatial memory tasks, are likely to have an enlarged hippocampus (Clayton and Krebs 1995; Maguire et al. 2000). However, the lack of a hippocampal structure in other animal classes does not necessarily mean that such species are incapable of flexible navigation.
Fish are the most numerous of all vertebrate species (Casebolt et al. 1998), and many live in complex environments, some of which are subject to instability and therefore require the animals to have powerful spatial memory capabilities (Brown 2014). Rodriguez and colleagues showed that, like mammals and birds, goldfish are capable of navigating using both egocentric and allocentric processes during maze tests (Rodriguez et al. 1994). The same authors have also been able to manipulate navigational strategy by ablating brain regions of fish, directly suggesting that there are specific areas of the fish brain responsible for different navigational processes (Salas et al. 1996; López et al. 2000; Broglio et al. 2010). Despite these suggestions, a recent search on Web of Knowledge (January 2015) using “spatial memory” as the search criterion reported 54,109 articles with only 216 citing fish as the study species. Furthermore, many of these articles focus on the large-scale navigation abilities (i.e. migration) of particular species rather than on which strategies fish use to solve spatial tasks (Dittman and Quinn 1996; Saito and Watanabe 2005). Finally, there have been limited studies comparing the similarities and differences in spatial memory across species of fish with many experiments focusing on only one study species (e.g. Braithwaite and De Perera 2006; Shapiro and Jensen 2009; Lamb et al. 2012). Such research would highlight that complex spatial memory is an attribute of fish as a taxonomic group rather than just of specific species.
This study used four different species of fish to assess the length of time each took to learn a spatial task. It also analysed which strategy each species preferred (egocentric or allocentric) for spatial memory. The four species were goldfish, Siamese fighting fish, zebrafish and a type of killifish. Each of these species is teleost (from the class Actinopterygii) with three from the order Cypriniformes and Siamese fighting fish from the order Perciformes (www.fishbase.org). It is important that they are all from the same class, as the teleost brain is thought to be very similar across fish species and is comparable on some levels to the mammalian brain (Tropepe and Sive 2003). As mentioned previously, the hippocampus is thought to be crucial for allocentric spatial memory performance in mammals, the area associated with similar spatial memory abilities in teleost fish has been identified as the lateral pallium area of the telencephalon, and impairment to this area results in a marked reduction in allocentric spatial learning in these animals (see Broglio et al. 2003 for a review).
All but one of these species (killifish) are thought of as domesticated fish (Gordon and Axelrod 1968; Andrews 2002; Spence et al. 2011) and popular laboratory species in the studies of a variety of both physiological and cognitive experiments (Roitblat et al. 1982; Kishi 2004; Portavella and Vargas 2005). The final species was chosen as it is gaining popularity within similar fields (Herrera and Jagadeeswaran 2004). Goldfish are the most domesticated of all, and therefore, there is limited available information about their natural habitat. However, as descendants of carp, it is assumed they tend to occupy ponds and other such bodies of water (Andrews 2002). More is known about the natural habitats of the other three species, each of which also prefer still or slow-moving pools of water (Gordon and Axelrod 1968; Genade et al. 2005; Spence et al. 2011). This is important in a comparison, as a previous study has indicated that even within the same species, fish from different environments (fast flowing vs. still) may use different strategies for navigation (Odling-Smee and Braithwaite 2003). Finally, the species of fish range in sociality with zebrafish having an innate tendency to shoal (Spence et al. 2011) through to the killifish and Siamese fighting fish where it is recommended that the males are housed separately.
Materials and methods
Subjects
Twenty fish from the following four species were used, giving a total of 80 individuals; Carassius auratus auratus (common goldfish), Nothobranchius guentheri (killifish), Betta speldens (Siamese fighting fish) and Danio rerio (zebrafish). The mean length of each species (with standard deviation) was 7.63 ± 2.47, 3.58 ± 1.08, 8.63 ± 1.12 and 4.14 ± 0.88 cm, respectively. All Siamese fighting fish were male, six of the killifish were male and fourteen female, and sex of goldfish and zebrafish was unknown. All animals were commercially sourced (goldfish, Siamese fighting fish and zebrafish from Exotic Aquatics, Belfast, N. Ireland; killifish from Maidenhead Aquatics, Newtownabbey, N. Ireland). All animals were experimentally naïve.
