Abstract
This paper explores the impact of encapsulation techniques on bioactive compounds, vitamins, and minerals, which are crucial for delivering bioactive compounds. Due to their instability and reactivity with the environment, encapsulation is often necessary to make these compounds suitable for medical or dietary applications. The evaluation of the kinetic model of bioactives reveals that encapsulation can significantly enhance their stability. However, encapsulation is not without its drawbacks. Incomplete encapsulation can reduce the effectiveness of the bioactives, and complexity of encapsulation processes can hinder widespread adoption. Interactions between the encapsulated materials and the encapsulating agents may also impact the release and bioavailability of the bioactives. It also presents perspectives for future research aimed at overcoming the limitations and enhancing the effectiveness of encapsulation. As research continues to advance, encapsulation is poised to play critical role in improving the delivery and stability of bioactive compounds, benefiting the food, pharmaceutical, and cosmetic industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to prolong the shelf life of delicate substances like vitamins, minerals, and bioactives and prevent oxidation and physical deterioration, encapsulation is a technology employed in the food and pharmaceutical industries (Kopecna et al., 2020). By covering the sensitive components in a protective shell, this approach helps to regulate the release of the bioactive molecules during different phases of digestion. Moreover, encapsulating bioactives can help with formulation of complicated combinations, pharmacokinetic property customization, and other issues related to non-bioavailability of different constituents (Mandaliya et al., 2020). A thorough review of the effects of encapsulation techniques on the properties of bioactives, vitamins, and minerals is still necessary to close the knowledge gap and identify the main research trends in this field, even though there have been studies on the effects of various encapsulation techniques on the various properties of bioactives, vitamins, and minerals. An assessment of the impact of encapsulating techniques on the various properties of bioactives, vitamins, and minerals will be the main goal of this review study. It will go over the different encapsulation strategies that are employed as well as the variables that affect how effective are these strategies. The different impacts of encapsulation on the characteristics of vitamins, minerals, and bioactives will next be examined. These effects will include things like increased food intake, higher bioavailability, and lower metabolic activity. Encapsulation is a crucial step in the production of pharmaceutical and food products. It can be used to enhance a formulation's physical characteristics without compromising the active components' ability to operate (Insomnia et al., 2020). Because encapsulation helps preserve nutrients from deterioration and improves nutritional content for increased absorption and bioavailability, it is an excellent delivery system vehicle. Encapsulation is a technique used in food to deliver and shield vitamins, minerals, and bioactives from a variety of external elements, including light, moisture, temperature, mechanical stress, etc. The aim of this paper is to find the effects on properties of vitamins, minerals, and bioactives of various encapsulating techniques, including emulsifying-coating, layer-by-layer, atactic, and interference layer (ILC), as well as the instruments utilized in their development. It will also explore the several advantages that come with using these methods, like better digestion, increased bioavailability, and qualities that might prevent oxidative damage. Also, it will look at the several aspects like cost, accessibility, and scale-up strategies that affect effectiveness of encapsulation. Lastly, it will compare the efficacy of encapsulating techniques with current encapsulation technologies and explore the possible uses of encapsulation techniques in the manufacturing of food goods and nutritional supplements. A growing number of people are interested in studying various strategies that could facilitate the effective delivery and preservation of bioactive compounds, given the swift advancements in science and technology. Out of all the various approaches looked at, encapsulation has shown to be one of the most promising and extensively researched ways to create edible materials with better qualities and functionality (Huang et al., 2020). Encapsulation technology is thought to be an effective way to formulate and deliver vitamins, minerals, and bioactives while preventing their degradation, inactivation, loss, and instability before they reach their intended location (Matras et al., 2020). While traditional encapsulation techniques like coating and spray-drying have significantly improved the stability and bioavailability of bioactives, recent research has pushed the boundaries further with nano-encapsulation (Aktas et al., 2024). This cutting-edge technology allows for even finer control over the encapsulation process, enhancing the solubility and bioavailability of bioactive compounds at a molecular level. Studies have demonstrated the superior performance of nano-encapsulation in protecting sensitive bioactives from degradation and improving their targeted delivery (El-Sayed et al., 2024).
Traditional techniques like coating and spray-drying have greatly improved the stability and bioavailability of bioactives, but they are not without drawbacks. These methods can sometimes result in incomplete encapsulation and reduced effectiveness. Techniques can be expensive and complex, making them less accessible for widespread use. Additionally, interactions between the encapsulated materials and the encapsulating agents can negatively impact the release and bioavailability of bioactives (Raj et al., 2024). Recent research has advanced to nano-encapsulation, a cutting-edge technology that provides even more precise control over the encapsulation process. Nano-encapsulation enhances the solubility and bioavailability of bioactive compounds at a molecular level, offering superior protection from degradation and improving targeted delivery. However, nano-encapsulation also faces challenges, such as high production costs and technical difficulties in maintaining consistent particle size and distribution (Li et al., 2024). This review examines these latest advancements and addresses the limitations of existing methods, providing a comprehensive overview of the current state of encapsulation technology and its potential future applications.
This review study aims to give an up-to-date overview of the state of the art and best practices on the efficacy of encapsulation techniques to enhance the functional characteristics and chemical stability of vitamins, minerals, and bioactive compounds. It will go over the many encapsulating material types that are utilized, including polysaccharides, proteins, lipids, and polymer-based capsules, as well as the production methods that include coacervation, emulsification-based procedures, and spray-drying.
The numerous approaches used to increase the effectiveness of vitamins, minerals, and bioactives by encapsulation will also be reviewed in this study. The process of encapsulation involves binding, coating, or enveloping food or food ingredients in a carrier substance. Because it has numerous benefits for ingredient stability, preservation, improved solubility, controlled release, and active targeted delivery, it has become a crucial component of the food business (Sheng et al., 2020). To enhance the transport of bioactives, vitamins, and minerals to desired locations while preventing chemical and enzymatic degradation, encapsulation techniques can be used (Tian et al., 2020). Furthermore, it may help lower the dosage of these substances while simultaneously raising their bioavailability. The many encapsulation methods currently in use will be covered in this paper, along with their benefits and drawbacks and a summary of the research on how they can alter the characteristics of different bioactive compounds, vitamins, and minerals. Additionally, it will outline the many tactics used to maximize these chemical loading efficiency, stability, and bioavailability. The paper will also cover the possible applications of new and cutting-edge methods for encapsulating these components, such as nanotechnology.
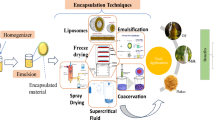
Microencapsulation technologies used for bioactive food ingredients
Encapsulation by spray-drying
A quick, continuous, scalable, repeatable, and economical method for turning liquids into dry powders is spray drying. Spray droplets evaporate in the drying chamber when the liquid is sprayed into a heated drying gas medium, usually air, using an atomizer. After that, the solid product is taken out of the air stream and gathered. Spray drying makes cells more resilient to stress, enabling them to endure thereafter harsh conditions like high heat, acidic surroundings, or the presence of bile salts (Deng et al., 2020).
A popular and commonly used technique called spray drying is a simple, flexible, quick, and cost-effective method for producing microcapsules and involves dispersing the core particles in a polymer solution and spraying them inside a heated chamber (He et al., 2020). The process starts with the carrier material solutions being sprayed into a heated chamber, where the core chemical dispersion is dissolved and emulsified. Later the solvent evaporates and the wall material covers the core to generate polynuclear or matrix microcapsules.Thus, this method comprises extracting the dehydrated microcapsules after atomizing an emulsion or solution of core-carrier agents (Li et al., 2020a, b, c, d).Usually, this process produces microcapsules that are of excellent quality. The use of water-soluble carriers and the high temperatures needed to evaporate the solvent are some of the disadvantages of this approach (Kahsai et al., 2020).
The unpleasant odour of these extracts was also masked by microencapsulation, making them suitable for nutraceutical and food applications. Alginates as a natural bioactive compound transporter and delivery method have been thoroughly researched (Gul et al., 2020). It can alter the characteristics of matrices for a range of applications, including food, by cross-linking with different cations. Alginate matrices have also been used to encapsulate enzymes such as cellulases and hemicelluloses. Gums are used in the microencapsulation of many different bioactive substances due to their superior film-forming and emulsion stabilization qualities (Singh et al., 2020). A superior wall material for lipid encapsulation, gum arabic creates stable emulsions with several oils across a broad pH range. The microencapsulation of rosemary essential oil was carried out, exhibiting a range of 17.25–33.96%, 0.03–0.15%, 7.15–47.57%, and 15.87–18.90% for powder recovery, oil retention, and hygroscopicity, respectively (Shindou et al., 2021). Supercritical fluid extraction was used to extract vitamin E and provitamin A from residual red pepper. To stop the extracts from degrading during storage, they were subsequently spray-dried and microencapsulated using gum arabic as a wall material. Cortés-Rojas et al. (2015) encapsulated Biden Pilosa leaf extract at 13.37% concentration in cellulose as the wall material. The powder has favorable features with respect to flow, drying efficiency, solubility, residual moisture content, and particle size. Enhancing microcapsule encapsulation efficiency and stability can also be achieved by using a combination of several compounds as the wall material, as no one compound can supply all of the necessary properties for microcapsules with the appropriate quality features (Lee et al., 2020). Gum arabic and maltodextrin were combined as the wall material, and the spray drying process was utilized to microencapsulate essential oil from the fruits of Pterodonemarginatus. The primary component of these essential oils, caryophyllene provided the best protection, and the researchers found that a 1:3:3.6 ratio of essential oil, gum arabic, and maltodextrin produced an efficiency of about 98.63% for encapsulation and entrapment (Fontana et al., 2020).