Housing conditions
All apparatus was commercially sourced (Maidenhead Aquatics, Newtownabbey, N. Ireland and Exotic Aquatics, Belfast, N. Ireland). Goldfish were kept in 80 cm × 30 cm × 40 cm tanks with a stocking density of five in each. Temperatures were maintained at an average of 20 °C. During experimentation, killifish, Siamese fighting fish and zebrafish were housed individually (for identification purposes) at an average temperature of 25 °C. Individual housing jars were made of transparent glass and were kept beside each other allowing fish to have visual access to conspecifics. When not completing experiments, all fish were fed commercial flaked food. pH and waste levels in all tanks were monitored regularly using API Freshwater Master Test Kit and water changes were carried out on a regular basis. Waste levels were kept within safe ranges (0 ppm ammonia and nitrite; <40 ppm nitrate). pH range for goldfish was maintained at a range of 7.6 ± 0.2; 7.7 ± 0.3 for killifish and zebrafish; and 8.1 ± 0.1 for Siamese fighting fish. All fish were maintained in a 13:11-h light–dark cycle at all times during the laboratory.
Experimental design
Apparatus
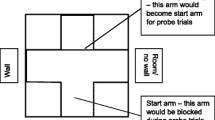
A square tank measuring 63 cm × 63 cm × 43 cm was used for all trials. Water temperatures were similar to housing conditions for each species, and waste levels were kept within the same safe limits. pH was maintained at a range of 7.8 ± 0.4 (depending on species). A plus maze (similar to that used in Odling-Smee and Braithwaite 2003) was made using pieces of white opaque Perspex, 21 cm in length with each arm attached to the inner walls of the tank (see Fig. 1).
Experimental design
Fish were placed into the arm of the maze designated as the “start arm” (see Fig. 1). Both the start arm and the arm opposite were blocked with removable pieces of Perspex. Trials would begin by unblocking the start arm to allow fish access to the remaining two arms of the maze (a T would be formed by keeping the opposite arm blocked; see Fig. 1). All trials (training and probe) were timed using a standard stopwatch and recorded using a Sony HDR-CX190E handycam video camera. The position of the camera and that of the experimenter were varied during experiments to help eliminate any unwanted cues. Similarly, the source of illumination was not in the vicinity of the experimental tank.
Training trials
Fish were randomly assigned to receive a bloodworm reward in the arm either to the left or to the right of the start arm (n = 10 for each side within each species). A trial was considered complete when the tail fin of the fish had passed fully into either arm of the maze. If the fish swam to its assigned rewarded arm, it would receive bloodworm immediately and be moved back to the start arm for the next trial. If the fish swam to the unrewarded arm, exit from that arm would be blocked and the fish would receive a two-min “timeout” (no reward given) before being moved back to the start arm for the next trial. Bloodworm was the recommended food reward for each of the four species by the commercial suppliers and has been used for feeding and reward in a variety of experiments involving fish (e.g. Miklósi and Andrew 1999; Saito and Watanabe 2005; Thomas et al. 2008; Pike et al. 2010). Each fish would receive 10 trials per day (one block). Training criterion was a minimum of 8 out of 10 trials correct for three consecutive blocks. For each incorrect block (7 or fewer trials correct), the training was reset to block one. The water, along with any substrate, in the tank was disturbed between each trial and the tank filtered for a minimum of 20 min between each fish to help reduce the risk of olfactory cues. Potential cues within the maze were controlled where possible, e.g. by mirroring the layout so that the external filter tube and the heater (both turned off during experiments) were at opposing corners of the tank and a tube was placed at the opposite wall of the tank from the output tube of the filter. Outside the maze, there was a wall at the end of the left-hand arm, while there was no wall at the end of the right arm. Potential global cues (i.e. those external to the maze) were not controlled in order to allow fish to avail of cues external to the tank. Such cues consisted of features on the surrounding walls (such as paper record sheets) and housing tanks.
Probe trial
When a fish had reached training criteria, it was immediately transferred to the previously blocked arm, which would now become the start arm; the training start arm would now be blocked to form a “T”. The probe trial would begin and end in the same manner as in training trials. During the probe trial, all fish were rewarded regardless of choice of arm. If a fish swam to the same location as before, a “place strategy” was recorded; if they chose to swim using the same turning-direction as they were trained, a “response strategy” was recorded. Following the probe trial, each fish was returned to its housing tank and experimentation for that individual animal was complete.
Statistical analyses
Data were analysed with the SPSS statistical package (20.0 version) and Microsoft Excel 2010. Generalized linear models were used to assess the effect of species on number of blocks to reach training criteria (Poisson log-linear model) and to assess the effect of species on time taken to complete the training and probe trials (linear scale response). Pairwise comparisons (Fisher’s LSD method) were performed to analyse differences between species following each generalised linear model. Paired-sample t tests were used to assess the difference in time taken to complete the first day versus last day of training within each species. Individual binomial tests and Bayesian inferences were carried out to assess whether the number of fish within each species adopting a place versus response strategy differed from chance.
Ethical note
No invasive procedures were performed, and no animals were harmed. Fish numbers were the minimum required for sufficient data collection and analyses. Strict procedures were followed in accordance with the “Guidelines for the treatment of animals in behavioural research and teaching” (2012). Complete water changes were avoided as these can be harmful and stressful to animals (see above for more information regarding water changes). Furthermore, fish were always transferred in water via containers. Laboratory conditions were inspected by the Veterinary Services Division of the DHSSPS, Northern Ireland, who deemed no licence was required for this series of experiments.
Results
Acquisition time
Acquisition time was based on the number of blocks it took each fish to reach training criteria.
There was a significant main effect of species on task acquisition time: Wald χ 2 (df = 3) = 25.59; P < 0.001. Further analyses suggest that zebrafish required significantly more blocks to learn the task than the other three species (goldfish, P = 0.003; killifish, P = 0.02; Siamese fighting fish P < 0.001) and that Siamese fighting fish also took significantly fewer blocks than both goldfish (P = 0.05) and killifish (P = 0.013). There was no significant difference between goldfish and killifish (P = 0.57) (see Fig. 2).
Boxplot showing task acquisition time for each species. Rectangular boxes display 25th and 75th quartiles and the median. Whiskers display 90th percentile of the data with outliers outside this range marked with an X on the plot. The dotted line displays the minimum number of blocks to reach training criteria within each species (3 blocks in all species). On the graphical display, asterisks are used to show significant differences between species [a single asterisk (*) indicates P ≤ 0.05 and double asterisks (**) indicate P < 0.001]
Time taken during training trials
Mean time taken (all trials)
There was a significant main effect of species on the time taken (in seconds) to complete individual training trials (both correct and incorrect): Wald χ 2 (df = 3) = 303.52; P < 0.001. Post hoc analyses showed that zebrafish took significantly less time to complete trials than the other three species (P < 0.001 in all cases). Furthermore, goldfish took significantly less time during training trials than both killifish and Siamese fighting fish (both, P < 0.001), and killifish took less time than Siamese fighting fish (P = 0.006) (see Fig. 3).
Difference in time taken on first and last day of training
Goldfish (P < 0.001), Siamese fighting fish (P = 0.001) and zebrafish (P < 0.001) each took significant more time (seconds) to complete trials on the first day of training compared to the last day of training. However, there was no significant difference in the time taken by killifish on the first and last day of training (P = 0.883) (see Fig. 4).
Navigational strategy
Figure 5 shows the number of individual fish in each species that adopted either a place or response strategy during the probe trial.
Bar chart showing the number of fish in each species that adopted either a place or a response strategy during probe trials. An asterisk (*) indicates that there was a significant difference in the number of fish that adopted a place strategy over a response strategy (P < 0.05) (n = 20 in all species)
Goldfish, killifish and Siamese fighting fish showed a significant preference for adopting a place strategy (goldfish and killifish, P = 0.041; Siamese fighting fish, P = 0.012), but zebrafish showed no significant preference (P = 0.507). Bayesian inferences, however, showed no significant support for choosing the null hypothesis (performance at chance level) over alternative hypothesis (of place learning) for zebrafish as probability estimates of the null hypothesis lie within the estimates of the alternatives with probabilities of between 0.6 and 0.8 (see Table 1).