Encapsulation by spray-drying offers several advantages, including improved stability and shelf-life of bioactive compounds, enhanced solubility, and controlled release. It also helps in masking undesirable flavors and protecting nutrients from environmental factors like heat and light (Bińkowska et al., 2024). The process can be costly and complex, and it sometimes results in incomplete encapsulation, which reduces effectiveness. Additionally, maintaining consistent particle size and distribution can be challenging. These limitations can impact the overall efficiency and scalability of the technique, making it less accessible for widespread application in the food and pharmaceutical industries (Paes et al., 2024) (Fig. 1) and (Table 1).
Encapsulation by freeze-drying
For core materials that are heat-sensitive, spray-freeze drying is suitable. Using this technique, the solvent is instantly sublimed (lyophilized) from atomized droplets. Sprays of liquid nitrogen are directed into a cold chamber. These droplets sublimate at room pressure in a fluidized bed, producing encapsulates with a microstructure of a matrix (Hou et al., 2020). Thinner shells produced by higher core loads allow core material droplets to leak on the surface of encapsulates because core droplets next to the shell are more prone to deterioration. While spray drying method yields encapsulates with extremely high solubility and reconstitution properties, low water activity, and ease of transportation and storage, its application is still restricted by the absence of ideal wall materials and microcapsule agglomeration(Li et al., 2020a, b, c, d).Several heat-labile active compounds were shown to be lost during the high-temperature spray drying procedure by Hou et al. (2020). Furthermore, the spray drying process results in low yield because of a number of factors including poor control over droplet size, limitations of common wall materials and low glass transition temperature (Li et al., 2020a, b, c, d). For microencapsulation to achieve, improved encapsulation efficiency and microcapsule stability, wall material selection is essential. Water soluble wall material is appropriate since nearly all spray drying procedures in the food sector are carried out in aqueous feed compositions. The most widely employed wall components are hydrocolloids, which comprise gum acacia, milk proteins, gelatin, and low molecular mass carbohydrates (Li et al., 2020a, b, c, d).
Another name for spray freezing, which is also called spray congealing, is a convenient, affordable, and easily scalable microencapsulation method. Being free of organic solvents and high temperatures, it is perfect for bioactive substances that are sensitive to temperature changes, such as probiotics, vitamins, and enzymes (Kaushik et al., 2021). In spray coagulation, alginate is commonly employed as a thickening and stabilising agent. Calcium carbonate is used as an internal gelation source to aid alginate coagulation, or calcium chloride is used as an outer gelation source. Martins et al. microencapsulated phenolic extracts from Rubusulmifolius flower buds using an alginate-based matrix. Antioxidant activity was marginally higher in the yoghurt containing Rubusulmifolius flower buds extract microcapsules than in the other samples (Sheng et al., 2020).
Encapsulation by freeze-drying offers several advantages. It effectively preserves the stability and bioactivity of sensitive compounds, such as vitamins and probiotics, by removing water under low temperatures. This method enhances shelf life and maintains nutritional quality (Kaur et al., 2024). Freeze-drying is an expensive and time-consuming process, requiring specialized equipment. The texture and taste of the encapsulated product can also be altered. Limiting factors include high energy consumption and potential structural changes during rehydration. Despite these challenges, freeze-drying remains a valuable technique for protecting and extending the shelf life of bioactive compounds (Tkacz et al., 2024).
Encapsulation by electro spraying
Electro spraying, also called electrohydrodynamic atomization, is a method of encapsulating and drying nano/microstructures at room temperature by means of an electric field. The principle of electro spraying involves the conductive material capillary injector/needle being used to pump a solution, dispersion, or emulsion containing the protein-based bioactive (Sun et al., 2021). A predetermined distance is set off from the needle in order to generate an electric field between the injector and the grounded collector. When the solution is pumped through the needle at a constant flow rate without any voltage, a meniscus forms when the solution drop exits the needle (Kadariya et al., 2020).The meniscus polarises at the air–liquid contact and deforms into a conical shape called a Taylor cone when the electrostatic field is high enough. The liquid in the droplet surface tension gives way under the increasing voltage, causing a jet to be released from the tip of cone and travel in the direction of the collector. Due to the electrostatic repulsion forces at work and the low viscoelasticity of the solution, the jet breaks into a spray of charged particles. The solvent(s) used evaporate as the droplets travel towards the collector, producing dry nano/microparticles (Anjum et al., 2020).
Based on the characteristics of the solution and the processing settings employed, two distinct approaches can be employed: One method of electrospraying involves lowering the fluid's intermolecular cohesion to a point where electrostatic forces fracture the jet released from the solution into tiny droplets, forming nano/microparticles following solvent evaporation; another method involves electrospinning, where high molecular cohesion prevents jet fragmentation and The best way to obtain food ingredients is via electro spraying, as electro spun fibres, in contrast to nano/microcapsules, generate continuous mats that are difficult to disseminate in any food matrix without prior breakdown (Kouzouli et al., 2020).
Electrospraying can be employed in coaxial or mono-configurations, just like spray-drying. Protein-based bioactives are usually distributed throughout the carrier matrix of amorphous solid dispersions created by monoaxial electro spraying (Bai et al., 2020). Conversely, coaxial electro spraying is a tailored form of electro spraying where two distinct liquids are dispensed using separate coaxial capillary needles. The inner needle (core) is used to inject the bioactive components and encapsulating agent, while the outer concentrical shell is used to inject a different encapsulating agent (Lopez et al., 2020). The advantages of monoaxial electro spraying are combined with coaxial electro spraying capacity to accurately control the core–shell structure and prevent the aggregation and denaturation of bioactive peptides (Donetti et al., 2021).
Environmental factors, processing variables, solution characteristics, and electrospraying parameters can all have an impact on the morphology, particle size, and EE. Greater density and viscosity of the solution at high encapsulating agent concentrations lead to larger particles, whereas higher electrical conductivity of the solution produces smaller particles (Scott et al., 2021). The cross-sectional area of nano/microcapsules generated using coaxial electro spraying in theory. Environmental factors, processing variables, solution characteristics, and electrospraying parameters can all have an impact on the morphology, particle size, and EE. Larger particles may form in a solution with a high concentration of encapsulating agent due to its high viscosity and density, while smaller particles are produced when the electrical conductivity of solution is increased (Mansur et al., 2020). Regarding processing parameters, a higher solution flow rate produces larger particle size, whereas a higher electric potential between the injector and the collector produces smaller particle diameters. While shorter lengths may produce moist and compressed particles, longer injector-collector distances enable better solvent evaporation (Yang et al., 2020). Due to their role in establishing the forces that propel the drying process, temperature and humidity are additional environmental elements that impact drying kinetics. Additional things to think about when encasing protein-based bioactives using electrospraying are as follows: (1) using proteins produces highly conductive solutions that impede charge formation and decrease the stability of the Taylor cone; and (2) using food-grade solvents, like water, produces solutions with high surface tension that impede jetting (Ronconi et al., 2021).Electro spraying encapsulation has been employed extensively in the field of pharmacology due to its inexpensive cost, simplicity of application, and enhanced bioaccessibility of the nano/microcapsules created. But because of its poor production capacity, its application in food applications is still restricted (Kumar et al., 2020). A number of adjustments, such as (i) multineedle electro spraying systems, (ii) free surface electro spraying systems, and (iii) pressurized-gas-assisted electro spraying, have been documented to get around this restriction. This lack of relevance to the food sector is, however, reflected in the paucity of literature on the issue. There are no data on bioactive protein hydrolysates; instead, the majority of the existing research focuses on oral pharmaceutical supplements. Thus, further investigation into the application of electrospraying encapsulation in food products is required (Sirilegar et al., 2020). Encapsulation by electro spraying offers several advantages, such as precise control over particle size and distribution, and the ability to create uniform and stable encapsulates. This method enhances the solubility and bioavailability of bioactive compounds, providing better protection from environmental factors. Electro spraying has its disadvantages, including high production costs and technical complexity, which can limit its scalability. Additionally, maintaining consistent particle size and distribution can be challenging. Limiting factors for this technique include the need for specialized equipment and expertise, as well as the potential for clogging and low throughput in large-scale applications (Kurek et al., 2024) (Fig. 2).