Time taken on probe trial
There was a significant main effect of species on time taken during the probe trial: Wald χ 2 (df = 3) = 30.92; P < 0.001. Post hoc analyses showed Siamese fighting fish took significantly more time (in seconds) than the other three species to complete the probe trial (P < 0.001 in all cases). There were no other significant differences (goldfish vs. killifish; P = 0.648, goldfish vs. zebrafish; P = 0.250, killifish vs. zebrafish; P = 0.108) (see Fig. 6).
Discussion
The aims of this study were to assess the length of time it took four species of fish to learn a spatial memory task and to see what navigational strategy each species preferentially employed in doing so. Results show that zebrafish took significantly more blocks to reach training criterion than the other three species (goldfish, killifish and Siamese fighting fish). Goldfish and killifish also took significantly more blocks than Siamese fighting fish to learn the training task. Such findings correspond with previous studies (Roitblat et al. 1982; Rodriguez et al. 1994) which found that both goldfish and Siamese fighting fish are capable of learning a variety of spatial memory tasks. Although little is known about the spatial memory of Nothobranchius guentheri, the findings in this study do suggest that these fish are also able to learn a spatial memory task and can do so at a faster rate than a more commonly used study species (zebrafish).
Other results from this study suggest that the mean time taken (in seconds) to complete an individual trial by the Siamese fighting fish (the largest of the four species and the quickest to learn) was significantly longer in both training (P < 0.01) and probe trials (P < 0.001) than the other three species. Similarly, the mean time to complete a training trial by zebrafish (the slowest to learn) was significantly shorter (P < 0.001) than all the other fish used in these experiments. These findings suggest that taking time to explore the environment may improve the learning process, by allowing the animal to learn from both correct and incorrect trials. In other contexts, it has been proposed that animals that are faster explorers and “proactive” might be less accurate, whereas animals that are slower explorers and “reactive” might be more accurate in their decision-making (Sih et al. 2004). Previous studies have also suggested that fish which are slower to explore their environment are more flexible in their behaviour and are quicker to adapt to changes (see Magnhagen 2012 for a review). However, it should be noted that these studies have focused on individual differences within a species and have not compared differences in behavioural flexibility and plasticity across species. We are cautious in our interpretation as another reason for this difference may be simply that Siamese fighting fish are from a different order than the other three species which may have an effect on their adaptation of spatial navigation (Perciformes and Cypriniformes, respectively). Further studies manipulating time allowed to complete a trial and its influence on learning speed would also be interesting. Likewise adopting similar analyses used in this study to assess the effects of time taken to make a decision could be applied to explore behavioural outcomes in a variety of species.
Although our findings, which show that three species (goldfish, Siamese fighting fish and zebrafish) took significantly less time to complete trials on the last day of training versus the first day of training, would be expected during a learning task, it is unclear why there was no significant difference in the killifish species. This may have been because the killifish were disadvantaged by being the smallest of the four species. However, further research is required to fully understand these differences.
The findings that three of the four species showed a preference for the adoption of a place strategy suggest that fish, like mammals and birds, are capable of complex spatial memory and that the lack of a hippocampal structure is not necessarily detrimental to the navigational abilities of fish. This has also been argued by others who suggest that the telencephalon in fish, in particular the lateral pallium, is homologous to the hippocampus of mammals and birds (Saito and Watanabe 2006; Spence et al. 2011). Zebrafish did not show a significant preference for the adoption of either a response or a place strategy despite their popularity in previous behavioural and memory studies (Williams et al. 2002; Miklosi and Andrew 2006; Sison and Gerlai 2010; Miller and Gerlai 2012b). However, results from Bayesian inference would suggest an increase in sample size may be required in order to establish whether these fish truly have a preference for either a place or a response strategy.
It is possible that the fish were using salient geometric cues external to the maze in order to navigate using what seemed to be a place strategy; this has been suggested to be the case in previous studies using rats as subjects (Ritchie 1947; Cheng 1986). The possible reason why some animals, particularly the zebrafish, did not show a preference for a place strategy may be that they were using geometrical information internal to the maze therefore causing a rotational error on the probe trial and eliciting what seemed to be a response strategy (Ritchie 1947; Cheng 1986). Further experiments could be completed in order to assess the effects of geometrical information on strategy choice.
Another possible reason for the zebrafish species performing differently could be based on zebrafish being a shoaling species which means they are more likely to navigate through their environment in groups (Wright and Krause 2006; Miller and Gerlai 2012a; Butail et al. 2013). Again, further research is needed to assess whether zebrafish show a difference in navigation or a preference for either strategy when completing the task in shoals.