Encapsulation by monoaxial
By drying just one solution containing the bioactive, monoaxial electro spraying encapsulates protein-based bioactives. Creating this feed stream is most commonly achieved by blending, i.e., bioactives dissolved in a carrier containing solution. In a study, Musaei et al. (2017) used PLGA as an encapsulating material to construct a blend feed stream including an ethanol-acetic acid mixture to encapsulate BSA. Even bigger molecules have been encapsulated via blend electro spraying; for example, the hormone angiotensin II uses N-octyl-O-sulfate chitosan (NOSC) as a carrier, or enzymes like streptokinase and alkaline phosphatase use poly(ethylene oxide) (PEO) and PLGA, respectively. While electrospraying is carried out at room temperature to prevent thermal deterioration of thermosensitive components, prolonged exposure to particular solvents may result in protein denaturation and loss of function (Mao et al., 2021).Therefore, a different strategy of using a blend to produce the feed stream is to develop emulsions that block contact between particular solvents and the bioactives. Emulsion electro spraying encapsulation enables the creation of particles with core–shell structures resembling those achieved by coaxial electro spraying, as demonstrated by earlier studies. Often using a single W/O or double W1/O/W2 emulsion, this technique is used to mix two immiscible fluids (Garcia et al., 2021). The drying of water-in-water (W/W) emulsions was the main objective of the two experiments that used emulsion electro spraying in the literature. Using this method, Yao et al. (2016) encapsulated BSA in PLGA. PLGA in chloroform made up the organic phase, and BSA dissolved in water made up the aqueous phase of the two immiscible solutions that were created for this purpose. It should be noted that the manufacture of emulsion feed is less common since it is difficult to produce stable emulsions and because the shear stress of mechanical mixing required for emulsion preparation may alter the protein-based bioactives (Chanutin et al., 2020). The type of carrier and solvent utilised determines the primary characteristics of the input stream that affect the electro spraying process, such as surface tension, conductivity, and viscoelasticity. In electrospraying, encapsulating agents like poly (lactic acid) (PLA), dextran, gelatin, glucose syrup, hyaluronan, MD, pullulan, CS, PLGA, alginate, PCL, PEG PEO, and NOSC are used..These polymers are also biocompatible and biodegradable (Chauce et al., 2022). Alginate, PEO, and NOSC were often utilised carriers; because of their safety and biocompatibility, these materials are very helpful when formulating oral administration medications. The calcitonin gene-related peptide (-CGRP) and BSA/porcine interleukin-1 (pIL-1) were encapsulated in alginate by mix electrospraying, yielding particles with widely varied diameters ranging from 10.08 to 20 µm, respectively (Metin et al., 2020). Alkaline phosphatase was electrosprayed using PEO, a synthetic semicrystalline polymer that is frequently utilized for electrospinning because of its rheological properties, in just one work. Similarly, NOSC could only encapsulate the hormone angiotensin II. The USFDA has approved PLGA, a biocompatible copolymer that is widely utilized in biomedical devices with great in vivo application records. The size of the nano capsules increased from 120 to 225 nm, which is associated to an increase in EE. It was found that increasing PLGA concentration did in fact influence capsule particle size (Musaei et al. (2017). Studies have mostly concentrated on drug release formulation, with minimal attention paid to food applications, despite the fact that all of these biopolymers have shown high encapsulating capacity (Quiroz et al., 2021).Promising findings have been observed in the study of PLGA as a potential dietary fortifier. Although polysaccharide and protein-based carriers are frequently employed as encapsulating agents for spray-drying protein-based bioactives, there hasn't been any research on their application for electrospraying these bioactives (Hudson et al., 2020).Due to their food-grade nature and water solubility, these carriers are perfect for the food sector as they do not require non-food-grade solvents. Further study is therefore needed to determine whether food-grade, inexpensive biopolymers may be used to electrospray encapsulate protein-based bioactives (Quintas et al., 2021).
Encapsulation by monoaxial methods offers significant advantages in protecting bioactive compounds from degradation and enhancing their stability. It enables controlled release over time, ensuring sustained efficacy in applications like pharmaceuticals and functional foods. Monoaxial encapsulation can face challenges such as inconsistent particle size distribution, which affects uniformity in product quality. The process can also be sensitive to environmental factors like temperature and humidity, impacting the final product's performance. These limitations necessitate careful optimization of process parameters and materials to achieve desired encapsulation efficiency and reliability in various industrial applications (Bodbodak et al., 2024).
Encapsulation by coaxial electrospraying
While protein-and polysaccharide-based carriers are frequently employed as encapsulating agents for spray-drying protein-based bioactives, there hasn't been any research on their use for electrospraying these bioactives. Because of their solubility in water, these carriers are ideal for the food industry, eliminating the need for non-food-grade solvents (Aklipinar et al., 2020). As a result, more research is needed on the use of food-grade, low-cost biopolymers for electrospraying encapsulation of protein-based bioactives. The protein medication ranibizumab, which is used to treat age-related macular degeneration, was encapsulated in the other study using PLGA dissolved in a mixture of DCM and acetonitrile as the outer solution (Sarkar et al., 2020). Organic solvents were used in six out of the eight operations, mostly for the outer feed. The reason for this is because by reducing interdiffusion between layers, the use of two immiscible solutions enhances core–shell separation. An ethyl acetate and n-butanol solution were used as the outer feed for encapsulating anthrax protective antigens that were dissolved in the inner water solution, with acetylated dextran acting as the carrier (Mohanty et al., 2021). Rasekh et al. (2015) electrosprayed angiotensin II coaxially using tristearin dissolved in DCM as the outer solution and NOSC as the inner solution carrier. Since the majority of the literature found was focused on the creation of oral medicines, consideration would need to be given to the requirement of using two fully immiscible food-grade solvents in order to make encapsulates oriented for food fortification (Piilocie et al., 2020).
The applied voltages varied from 5 to 22.5 kV, which is comparable to the values used for monoaxial electrospraying (2.67–20 kV). The effect of voltage on angiotensin II encapsulation was investigated using tristearin and NOSC as the inner and outer carriers, respectively. The scientists compared the stability of the enzyme using an ELISA and found that the concentration of angiotensin II in the microparticles dropped by about 20% at 30 kV. PEO was used as the external carrier to encapsulate alkaline phosphatase, and the voltage was adjusted to 22.5 kV (Bang et al., 2017). Similarly, when compared to monoaxial electrospraying at 15.5 kV, these authors found that this high voltage resulted in a decrease of enzyme activity of up to 40%. Nozzle sizes and the inner and outer feed flow rates have an impact on particle characteristics as well. Feed flow rates for the inner solutions (core) were 0.02–3.6 mL/h, and for the outer solutions (shell), 0.1–18 mL/h (Kang et al., 2015). The nozzle sizes of outer capillary ranged from 603 to 2000 µm, while the inner capillary spanned from 184 to 1000 µm. Larger particles are typically the outcome of increasing the nozzle diameters and feed flow rate, as was previously mentioned in the section above. This was consistent with research from Zhao et al. (2021), who encapsulated alkaline phosphatase utilizing alginate and PEGDA as outer carriers and CMC as an interior carrier. The results of Zhao et al. (2021), who encapsulated alkaline phosphatase using alginate and PEGDA acting as the outside carriers and CMC acting as the inside carrier, were in line with this. The largest particles were formed at 440 m, and the greatest feed flow rates (3.8 mL/h for the shell and 3.96 mL/h for the core) were recorded in the literature. Nonetheless, the opposite result was observed when comparing the encapsulation of alkaline phosphatase with PEO as the carrier with the encapsulation of angiotensin II with NOSC as the inner carrier and tristearin as the outside carrier. Nozzle diameters of 1000 µm (inner)–2000 µm (outer) and 900 µm (inner)–1900 µm (outer) wasutilized in both investigations; however, the first research had feed flow rates that were ten times higher (Yao et al., 2021). The greater flow rates led to an 86% reduction in particle size, which is surprising because larger particles would be expected for angiotensin II encapsulation. In actuality, they produced the tiniest particles, which may have been caused by the 20 cm nozzle-collector distance and the marginally greater voltage applied (Liao et al., 2020).
When coaxially electrosprayed with bovine haemoglobin, tiny particles measuring 0.37 m were produced. In order to serve as oxygen carriers and prevent extravasation through the blood vessel wall, it was crucial to produce nano/microcapsules in the range of 0.1 to 3 m. According to Zamani et al. (2014), EE ranges of 46.7 4.3% to 74.6 2.9% were associated with high BSA concentrations in the core and inadequate encapsulation as a result of excessively high inner feed flow rates. When alkaline phosphatase was encapsulated using PEO as the exterior carrier, the greatest EE was achieved (Eleke et al., 2012).The EE in core–shell designs was confirmed to be enhanced by comparing the results of monoaxial and coaxial electrospraying.