Three of the four species were housed individually during experimentation (killifish, Siamese fighting fish and zebrafish). This isolation could have caused some level of stress, particularly for the shoaling zebrafish species which is highly social. Housing jars were, however, kept beside each other allowing each fish visual contact to conspecifics. Previous studies have suggested that this is satisfactory to reduce isolation stress in such animals and may also induce shoaling movements (e.g. Engeszer et al. 2004; Sison and Gerlai 2010; Karnik and Gerlai 2012). In the experimental protocol, fish would complete the trials individually which may have led to isolation stress, particularly in a novel environment. Additionally, it is possible that body size may have had an impact on the results, perhaps placing a disadvantage on the smaller species. There were, however, no observed signs of stress during experimentation with individual fish willingly eating the bloodworm reward on all correct trials, a further indicator that stress levels were minimal during experiments (Carr 2002).
As each fish would receive ten trials per block and multiple fish would be tested on each experimental day, it could be argued that olfactory cues may have been important in the learning of the task and the performance of the probe trial. These effects were controlled for where possible by disturbing the water and any substrate in the tank between each correct trial and by filtering the tank for a minimum of 20 min between each fish. Furthermore, a previous study using bloodworm reward and goldfish as a study species suggests that olfaction is not often used by fish during similar maze tests (Saito and Watanabe 2005). Finally, as place learning was not employed by all fish, it is unlikely that olfactory cues played any significant role on the performance of fish during these experiments.
A previous study suggested that the number of training days has an effect on whether an animal uses a place or response strategy for navigation (Packard and McGaugh 1996). This study found that rats were more likely to choose a response over a place strategy when they had more training in the experimental set-up. This could explain why the zebrafish, who showed the greatest variation in the number of blocks of trials taken to reach training criteria, showed no significant preference for the adoption of either a place or a response strategy. However, it should be noted that the difference in the set of experiments presented here is that not all fish received the same amount of training. This differed from the previous study (Packard and McGaugh 1996) where there was a fixed training schedule before each probe trial. The suggestion that more training could have an impact on navigational strategy also requires further investigation.
It is possible that the fish could have used the wall at the end of one of the goal arms (or the lack of wall at the end of the other) as a navigation beacon. If this was the case, then one would expect the animals to use a simpler US-CS link to learn the task rather than a more complex spatial memory strategy (Karnik and Gerlai 2012). As global cues external to the maze were not controlled for or analysed during experimentation, it is not possible to conclude what cues the fish used in order to display place learning. Indeed, it has been argued that it is difficult to exclude simpler strategies from any experiment that investigates complex spatial memory processes (Bennett 1996). However, it is surprising that the zebrafish did not show a preference for the training location if a simple, salient feature was indicating this and could explain performance of the other species. Thus, whether it was beaconing to a cue external to the maze or a set of more complex allocentric features that explain the performance of the other three species, it is clear that the zebrafish were not able to consistently use the same strategy as the other species to learn the location of the food reward. While our experimental set-up cannot distinguish between beaconing to a cue and an allocentric strategy, it is worth noting that other experiments, using the same experimental set-up (plus maze), have indicated that when the lateral pallium area of the telencephalon is ablated, fish are no longer able to learn place, but retain an ability to use a cue-based strategy (Rodriguez et al. 1994; Salas et al. 1996; López et al. 2000; Broglio et al. 2010). Experiments using a controlled salient cue at the rewarded arm would indicate whether zebrafish can learn this task and use it from a novel start point and thus provide further insight into the strategies used in the current experiment.
The findings in this study suggest that the spatial memory of fish may be comparable to other animals as three species out of four demonstrated a preference for using a strategy that is often associated with more complex and flexible allocentric navigation when completing the plus maze task. Zebrafish did not demonstrate place learning and showed no significant preference for either a place or response strategy. Further experiments using shoals of zebrafish should be completed to assess whether navigation is facilitated by collective decision-making in this species (Couzin et al. 2005). Regardless of strategy choice, these findings suggest that all four species are able to learn a spatial memory task, reinforcing previous findings that fish are a useful animal model for the study of cognitive behavioural tasks (Rodriguez et al. 1994; Braithwaite and De Perera 2006; White and Brown 2014).