Similar to the 2.67–20 kV range used for monoaxial electrospraying, the applied voltages ranged from 5 to 22.5 kV. The effect of voltage on angiotensin II encapsulation was investigated using tristearin and NOSC as inner and outer carriers, respectively. Based on an ELISA study of the enzyme stability, the scientists found that the microparticles concentration of angiotensin II dropped by about 20% at 30 kV (Masuko et al., 2020). Using PEO as the external carrier, alkaline phosphatase was encapsulated, and the voltage was adjusted to 22.5 kV. Similarly, these scientists discovered that this high voltage led to as up to 40% reduction in enzyme activity when compared to monoaxial electrospraying at 15.5 kV. Nozzle diameters and the inner and outer feed flow rates also affect the characteristics of the particles. Feed flow rates for the inner solutions (core) were 0.02–3.6 mL/h, and for the outer solutions (shell), 0.1–18 mL/h (Wang et al., 2020). The outer nozzle sizes of the capillary ranged from 603 to 2000 µm, while the inner capillary spanned from 184 to 1000 µm. Larger particles are typically the outcome of increasing the nozzle diameters and feed flow rate, as was previously mentioned in the section above. The results of Zhao et al. (2021), who encapsulated alkaline phosphatase using alginate and PEGDA acting as the outside carriers and CMC acting as the inside carrier, were in line with this. The largest particles were formed at 440 m, and the greatest feed flow rates (3.8 mL/h for the shell and 3.96 mL/h for the core) were recorded in the literature. However, the opposite outcome was seen when the encapsulation of angiotensin II (with tristearin as the outer carrier and NOSC as the inner carrier) and that of alkaline phosphatase (with PEO as the carrier) were examined (Chang et al., 2017). The greater flow rates led to an 86% reduction in particle size, which is surprising because larger particles would be expected for angiotensin II encapsulation. The nozzle-collector distance of 20 cm and the slightly higher voltage utilized may have contributed to the fact that they actually achieved the tiniest particles. Because it was essential to acquire nano/microcapsules in the range of 0.1 to 3 m to successfully prevent extravasation through the blood vessel wall and act as a barrier, coaxial electrospraying of bovine haemoglobin produced small particles of 0.37 m. According to Zamani et al. (2014), EE ranges of 46.7 4.3% to 74.6 2.9% were associated with high BSA concentrations in the core and inadequate encapsulation as a result of excessively high inner feed flow rates. When PEO was used as the outer carrier to encapsulate alkaline phosphatase, the maximum EE was achieved. Additionally, they contrasted the results of coaxial and monoaxial electrospraying, demonstrating that the EE in core–shell structures was raised. The encapsulation of bioactive protein hydrolysates or peptides has not gotten much attention, despite the encouraging results that coaxial electrospraying has produced. To properly evaluate the viability of this method for the encapsulation of bioactive peptides, more investigation is therefore required (Peres et al., 2010).
Encapsulation by coaxial electrospraying offers precise control over the encapsulation process, enhancing the protection and controlled release of bioactive compounds. It allows for the creation of core–shell structures that can shield sensitive ingredients from environmental factors like light and oxygen, thereby extending shelf life. However, this technique can be technically demanding and costly due to the intricate setup required for maintaining the coaxial flow and ensuring uniform particle size distribution. Limiting factors include potential instability in operational parameters and challenges in scaling up production to industrial levels, which currently restrict widespread adoption in commercial applications (Lima et al., 2024).
Encapsulation by complex coacervation
Coacervation is the term used to describe the fundamental process of capsule wall development. A colloid phenomenon called coacervation encapsulates liquids and solids under constant agitation. Through modifications of the medium's physicochemical properties, including temperature, ionic strength, pH, and polarity, the process of "coacervation" allows polymer to be deposited around the core (Wang et al., 2012). The low-cost, low-temperature technique of coalescing eliminates the need for high temperatures and organic solvents. The most popular application for it is to encapsulate flavour oils. The fact that coacervation only happens within particular pH, colloid concentration, and/or electrolyte concentration limits is one of its main drawbacks. Simple coacervation involves only one colloidal solute, whereas complicated coacervation necessitates the presence of two or more colloidal solutes in the continuous phase of the fluid system. While cellulose derivatives and gelatin are the most often utilized polymers in basic coacervation, other polymers have also been employed in pharmaceutical practice to create microcapsules (Ramirez et al., 2012).
Coacervation occurs when core materials are combined with charged biopolymer solutions, causing complexes between oppositely charged biopolymers to form. Here, the introduction of an oppositely charged biopolymer causes a complex to develop that trap and precipitates the core material. These are gathered and dried in carefully regulated settings, producing complexes that have a sphere-like or uneven shape (Zhang et al., 2018). The process of coacervation involves dividing colloidal fluids into two phases: rich in colloid and deficient in colloid. By changing the pH or temperature, introducing electrolyte or non-solvent chemicals, or all three, the wall components surrounding the active core precipitate. Depending on the phase separation technique, the aqueous coacervation can be categorized as simple or complex. By encouraging macromolecule-macromolecule connections at the expense of macromolecule-solvent interactions, simple coacervation—which involves the addition of solvents like ethanol or sodium sulphate or the modification of temperature—facilitates phase separation. Hydrocolloids with opposing charges, such as pea proteins, gelatin, alginates, gum arabic, pectins, and others, are attracted to one another and help form a matrix wall around the active component that was suspended or emulsified in the wall materials. This is a popular method of encasing antibacterial substances (Wang et al., 2015).
The usage of glutaraldehyde as a cross-linking substance, high cost, and complicated method were the primary obstacles to the adoption of coacervation technology in the industry, as noted by Yaseen et al. (2021). Its technology, on the other hand, has many benefits, including low operating temperatures that lead to very low evaporative losses and thermal degradation, high loading capacity, simplicity of operation, lack of sophisticated equipment requirements, low stirring requirements, and adaptability to a range of raw materials (Wang et al., 2015). One kind of phase separation encapsulation that is frequently employed following spray drying is called coacervation. This is the first account of microcapsules being produced industrially. The coacervation method generates gelatin and gelatin-acacia microcapsules quite regularly. Its triggered controlled release and excellent encapsulation efficiency increase its application in food systems. A simple coacervation utilizes a single polymer, whereas in complex coacervation two polymeric materials are employed. Wall components for simple coacervation are frequently used, including pectin, alginate, and milk proteins. Complicated coacervation involves three basic steps: rigorizing the coatings, deposition, and formation of three immiscible phases (Reddy et al., 2014).
In order to microencapsulate tuna oil enriched with vitamins A, D3, E, K2, curcumin, and coenzymes Q10, Wang et al. (2015) developed complicated gelatin-sodium hexametaphosphate coacervates. They characterized this technique as an effective means of encasing bioactive chemicals that are soluble in lipids. Naik et al. (2014) microencapsulated Lepidium sativum seed oil high in linolenic acid using the coacervation process. The soy protein isolate, gum ghatti, and gum arabic were used to make an oil-in-water emulsion. The novel food products could be developed using optimised oil capsules rich in linolenic acid. Ostertag et al. (2012) encapsulated orange and limonene flavour oils in low-energy nanoemulsions by using the emulsion phase inversion technique. With this type of emulsion, lipophilic bioactive compounds can be administered as a nutraceutical agent. Gelatin and pectin were used in a complex coacervation method to encapsulate an oily lycopene dispersion. Encapsulated lycopene degraded linearly, losing 14% on average each week.
In order to prevent the growth of Listeria monocytogenes, Huq et al. (2014) developed nisin-microencapsulated edible beads. They then observed a 20-fold increase in nisin availability in encapsulated form during the 28-day refrigerator storage of ready-to-eat (RTE) gammon, without compromising pH or color. Since it balances the electrostatic force between biopolymers that are oppositely charged, the zeta potential (-potential) is an important quantity to take into account during coacervation. Stability is enhanced when biopolymers with opposing charges are completely neutralized and an enhanced microstructure while also increasing the retention of the bioactive ingredient. It is important to keep in mind that this approach is still relatively new and hasn't been used widely in the food industry (Reddy et al., 2014).
Encapsulation by complex coacervation offers significant advantages in food and pharmaceutical industries by providing efficient protection and controlled release of bioactive compounds. It enhances stability against environmental factors like sunlight and oxidation, thereby extending shelf life and maintaining potency. However, this technique can be sensitive to pH and temperature variations, affecting encapsulation efficiency. It also requires careful selection of materials to achieve desired encapsulation properties, which can increase production costs. Achieving uniform particle size and controlling release rates can be challenging, limiting its application in precision delivery systems. Despite these challenges, complex coacervation remains a valuable tool for enhancing product performance and consumer satisfaction (Coelho et al., 2024) (Fig. 3).
Delivery of bioactive ingredients into foods and to the GI tract
The process by which certain beneficial compounds found in foods, such as vitamins, minerals, dietary fibre, and antioxidants, are made available to our bodies so that they can absorb and use them for their intended purposes is referred to as delivery of bioactive ingredients into foods and to the GI tract (Rao et al., 2014). This is frequently accomplished through the deliberate addition of ingredients, such as fortified foods or functional ingredients. Also, certain processing techniques, such as emulsification and encapsulation, can aid in bioactive ingredient delivery. When the ingredients reach the GI tract, the small intestine absorbs them and distributes them throughout the body (Burgal et al., 2016).
Bioactive components are substances that, through controlling cellular or physiological processes, can improve the health of the organism. Studies have demonstrated that certain food-derived bioactive components, such as polyphenols, essential oils, carotenoids, vitamins, minerals, bioactive peptides, and probiotics, possess anticancer, anti-inflammatory, and antioxidant characteristics. These substances can be regularly ingested. (Khoddami et al., 2015). However, because of their low oral bioavailability and bioaccessibility and high vulnerability to hostile conditions, their application is limited. The gastrointestinal (GI) destiny of bioactive chemicals, the process of digestion, and the fundamentals of molecular absorption are covered in this section.
The release of bioactive compounds
Digestion
There are obvious differences between the microenvironments in the different sections of the GI tract. Bioactive chemicals must first overcome a number of physiological and chemical obstacles in the GI tract in order to be absorbed into the systemic and lymphatic circulations. The oral cavity presents the first hurdle for bioactive substances after consumption. Saliva normally contains salts and amylases and has a neutral pH, so it is combined with food (Boza et al., 2019). Chyme, a viscous semifluid substance that is moved to the intestine for additional digestion, is the result of the physical breakdown and digestion of solid foods in the stomach. The materials undergo a sharp pH decrease during this process, going from around 6.8 to 2.0, and as they reach the tiny size, they move from an extremely acidic to a somewhat basic environment. Three to four hours is the typical residence time in the small intestine and two to three hours is the usual transit time in the stomach.In addition to the severe acidic environment of the stomach, enzymatic nutritional degradation presents a hurdle. Due to the action of gastric pepsin and lipase, certain proteins are broken down into peptides in the stomach, while some lipids are broken down into monoacylglycerols and free fatty acids (Shukla et al., 2020).
Trypsin, elastase, lipase, and carboxypeptidases A and B are a few of the pancreatic enzymes that aid in the breakdown in the duodenum and ileum. While amylases hydrolyze the majority of tri- and diacylglycerols into oligosaccharides and glucoses, most digestible polysaccharidessuch as starchare broken down by enzymes into free fatty acids and monoacylglycerols, which are then absorbed and metabolised by epithelial cells (Baharoglu et al., 2021). Most meal components are processed and absorbed by the upper gastrointestinal tract; however, some, including mineral oil and dietary fibre, cannot be processed due to their distinct shapes and compositions. Once a component enters the colon, different bacteria break it down (Yadav et al., 2022) (Fig. 4) and (Table 2).
Absorption
Ingested dietary ingredients and nutraceuticals are mostly absorbed in small intestines. Small intestines are separated into two sections: epithelial cell layers and the mucus layer. Mucus layer is made up of glycoproteins and polysaccharides (Kobo et al., 2023). Ingested materials have to cross mucous membranes before they can be absorbed by epithelial cells. The physicochemical characteristics of the nutrients—such as hydrogen bonding, hydrophobic and electrostatic interactions, and polymer entanglement—determine how well the nutrients diffuse through the mucus layer. The interactions between the mucus layer and pore size affect how nutrients reach epithelial cells. Substances cannot readily pass through the mucus layer if their size is greater than the mucus pore size, which is a few hundred nanometers (Irizar et al., 2020). The three distinct cell types that make up the intestinal cell layer, where absorption takes place, are in contact with the digested components. First, enterocytes which are the most prevalent cells in the small intestinehave microvilli on the lumen side and are in charge of both active and passive transport-based nutrition absorption. Second, the goblet cells that are present in all intestinal cells secrete mucus. Third, the microfold (M) cells that are present in Peyer's patches are highly capable of transcytosis and are involved in immunological reactions (Razavi et al., 2020).
Transportation
There are four main transport pathways for substances that are swallowed and make it pass the gut. Passive diffusion is the first pathway process that happens through the paracellular or transcellular channel in response to osmotic pressure. Small molecules that are hydrophobic enter the cell through transcellular diffusion, while hydrophilic molecules are transferred through paracellular diffusion (Abdullaeva et al., 2020).The precise binding of nutrient ligands to their matching receptors on the surface of intestinal cells initiates the second mechanism, known as receptor-mediated transport, an energy-dependent system. Thirdly, substances can enter or exit cells through cellular protein transporters through a process known as carrier-mediated transport, which involves both assisted diffusion and energy-dependent active transport. As absorbed bioactive substances can be pumped out to the luminal side of the intestine via these efflux pumps, resulting in relatively poor bioavailability, the final process involves the efflux pump, an energy-dependent pathway that causes drug resistance (Tekant et al., 2021).
Release kinetics model and various studies of bioactive compound
A release kinetics model describes the relationship between the concentration of a bioactive compound over time, allowing us to predict the release rate of compound. It is based on the physical properties of compoundsuch as solubility and diffusivity. In drug development, release kinetics models are typically used to understand how compounds are released in specific delivery systems or to predict the dose-dependent efficacy of compound. Several studies on bioactive compounds have been conducted in order to better understand their effects on the physiological and biochemical processes of the body. These studies are aimed at identifying the active components of these compounds, as well as their biochemical pathways, pharmacokinetics, and potential therapeutic effects. They can also be used to learn about the safety and efficacy of compounds, as well as their potential applications in disease treatment (Matha et al., 2020).
A release kinetics model is a mathematical model that describes how bioactive compounds are released from a matrix, such as a drug. It predicts the rate and amount of bioactive compound released from a drug based on variables such as temperature, pH, mechanical agitation, and diffusion. Several studies have shown that the release kinetics model can predict the release of bioactive compounds from a drug with high accuracy (Anwar et al., 2022). Several studies, for example, have shown that release kinetics models can accurately describe the release of drugs containing multiple active ingredients. Other studies compared the release kinetics of different bioactive compounds to determine which formulation has the best bioactivity (Harper et al., 2020).
Three stages of bioactive component release are observed in encapsulants: surface release, diffusion through an expanding matrix, and matrix erosion (Bule et al., 2020). Other mechanisms that were investigated included fissure formation, hydrostatic pressure, changes in geometry brought on by shear pressures, pH, and enzyme-mediated matrix degradation. A polar bioactive compound's propensity to partition towards an emulsion's hydrophilic surface or inadequate trapping inside the matrix can both result in surface release (Chand et al., 2020). A number of mathematical models have been created to explain how bioactive components release (Fotouhifar et al., 2021).
Zero order (Eq. (1)), first order (Eq. (2)), Hixson-Crowell (Eq. (3)), and Korsmeyer-Peppas (Eq. (4)) kinetic models were used:
where Qt denotes the quantity released after time t; Q0denotes the initial quantity, which is usually zero; Mt denotes the cumulative release at time t; M denotes the cumulative release at infinite time; The kinetic constants K0, K1, KHC, and k represent zero-order, first-order, Hixson-Crowell, and Korsmeyer-Peppas, respectively; n represents the diffusion exponent in the Korsmeyer-Peppas model. Characterizing release from porous matrices is often done using the zero-order and first-order models. The Hixon-Crowell model's linear graph of the cubic root of the unreleased fraction of capsule vs. time demonstrates how the surface area of composite varies as the release process does. R2 values for the Hixson-Crowell model were similar to those for first-order and zero-order kinetics. The Korsmeyer-Peppas model is commonly employed in situations when the release mechanism is unclear or if multiple release mechanisms are controlling the release process (Luong et al., 2020).
Consequently, a number of internal and external stimuli are known to initiate the releasing action (Abbasi et al., 2021). Various models have been created to forecast the release of bioactive agents while taking into consideration the possibility of varying kinetic rates (Chanutin et al., 2020). Using models like zero-order, first-order, Korsmeyer-Peppas, and Hixson Crowell, a number of academics have tried to forecast experimental release data. The Korsmeyer-Peppas model of bioactive component release is used in most studies on the release of corrosion inhibitor from nanocontainers (Laleta et al., 2022).
The encapsulation efficiency, bioactive component release kinetics, capsule stability, and matrix properties are all influenced by the encapsulation method and materials. Although the size of the encapsulating material influences the properties of encapsulants and the regulated release of bioactive substances, without any effect on the encapsulation efficiency (Ghazizadeh et al., 2020).
Ultimately, research has shown that the majority of studies report the encapsulation efficiency since kinetic models are more commonly used to implement simulations and evaluate the diffusion process. Although one of the most important factors in determining how well the core material (bioactive component) is preserved, there aren't many researches that discuss them in food applications (Cheng et al., 2023).
Future work and conclusion
Microencapsulating bioactive compounds, vitamins, and minerals has shown significant benefits, enhancing their solubility, stability, bioavailability, and controlled release. Compared to traditional methods, microencapsulation offers improved compatibility, new delivery formats, better taste, and increased safety. This technology has advanced rapidly and is set to become a key player in the nutrition and food industries. The primary goals of encapsulation include improving stability, solubility, bioavailability, sensory attributes, preserving microstructure and bioactive qualities, reducing hygroscopicity, and extending shelf life. However, the bitter taste and breakdown during digestion have limited their use. This paper explores encapsulation technologies like spray-drying and electro-spraying for bioactive peptides, considering both coaxial and monoaxial versions. It examines unique process parameters for each technology, such as feed flow, electrical potential, drying air inlet and outlet temperature, and injector-collector distance. Despite the development of new bioactive peptide sequences, their stabilization has received little attention. Research on encapsulating protein-based bioactives using monoaxial spray-drying is scarce, and almost non-existent for coaxial spray-drying. Most studies have focused on parenteral nutritional supplements and medicinal uses, leaving a significant gap in research on oral supplements and food applications. The encapsulation process is crucial for maintaining the effectiveness of functional foods containing bioactive proteins and peptides. The potential of coaxial spray-drying has not been fully explored, as current research has primarily focused on the monoaxial arrangement. While advanced techniques like electro-spraying have shown promise in pharmaceutical production, their potential in the food industry requires further investigation. As research continues, we anticipate more advancements in the nano/microencapsulation of bioactive peptides for functional foods. Future improvements in the properties of vitamins, minerals, and bioactive compounds may arise from combining microencapsulation with other technologies, enabling new capabilities. Microencapsulating bioactive compounds is expected to benefit the food and beverage, cosmetics, and pharmaceutical industries as more precise and efficient encapsulation technologies emerge. With its broad range of applications, microencapsulation will be essential in delivering various food and health ingredients.