References
Andrews C (2002) An interpet guide to fancy goldfish. Interpret Publishing, Surrey
Bennett AT (1996) Do animals have cognitive maps? J Exp Biol 199:219–224
Braithwaite VA, De Perera TB (2006) Short-range orientation in fish: how fish map space. Mar Freshw Behav Physiol 39(1):37–47
Broglio C, Rodriguez F, Salas C (2003) Spatial cognition and its neural basis in teleost fishes. Fish Fish 4:247–255
Broglio C et al (2010) Selective involvement of the goldfish lateral pallium in spatial memory. Behav Brain Res 210(2):191–201
Brown C (2014) Fish intelligence, sentience and ethics. Anim Cogn 18:1–17
Butail S, Bartolini T, Porfiri M (2013) Collective response of zebrafish shoals to a free-swimming robotic fish. PLoS ONE 8(10):e76123
Carr JA (2002) Stress, neuropeptides, and feeding behavior: a comparative perspective. Integr Comp Biol 42(3):582–590
Casebolt D, Speare D, Horney B (1998) Care and use of fish as laboratory animals: current state of knowledge. Lab Anim Sci 48(2):124–135
Cheeseman JF et al (2014) Way-finding in displaced clock-shifted bees proves bees use a cognitive map. Proc Natl Acad Sci USA 111(24):8949–8954
Cheng K (1986) A purely geometric module in the rat’s spatial representation. Cognition 23:149–178
Clayton NS, Krebs JR (1995) Memory in food-storing birds: from behaviour to brain. Curr Opin Neurobiol 5(2):149–154
Couzin ID et al (2005) Effective leadership and decision-making in animal groups on the move. Nature 433(7025):513–516
Dittman AH, Quinn TP (1996) Homing in pacific salmon: mechanisms and ecological basis. J Exp Biol 199:83–91
Engeszer RE, Ryan MJ, Parichy DM (2004) Learned social preference in zebrafish. Curr Biol 14(10):881–884
Genade T et al (2005) Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell 4(5):223–233
Gordon M, Axelrod H (1968) Siamese fighting fish. TFH Publications Inc, New Jersey
Guidelines for the Use of Animals (2012) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 83:301–309
Hamilton DA et al (2009) Control of rodent and human spatial navigation by room and apparatus cues. Behav Process 81(2):154–169
Herrera M, Jagadeeswaran P (2004) Annual fish as a genetic model for aging. J Gerontol A Biol Sci Med Sci 59(2):101–107
Iaria G et al (2009) Age differences in the formation and use of cognitive maps. Behav Brain Res 196(2):187–191
Karnik I, Gerlai R (2012) Can zebrafish learn spatial tasks? An empirical analysis of place and single CS-US associative learning. Behav Brain Res 233(2):415–421
Kishi S (2004) Functional aging and gradual senescence in zebrafish. Ann NY Acad Sci 1019:521–526
Lamb EA, Echevarria DJ, Jouandot DJ (2012) The utility of the T-maze in assessing learning, memory, and models of neurological disorders in the zebrafish. Behaviour 149(10–12):1081–1097
López JC et al (2000) Dissociation of place and cue learning by telencephalic ablation in goldfish. Behav Neurosci 114(4):687–699
Magnhagen C (2012) Personalities in a crowd: what shapes the behaviour of Eurasian perch and other shoaling fishes? Curr Zoo 58(1):35–44
Maguire E et al (2000) Navigation-related structural change in the hippocampi of taxi drivers. PNAS 97(8):4398–4403
Miklosi A, Andrew R (2006) The zebrafish as a model for behavioral studies. Zebrafish 3(2):227–234
Miklósi A, Andrew RJ (1999) Right eye use associated with decision to bite in zebrafish. Behav Brain Res 105(2):199–205
Miller N, Gerlai R (2012a) From schooling to shoaling: patterns of collective motion in zebrafish (Danio rerio). PLoS ONE 7(11):e48865
Miller N, Gerlai R (2012b) Automated tracking of zebrafish shoals and the analysis of shoaling behaviour. In: Kalueff AV, Steward AM (eds) Zebrafish protocols for neurobehavioral research. Humana Press, New Jersey, pp 217–230
O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford
Odling-Smee L, Braithwaite VA (2003) The influence of habitat stability on landmark use during spatial learning in the three-spined stickleback. Anim Behav 65(4):701–707
Packard MG, McGaugh JL (1996) Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65(1):65–72