References
Abbasi S, Hemmati S, Emami, S. Microencapsulation of Bioactive Compounds: A Technical Review. International Food Research Journal. 28(2): 708-724 (2021)
Abdullaeva K, Hövelmann, H. Microencapsulation of Vitamins and Minerals: A Novel Approach Toward Addressing Micronutrient Deficiencies. Journal of Food Science. 85(1): 13–22 (2020)
Afo B, Havulinna AS, Sarkanen S, Linko P. The role of microencapsulation of bioactives on functional food engineering. Food Control. 103: 136–142 (2019)
Aklipinar N, Aydin S, Yazici A. Tuncel G. Microencapsulation of vitamins and minerals: Recent trends and applications. Trends in Food Science & Technology. 100: 97-107 (2020)
Aktas H, Napiórkowska A, Szpicer A, Custodio-Mendoza JA, Paraskevopoulou A, Pavlidou E, Kurek MA. Microencapsulation of green tea polyphenols: Utilizing oat oil and starch-based double emulsions for improved delivery. International Journal of Biological Macromolecules. 133295 (2024)
Anjum S, di-Saala N, Tannery A. A review on micro-encapsulation of lipids in food: Encapsulation techniques, polymeric materials, properties and applications. Food Technology and Biotechnology. 58(3): 426-445 (2020).
Anwar S, Akhtar MA. Microencapsulated nutraceuticals: A comprehensive review. The Journal of Pharmaceutical Sciences and Research. 4(5): 57-65 (2022).
Baharoglu G, Alemdaroglu H, Ozkutlu F, Velioglu YS. Recent advances in the encapsulation and controlled delivery of vitamins. Comprehensive Reviews in Food Science and Food Safety. 20(4): 1906-1920 (2021)
Bai S, Wang S, Song Y, Li Y, Gao W, Li J. Microencapsulation techniques for vitamin and mineral fortification of functional food. Trends in Food Science & Technology. 93: 441-461 (2020)
Bang YG, Chia LS, Tan CT, Chia TF, Ikehata K, Thongsawat S, Perumbilavil T, Mohan V, Moghadasian MH, Shahidi F. Microencapsulation of dietary lipids for enhancement of their structure, functional, and health properties. Food Structures, 6: 1-39 (2017)
Bińkowska W, Szpicer A, Wojtasik-Kalinowska I, Półtorak A. Innovative Methods of Encapsulation and Enrichment of Cereal-Based Pasta Products with Biofunctional Compounds. Applied Sciences. 14(4). 1442 (2024)
Bodbodak S, Nejatian M, Ghandehari Yazdi A P, Kamali Rousta L, Rafiee Z, Jalali-Jivan M, Jafari SM. Improving the thermal stability of natural bioactive ingredients via encapsulation technology. Critical Reviews in Food Science and Nutrition. 64(10). 2824-2846. (2024)
Boza JJ. Calderón-Montaño JM, Vallverdu-Queralt A. Benefits of encapsulating vitamins and minerals and their applicability in food. Comprehensive Reviews in Food Science and Food Safety. 18(4): 945-959 (2019)
Bule BT, Markese SK, Shukla K. Microencapsulations technology for encapsulation of phytochemical and functional food. International Food Research Journal. 27(6): 2409-2417 (2020)
Burgal L, Jimenez JM, Burgos MF. Advances in Microencapsulation Technologies for Incremental Preservation and Controlled Release of Vitamins, Minerals and Bioactive Compounds. Journal of Food Science. 81(7): 1873-1882 (2016)
Chand R, Ali I. Microencapsulation of Vitamin A and B2 as Nutraceuticals. Natural Product Research. 34(10): 1193-1201 (2020)
Chang YW, Wu ZH, Wang S, Yan YC, Zhang ZM. Microencapsulation techniques for potential delivery of bioactive compounds, vitamins, and minerals. Advances in food technology. 7(3): e00368 (2017).
Chanutin A, Ballesteros T, Tam NFY. Microencapsulation of Oil-Soluble Bioactive Compounds for Use in Functional Foods and Nutraceuticals. Nutrients. 12(11): 3397 (2020)
Chauce J, Mendoza C. Microencapsulation for improvement of taste masking and stability of bioactive compounds. Food Science & Nutrition. 10(2): 1005-1013 (2022)
Cheng J, Zhang Q, Han R, Wang Y, Liang J. Application of Microencapsulation Techniques in Dietary Supplement. Nutrition. 90: 63-70 (2023)
Coelho S C, Giron A, Rocha F, Estevinho B N. Electrosprayed B‐complex vitamins/zein microparticles for drug sustained release and antioxidant applications. Journal of Chemical Technology & Biotechnology 99(1). 217-226. (2024)
Cortés-Rojas DF, Souza CRF, Oliveira WP. Optimization of Spray Drying Conditions for Production of Bidens pilosa L. Dried Extract. Chem. Eng. Res. Des. 93: 366–376 (2015)
Deng W, Ma Y, Jia X, Zhang C, Yang X, Zhou L. Micro-encapsulation of differentially loaded dietary fiber microparticles for improving the stability of bioactive components. Food Control. 115: 107739 (2020)
Donetti A, Silva C, Oliveira D, Teixeira J. Microencapsulation of minerals for nutritional applications. Trends in Food Science & Technology. 105: 101-111 (2021)
Eleke SO, Igbokwe UV. Microencapsulation of food bioactive components and vitamins: A review. Food and Bioprocess Technology. 5(7): 1912–1929 (2012)
El-Sayed S M, El-Sayed H S, Youssef A M. Recent developments in encapsulation techniques for innovative and high-quality dairy products: Demands and challenges. Bioactive Carbohydrates and Dietary Fibre. 100406. (2024)
Fontana M, Morgen C, Lawrence A, Davidson K, Loeffler D, Rankin S. Encapsulation Technologies and Microencapsulation of Vitamins and Minerals: Advantages and Applications in TRPM8 Ion-Channel Modulation. Recipes in Pharmaceutical Science. 5(4): 482-497 (2020)
Fotouhifar KB, Seifi H, Serpooshan V. Microencapsulation of lipophilic Bioactives: A review. Journal of Food Bioactives. 18: 5-15 (2021)
Gallego M, Cabo M, Tateno T. (2023) Microencapsulation of vitamins and minerals for enhancing their stability and bioavailability. Journal of Biomolecular Structure and Dynamics, 364-375
García‐Morales AM, Peñalver RP, Sánchez‐Saiz MR, Suárez AA. Structured emulsions based on alginate‐monoglycerides systems for microencapsulation of macroencapsulated bioactives. Food Hydrocolloids. 105: 106160 (2021)
Ghazizadeh M, Asadi S, Alizadeh AM, Sobhani F. Microencapsulation of bioactive compounds: A Review. Food Science and Applied Technology. 5(1): 45-52 (2020)
Guil-Guerrero JL. Microencapsulation: An overview in food applications. Trends in Food Science & Technology. 98: 150-164 (2020)
Gul T, Barrie A, Fincato E, Biasutti M, Campiglia E, Venturelli E. Microencapsulation of vitamins and minerals in microparticles based on different polymers and oils for improved stability. International Journal of Molecular Sciences. 21(2): 502 (2020)
Harper HM, Dong J, Wu-Pong S, Fontana LJ. Novel and Functional Microencapsulation Techniques to Improve the Bioavailability, Stability, and Safety of Mineral Micronutrients. Nutrients. 12(3): 728 (2020)
He L, Yang X, Zhang S, Zhao C, Xian L, Li X. Controllable and Encapsulatable Ascorbic Acid Microcapsules Based on Bio-transformable Matrix. Materials. 13(17): 3681 (2020)
Hou Y, Sun HQ, Tang H, Chen Z, Liang Y, Zhao S. In situ preparation of vitamin B12 microcapsules by coacervation and their controlled release. Cold Chain Logistics. 9(4): 943-951 (2020)
Huang Y, Xu B, Xu Z, Pi J. Microencapsulation techniques for functional food fortification: Applications, benefits and advances. Food Research International. 132: 109246 (2020)
Hudson J, de Freitas V. Microencapsulation technologies in the functional food area: control of nutraceutical release. Trends in Food Science & Technology 91: 304-314 (2020)
Huq T, Riedl B, Bouchard J, Salmieri S, Lacroix M. Microencapsulation of Nisin in Alginate-Cellulose Nanocrystal (CNC) Microbeads for Prolonged Efficacy against Listeria Monocytogenes. Cellulose. 21: 4309–4321 (2014)
Insomnia G, Rasul S. Microencapsulation of Vitamins and Minerals: Challenges and Opportunities. Nutrition Reviews. 78(1): 13–23 (2020)
Irizar I, López R, Corredig M, Chiralt A. Microencapsulation of Bioactive Compounds: A Realistic and Sustainable Alternative for Food and Health. Comprehensive Reviews in Food Science and Food Safety. 19(4): 969–991 (2020)
Kadariya J, Kang Y, Batt R. Vitamin C Microencapsulation in Food Systems and Its Health Benefits. Nutrients. 12(12): 3750 (2020)
Kahsai AG, Belay T. Vegetable oil-based edible films for encapsulation and protection of bioactive compounds. A review. Trends in Food Science & Technology. 91: 721-729 (2020)
Kang BH, Park SH, Oh SH. Development and use of microencapsulation approaches for the protection and controlled delivery of minerals and vitamins. Food Reviews International. 31(3):193-202 (2015)
KAUR G. Encapsulation techniques and their applications in the food industry. Advanced Research Methods in Food Processing Technologies: Technology for Sustainable Food Production. 317. (2024)
Kaushik A, Mahato KK, Makhija S, Suleria HAR, Srinivasan P. Calcium microencapsulation using alginate-caseinate beads: The impact of incorporation of chitosan as a semi-permeable coating layer. Food Chemistry. 337:128881 (2021)
Khoddami V. Development and characterization of polyvinyl alcohol-gum kondagogu microcapsules containing bioactive fatty acid conjugates. Food Hydrocolloids. 46: 505-514 (2015)
Kim HJ, Tidiane C, Ba M. Microencapsulation of Bioactives: Approach for Nutraceuticals Formulation and Delivery. Trends in Food Science & Technology. 91: 30-44 (2023)
Kobo NM, Fagbohun JA, Onyimba GO, Audu OA, Raji SB, Antikainen T. (2023) Microencapsulation techniques: A review of application for minerals. Critical Reviews in Food Science and Nutrition. 63(2)
Kopecna K, Secanin B, Tešević V, Yelic M. Encapsulation techniques for improving stability and delivery of vitamins and minerals. International Journal of Molecular Sciences. 21(4): 1303 (2020)
Kouzouli E, Gallois P, Delmas M, Olivier-Bourbigou H. Characterization of Fish Oil Microencapsulated by Complex Coacervation Toward Potential Applications for Mineral and Vitamin Fortification. Metabolites. 10(2): 23 (2020)
Kumar D, Mukherjee S, Mahapatra T. A review on microencapsulation of bioactives. Journal of Microencapsulation 37(1): 82-104 (2020)
Kurek M A, Ogrodowska D, Tańska M, Šeregelj V, Vulić J. (2024) Encapsulation efficiency of food bioactive ingredients during spray drying. Spray Drying for the Food Industry, pp. 473–516. Woodhead Publishing
Laleta K, Seham D. Microencapsulation Techniques and Applications in Functional Foods. Foods Technology and Nutraceuticals. 5(1): 17-32 (2022)
Lee H, Zhong W, Kim E, Lee W. Microencapsulation of bioactive vitamins and minerals by coacervation. Food Science and Nutrition. 8(7): 2583-2589 (2020)
Li H, Li R, Wu N, Yuan Y, Li R, Zhang Y. Microencapsulation of various vitamins and minerals and their impact on bioavailability: A review. Journal of Food Science. 85(1): 216-223 (2020)
Li L, Yang H, Li Y, Bai M, Zhang Y. Preparation of soybean protein-pectin coacervates microcapsules containing vitamins and minerals based on an emulsion-crosslinking technique. Food Chemistry. 321: 126874 (2020)
Li W, Rangappa SS, Long J, Shen J, Vidya Sagar MP. Pseudolatex encapsulation of bioactives: A vehicle of sustained release for food, nutraceutical, and pharmaceutical applications. Trends in Food Science & Technology. 93: 156-166 (2020)
Li Y, Zhang W, Chen N, Chai W, Lin Y. Microencapsulation of lycopene using milk protein and edible starch: preparation, characterization, and lycopene bioaccessibility. Food Chemistry. 319: 126423 (2020)
Li X, Wang Y, Jiang Y, Liu C, Zhang W, Chen W, Bai W. Microencapsulation with fructooligosaccharides and whey protein enhances the antioxidant activity of anthocyanins and their ability to modulate gut microbiota in vitro. Food Research International. 181. 114082. (2024)
Liao C, Shi L, Zhou Z, Liu J, Zhang Y. Microencapsulation of Vitamins and Minerals: From Characteristics, Encapsulating Materials, and Techniques to Their Structures and Roles. Molecules. 25(13): 3083 (2020)
Lima N G, Lima G N, da Costa Abreu V G, Lopes P H S, da Costa J M G. Effects of native oat starch on vitamin B12 microencapsulation: New perspectives on encapsulants. Powder Technology. 434. 119325. (2024)
Lobo VDS, Maia MB, Wimmer MA. Microencapsulation of phenolic bioactives: Potential application for functional foods. Microbial Cell Factories. 19(1): 38 (2020)
López-Malo M, Palafox-Carlos H, de la Garza T, Rodríguez-Arco M, Hernández-Pando R, Lara-González J., ... Nieto-Hidalgo M. Development and characterization of stabilized goat milk ready-to-use fortified with vitamins and minerals using dry-encapsulation. LWT. 121: 109704 (2020)
Luong M, Antolovich M, Prenzler PD. Emerging Approaches to Microencapsulation of Bioactive Compounds. Foods. 9(6): 625 (2020)
Mandaliya V, Dutta S, Agarwal V. Microencapsulation of nutraceuticals for enhanced delivery and efficacy. Trends in Food Science & Technology. 92: 561–574 (2020)
Mansur H, Siuly P. Microencapsulation of Vitamin D3 in β-cyclodextrin/gum arabic complexes: Optimization and characterization. Food Bioscience. 33: 100295 (2020)
Mao W, Zhang J, Sun H, Liu H, Ding PH, Wang D. Microencapsulation capacity of polymers for vitamins optimization. Food Reviews International 37(2): 239-257 (2021)
Masuko S, Shimada T, Sakakibara N. Microencapsulation technologies for bioactive properties of vitamins and minerals. Comprehensive Reviews in Food Science and Food Safety 19(1): 114-126 (2020)
Matha VS, Jain R, Bawa AS. Microencapsulation: Encapsulating Actives for Enhanced Delivery. Current Nutraceuticals. 16(2): 172–180 (2020)
Matras E, Sali I, Tazzoli M. Role of Microencapsulation Technology in Ensuring Properties of Bioactive Compounds. Current Pharmaceutical Design 26(14): 1896–1913 (2020)
Metin H. (2020) Microencapsulation of bioactive components in nutrition and food processing, 19–29
Mohanty N, Ma Q, Parkash A, Chawla SK, Jain M, Sakar M. Microencapsulation: A method to preserve the bio-actives of medicinal plants. Pharmacognosy Reviews. 15(1): 9-22 (2021)
Musaei M, Mokhtari J, Nouri M, Rad ZP. Fabrication and Characterization of Nanocapsules of PLGA Containing BSA Using Electrospray Technique. Nanomed. Res. J. 2: 158–164 (2017)
Naik A, Meda V, Lele SS. Freeze Drying for Microencapsulation of $α$-Linolenic Acid Rich Oil: A Functional Ingredient from Lepidium Sativum Seeds. Eur. J. Lipid Sci. Technol. 116: 837–846 (2014)
Ostertag F, Weiss J, McClements DJ. Low-Energy Formation of Edible Nanoemulsions: Factors Influencing Droplet Size Produced by Emulsion Phase Inversion. J. Colloid Interface Sci. 388: 95–102 (2012)
Oubaert E, Muylaert K. Food Microencapsulation: Techniques, Food Matrixes, Coatings, Bioactives, Delivery Systems, and Nutraceutical–Pharmaceutical Applications. Pharmaceuticals. 13(10): 273 (2020)
Paes F E R, de Sousa Sabino L B, da Silva L M R, da Silva I J, Ricardo N M P S, de Brito D H A, de Figueiredo R W. Anthocyanins extracted from Jamelon fruits (Syzygium cumini L.): Effect of microencapsulation on the properties and bio accessibility. South African Journal of Botany. 166. 423-431. (2024)
Park S, Kim J. Microencapsulation of Nutraceuticals for Enhanced Stability and; Controlled Release. Current Drug Delivery. 10(4): 224-235 (2023)
Peres DN, Nogueira ACS, Monteiro SASM, Mantovani MS. Microencapsulation of vitamins and bioactives for food industry. Food Science and Technology International. 16(5): 403-411 (2010)
Pilocie T, Bogacka E, Szubrycht-Madej E, Karska-Rybicka E, Misiolek M. Microencapsulation: A Strategy to Enhance Nutritional and Functional Properties of Food Bioactives. Nutrition Research Reviews. 33(2): 269-286 (2020)
Quintas-Soriano T, González-Fandos E, Martin-Pozuelo T, Carrasco F. Nutraceutical-enriched microparticles obtained by microencapsulation for applications as functional food. European Food Research and Technology. 247(4): 947-952 (2021)