Pike TW et al (2010) Learning by proportional observation in a species of fish. Behav Ecol 21(3):570–575
Portavella M, Vargas J (2005) Emotional and spatial learning in goldfish is dependent on different telencephalic pallial systems. Eur J Neurosci 21:2800–2806
Pravosudov VV, Roth TC II (2013) Cognitive ecology of food hoarding: the evolution of spatial memory and the Hippocampus. Annu Rev Ecol Evol S 44(1):173–193
Ritchie BF (1947) Studies in spatial learning III: two paths to the same location and two paths to two different locations. J Expl Psychol 37(1):25–38
Rodriguez F et al (1994) Performance of goldfish trained in allocentric and egocentric maze procedures suggests the presence of a cognitive mapping system in fishes. Anim Learn Behav 22(4):409–420
Roitblat H, Tham W, Golub L (1982) Performance of Betta splendens in a radial arm maze. Anim Learn Behav 10(1):108–114
Saito K, Watanabe S (2005) Experimental analysis of spatial learning in goldfish. Psychol Rec 55:647–662
Saito K, Watanabe S (2006) Deficits in acquisition of spatial learning after dorsomedial telencephalon lesions in goldfish. Behav Brain Res 172(2):187–194
Salas C et al (1996) Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav Neurosci 110(5):965–980
Shapiro MS, Jensen AL (2009) Parameters of rewards on choice behavior in Siamese fighting fish (Betta splendens). Behav Process 82(1):30–38
Shelton AL, McNamara TP (2001) Systems of spatial reference in human memory. Cogn Psychol 43(4):274–310
Shettleworth SJ, Westwood RP (2002) Divided attention, memory, and spatial discrimination in food-storing and nonstoring birds, black-capped chickadees (Poecile atricapilla) and dark-eyed Juncos (Junco hyemalis). J Exp Psychol Anim B 28(3):227–241
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19(7):372–378
Sison M, Gerlai R (2010) Associative learning in zebrafish (Danio rerio) in the plus maze. Behav Brain Res 207(1):99–104
Spence R, Magurran AE, Smith C (2011) Spatial cognition in zebrafish: the role of strain and rearing environment. Anim Cogn 14(4):607–612
Thomas POR et al (2008) Does defection during predator inspection affect social structure in wild shoals of guppies? Anim Behav 75(1):43–53
Tolman EC (1948) Cognitive maps in rats and men (1). Psychol Rev 55(4):189–208
Tolman E, Ritchie B, Kalish D (1946) Studies in spatial learning. II. Place learning versus response learning. J Exp Psychol 36(3):221–229
Tropepe V, Sive HL (2003) Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav 2:268–281
Van Gerven DJH et al (2012) Direct measurement of spontaneous strategy selection in a virtual Morris water maze shows females choose an allocentric strategy at least as often as males do. Behav Neurosci 126(3):465–478
White GE, Brown C (2014) A comparison of spatial learning and memory capabilities in intertidal gobies. Behav Ecol Sociobiol 68(9):1393–1401
Williams FE, White D, Messer WS (2002) A simple spatial alternation task for assessing memory function in zebrafish. Behav Process 58(3):125–132
Wolbers T, Hegarty M (2010) What determines our navigational abilities? Trends Cogn Sci 14(3):138–146
Wright D, Krause J (2006) Repeated measures of shoaling tendency in zebrafish (Danio rerio) and other small teleost fishes. Nat Protoc 1(4):1828–1831
Acknowledgments
The authors would like to acknowledge the following individuals for assistance with laboratory set-up and fish husbandry: Georgina Glaser, Sinéad Smith, Chris Preshaw, Gillian Riddell and other members of the technical staff at the School of Biological Sciences, QUB. We would also like to thank the funding bodies of this research: The Department of Employment and Learning, Northern Ireland and Queen’s University Belfast. Finally, we would like to thank Ken Cheng and Hannah White for advice on statistics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
McAroe, C.L., Craig, C.M. & Holland, R.A. Place versus response learning in fish: a comparison between species. Anim Cogn 19, 153–161 (2016). https://doi.org/10.1007/s10071-015-0922-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-015-0922-9