Quiroz. A, Pisabarro G, Villegas D, Lefebvre D. Overview of microencapsulation technologies and products for functional food. Foods. 10(4): 568 (2021)
Raj R, Nizar S, Bhattacharyya C, Savanur M A. Advances in Microencapsulation and Nanoemulsion Techniques of Plant Pigments: Improving Stability, Bioavailability, and Bioactivity for Application in Food Industry. In Plant Specialized Metabolites: Phytochemistry, Ecology and Biotechnology. pp. 1-26. Cham: Springer Nature Switzerland. (2024)
Ramirez-Wong B, Duran-Montiel, DM, Barboza-Cano E, Arzate-Vázquez L, Ustarroz-Leyva MR, Anaya-Gallardo J. Vitamin microencapsulation for functional foods. Advances in Food Science and Technology. 2: 4-23 (2012)
Rao PP, Gupta A, Soni A. Effect of microencapsulation technique on properties of bioactives: a review. Critical Reviews in Food Science and Nutrition. 54(4): 485-493 (2014)
Rasekh M, Young C, Roldo M, Lancien F, Le Mével JC, Hafizi S, Ahmad Z, Barbu E, Gorecki D. Hollow-Layered Nanoparticles for Therapeutic Delivery of Peptide Prepared Using Electrospraying. J. Mater. Sci. Mater. Med.26: 256 (2015)
Razavi M, Mourad S, Reardon K, Mirzaei N. A comprehensive review on microencapsulation techniques for food fortification. Trends in Food Science & Technology. 95: 77–95 (2020)
Reddy UV, Khedkar G, Govekar S. Microencapsulation of minerals, vitamins and bioactives—A review. Trends in Food Science & Technology. 40(2): 252-262 (2014)
Reddy, T. R., Kumar, M., Sudhakar, T., & Narayana, K. (2023). Recent Advances in Microencapsulation of Nutraceuticals. Current Pharmaceutical Design, 19(30), 5539-5556. https://doi.org/10.2174/1381612824666231216151850
Ronconi E, Rastrelli S, Fattorini L, Ye Q, Testa I. Microencapsulation of Vitamin C by spray drying for osmotic drug delivery systems: Concentration, formulation and physicochemical properties. Food Research International. 138: 109423 (2021)
Saha S, Lin L. Recent Advances in Microencapsulation for Nutraceuticals. Current Reviews in Food Science & Technology. 10(1): 83-98 (2023)
Sarkar S, Mukherjee B, Seal A, Paul TK. Physico-chemical, functional and structural properties of micro-encapsulated bioactives—A review. Food Chem. 310: 126250 (2020)
Scott-Thomas C, Wassef R, Lacroix M. (2021) A look into the stability of microencapsulated bioactive compounds. In Vitamins & Minerals: Utilization and Applications. CRC Press.
Sharma G, Singh K. Microencapsulation for Nutraceuticals Fortification: A Review. Journal of Science and Food Science. 93(1): 120-127 (2023)
Sheng P, Zhang Z, Dong G, Yu M. Improvement of the antioxidant properties of carotenoids via microencapsulation technologies. Food & Function 11(3): 2036-2047 (2020)
Shindou Y, Bo R, Fukushima K, Omori T. Effect of Microencapsulation on Retention of Vitamin C and Mineral Content in Nutritional Supplements. Journal of Food Science. 86(3): 1016-1022 (2021)
Shukla R, Kapoor S, Verma A, Prakash O. Microencapsulation techniques of bioactive molecules: A review. Journal of Pharmaceutical Analysis. 10(2): 222-233 (2020)
Singh R, Sahoo N. Novel encapsulation and delivery of nutraceuticals - Recent advances in dietary supplement delivery systems. International Journal of Pharmaceutics. 577: 119125 (2020)
Sirilegar S, Hutchins W, Shah PG, Dzijak C. Microencapsulation of vitamins and minerals: Processing and evaluation criteria. International Journal of Food Properties. 23(9): 2504-2518 (2020)
Sun Y, Zhang X, Xiao C, Wang Y, Zhao T, Lv C., Gao C. Application and encapsulation technology of bioactive components in functional foods. Trends in Food Science & Technology. 112: 269-279 (2021)
Tekant B. Microencapsulation of bioactives and vitamins for food fortification and health benefits. Foods. 10(1): 21 (2021)
Tian L, Niu Y, Ran J, Gao Y. Preparation and application of nanostructured microencapsulation system for delivery of polyphenols. Polymers 12(7): 1745 (2020)
Tkacz K, Turkiewicz I P, Nowicka P, Wojdyło A. Microspheres as carriers of sea buckthorn carotenoids with antidiabetic potential: Effect of biopolymers, cross-linking and storage. Food Bioscience. 59. 103995. (2024)
Tomasini NS, Casarin F, Bellin AK, Gutkoski LC. The influence of microencapsulation on biological properties of nutritional compounds. Journal of Food and Nutritional Disorders. 8(3): 56–63 (2019)
Wang Y, Liu Y, Zhang P. Microencapsulation of food-grade vitamins, minerals and antioxidants: characteristics, technology, and application. Food engineering Reviews 4(1): 41-54 (2012)
Wang S, Feng Y, Zhang X, Shou Y, Li L, Liu Z. Microencapsulation of vitamins and minerals in protein matrixes by emulsion-w/o/w Processing. Advances in Food Science and Technology. 6(3): 62-67 (2015)
Wang B, Lu H, Ma X, Li P, Li J, Liu Y. Effects of microencapsulation on antioxidative abilities of vitamins and minerals in fruits and vegetables. LWT. 126: 109431 (2020)
Yadav A, Gogoi P. Role of microencapsulation techniques in biofortification of food. Critical Reviews in Food Science and Nutrition. 62(2): 306-316 (2022)
Yang Z, Duan Y, Liu S, Liu, B, Gao X, Xie J, Wei F. Preparation and evaluation of soybean oil microencapsulates and their application to margarines processed by dry extrusion technology. Food Hydrocolloids. 96: 105378 (2020)
Yao S, Liu H, Yu S, Li Y, Wang X, Wang L. Drug-Nanoencapsulated PLGA Microspheres Prepared by Emulsion Electrospray with Controlled Release Behavior. Regen. Biomater. 3: 309–317 (2016)
Yao X, Zhou B, Huang L. Microencapsulation Technology and Its Application in Stabilizing Bioactive Components, Vitamins, and Minerals. Comprehensive Reviews in Food Science and Food Safety. 20(2): 447–463 (2021)
Yaseen S, Amjad SF, Mansoora N, Kausar S, Shahid, H, Alamri SAM, Alrumman SA, Eid EM, Ansari MJ, Danish S et al. Supplemental Effects of Biochar and Foliar Application of Ascorbic Acid on Physio-Biochemical Attributes of Barley (Hordeum vulgare L.) under Cadmium-Contaminated Soil. Sustainability. 13: 9128 (2021)
Youssef MM, Saleh A. Application of Microencapsulation Technology in Enhancing Bioactivity of Nutraceuticals. International Journal of Drug Delivery. 15(3): 420-430 (2023)
Zamani M, Prabhakaran MP, Thian ES, Ramakrishna S. Protein Encapsulated Core–Shell Structured Particles Prepared by Coaxial Electrospraying: Investigation on Material and Processing Variables. Int. J. Pharm. 473: 134–143 (2014)
Zhang Y, Kit CS, Zhu Y, Guo, Y. Current advances in microencapsulation of vitamins, minerals and bioactives. Trends in Food Science & Technology. 77: 37-51 (2018)
Zhao C, Chen G, Wang H, Zhao Y, Chai R. Bio-Inspired Intestinal Scavenger from Microfluidic Electrospray for Detoxifying Lipopolysaccharide. Bioact. Mater 6: 1653–1662 (2021)
Zou M, Huang F. Controlled Release of Bioactives by Microencapsulation: A Review. Current Pharmaceutical Design. 19(18): 3207–3217 (2023)
Acknowledgements
The authors are thankful to Department of Food Technology, Islamic University of Science and Technology, Kashmir, India and Department of Food Technology, Harcourt Butler Technical University, Nawabganj, Kanpur, Uttar Pradesh, India for conducting this review. No public, commercial, or non-profit funding organization provided a specific grant for conducting this review.
Funding
No funding was availed for this review.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest between the authors.
Research involving human and animal rights
This review article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastava, S., Pandey, V.K., Dar, A.H. et al. Effect of microencapsulation techniques on the different properties of bioactives, vitamins and minerals. Food Sci Biotechnol (2024). https://doi.org/10.1007/s10068-024-01666-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10068-024-01666-1